Associations between Total and Added Sugar Intake and Diabetes among Chinese Adults: The Role of Body Mass Index
Abstract
1. Introduction
2. Methods
2.1. Study Sample
2.2. Assessment of Sugar Intake
2.3. Assessments of Covariates
2.4. Definition of Diabetes
2.5. Statistical Analysis
3. Results
3.1. Participant Basic Demographics and Characteristics by Total Sugar Intake Levels
3.2. Total and Added Sugar Intake of Diabetics and Non-Diabetics by Age, Gender, and BMI Category
3.3. Association between Total and Added Sugar Intake and Diabetes Status
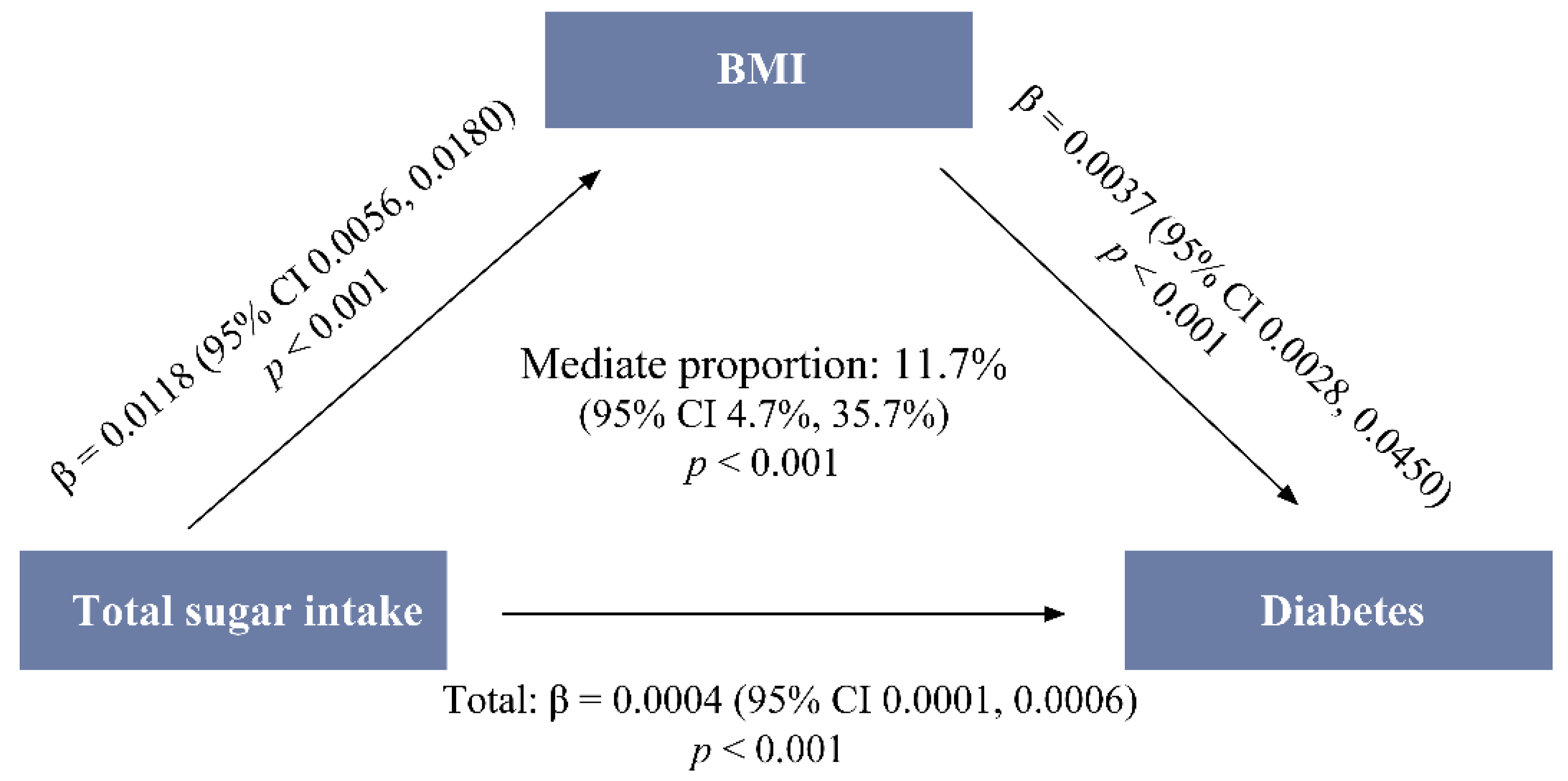
3.4. Mediating Effect of BMI on the Total-Sugar–Diabetes Association
3.5. Moderating Effect of the BMI on the Sugar–Diabetes Association
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 21 May 2023).
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.; Fazli, G.S.; Johns, A.; Booth, G.L. Evolving Trends in the Epidemiology, Risk Factors, and Prevention of Type 2 Diabetes: A Review. Can. J. Cardiol. 2018, 34, 552–564. [Google Scholar] [CrossRef]
- Al Amiri, E.; Abdullatif, M.; Abdulle, A.; Al Bitar, N.; Afandi, E.Z.; Parish, M.; Darwiche, G. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health 2015, 15, 1298. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Gui, Z.-H.; Zhu, Y.-N.; Cai, L.; Sun, F.-H.; Ma, Y.-H.; Jing, J.; Chen, Y.-J. Sugar-Sweetened Beverage Consumption and Risks of Obesity and Hypertension in Chinese Children and Adolescents: A National Cross-Sectional Analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol. 2020, 18, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes/Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Morenga, L.T. Sugar and Type 2 diabetes. Br. Med. Bull. 2016, 120, 43–53. [Google Scholar] [CrossRef]
- China Health and Nutrition Survey. 2022. Available online: https://www.cpc.unc.edu/projects/china (accessed on 6 May 2023).
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Leuk. Res. 2009, 39, 1435–1440. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, J.; Wan, L.; Chen, W. Total and Added Sugar Intakes Are Increasing among Children and Adolescents in China: Findings from CHNS 1997–2011. Nutrients 2022, 14, 3340. [Google Scholar] [CrossRef]
- Yuexin, Y. China Food Composition Tables 2004; Peking University Medical Press: Beijing, China, 2005. [Google Scholar]
- US Department of Agriculture, Agricultural Research Service. 2016. Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (Slightly revised). Version Current: May 2016. Available online: http://www.ars.usda.gov/nea/bhnrc/mafcl (accessed on 12 March 2019).
- Bowman, S.A.; Clemens, J.C.; Shimizu, M.; Friday, J.E.; Moshfegh, A.J. Food Patterns Equivalents Database 2015–2016: Methodology and User Guide Acknowledgement of Reviewers; U.S. Department of Agriculture Agricultural Research Service Beltsville Human Nutrition Research Center Food Surveys Research Group: Beltsville, MD, USA, 2018. [Google Scholar]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sport. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, J.; Lu, M.; Chen, Y.; Yang, Y.; Cao, W.; Meng, K.; Chen, Y.; Zhang, J.; Xu, C.; et al. Compilation of Physical Activities of Healthy Chinese Adults: Reference Values for Energy Expenditure. Chin. J. Sport. Med. 2022, 41, 335–349. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S, discussion in Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1229S–1231S. [Google Scholar] [CrossRef]
- Janket, S.-J.; Manson, J.E.; Sesso, H.; Buring, J.E.; Liu, S. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.-H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- The Chinese Nutrition Society. Dietary Guidelines for Chinese Residents. 2022. Available online: http://dg.cnsoc.org/article/2021b.html (accessed on 27 May 2023).
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P.H. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Ramage, S.; Farmer, A.; Eccles, K.A.; McCargar, L. Healthy strategies for successful weight loss and weight maintenance: A systematic review. Appl. Physiol. Nutr. Metab. 2014, 39, 1–20. [Google Scholar] [CrossRef]
| Total (n = 12,800) | Binaries of Total Sugar Intake | |||
|---|---|---|---|---|
| Group 1 (Lower; n = 6400) | Group 2 (Higher; n = 6400) | p-Value | ||
| Characteristics [mean ± SE 1 or median (25th, 75th)] | ||||
| Age (year) | 50.5 ± 0.1 | 51.4 ± 0.2 | 49.7 ± 0.2 | <0.001 |
| Physical activity (MET 1 h/week) | 194.2 (44.8, 609.1) | 148.6 (38.0, 612.3) | 269.4 (52.5, 604.7) | <0.001 |
| Total energy intake (kcal/d) | 1830.9 (1444.9, 2291.8) | 1668.8 (1305.3, 2133.1) | 1975.9 (1596.9, 2429.3) | <0.001 |
| Total fat intake (g/d) | 65.9 (46.3, 90.5) | 62.2 (43.3, 86.4) | 69.8 (49.6, 93.9) | <0.001 |
| Total protein intake (g/d) | 60.8 (47.1, 77.0) | 54.1 (41.6, 69.2) | 67.2 (53.8, 83.1) | <0.001 |
| Total carbohydrate intake (g/d) | 232.7 (170.2, 306.8) | 211.7 (149.6, 283.7) | 252.4 (193.9, 323.9) | <0.001 |
| Characteristics (n (%)) | ||||
| Age group (year) | <0.001 | |||
| 18–44 | 4544 (35.5) | 2167 (33.9) | 2377 (37.1) | |
| 45–59 | 4573 (35.7) | 2266 (35.4) | 2307 (36.0) | |
| ≥60 | 3683 (28.8) | 1967 (30.7) | 1716 (26.8) | |
| Gender | 0.017 | |||
| Male | 6025 (47.1) | 2945 (46.0) | 3080 (48.1) | |
| Female | 6775 (52.9) | 3455 (54.0) | 3320 (51.9) | |
| BMI 1 (kg/m2) | <0.001 | |||
| <24.0 | 6880 (55.4) | 3619 (58.7) | 3261 (52.2) | |
| 24.0~27.9 | 4038 (32.5) | 1882 (30.5) | 2156 (34.5) | |
| ≥28.0 | 1496 (12.1) | 665 (10.8) | 831 (13.3) | |
| Smoking status (%) | 0.001 | |||
| Non-smoker | 8873 (69.3) | 4395 (68.7) | 4478 (70.0) | |
| Ex-smoker | 555 (4.3) | 242 (3.8) | 313 (4.9) | |
| Current smoker | 3367 (26.3) | 1761 (27.5) | 1606 (25.1) | |
| Unknown | 5 (0.0) | 2 (0.0) | 3 (0.0) | |
| Drinking frequency (%) | <0.001 | |||
| Never | 8461 (66.1) | 4451 (69.6) | 4010 (62.7) | |
| Almost everyday | 1275 (10.0) | 604 (9.4) | 671 (10.5) | |
| 3–4 times per week | 491 (3.8) | 218 (3.4) | 273 (4.3) | |
| 1–2 times per week | 951 (7.4) | 416 (6.5) | 535 (8.4) | |
| 1–3 times per month | 854 (6.7) | 365 (5.7) | 489 (7.6) | |
| <1 time per month | 698 (5.5) | 312 (4.9) | 386 (6.0) | |
| Unknown | 69 (0.5) | 33 (0.5) | 36 (0.6) | |
| Total (n = 12,800) | Diabetes (n = 512) | Non-Diabetes (n = 12,288) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE 1 | Median (25th, 75th) | Mean ± SE | Median (25th, 75th) | Mean ± SE | Median (25th, 75th) | |||
| Age group (year) | ||||||||
| 18–44 | Total sugar intake (g/d) | 29.7 ± 0.4 | 23.3 (11.3, 41.6) | 25.3 ± 3.4 | 19.8 (13.0, 34.8) | 29.8 ± 0.4 | 23.3 (11.2, 41.7) | 0.630 |
| Total sugar (%E 1) | 6.3 ± 0.1 | 5.3 (2.6, 8.7) | 6.2 ± 0.8 | 5.4 (3.4, 7.4) | 6.3 ± 0.1 | 5.3 (2.6, 8.7) | 0.868 | |
| Added sugar intake (g/d) | 5.7 ± 0.2 | 0.3 (0.0, 5.0) | 3.6 ± 1.1 | 0.5 (0.0, 6.5) | 5.8 ± 0.2 | 0.3 (0.0, 5.0) | 0.766 | |
| Added sugar (%E) | 1.2 ± 0.0 | 0.1 (0.0, 1.1) | 0.8 ± 0.2 | 0.1 (0.0, 0.9) | 1.2 ± 0.0 | 0.0 (0.0, 1.1) | 0.098 | |
| 45–59 | Total sugar intake (g/d) | 28.3 ± 0.4 | 22.2 (10.9, 39.1) | 30.5 ± 1.8 | 25.3 (12.3, 43.6) | 28.2 ± 0.4 | 22.1 (10.8, 39.0) | 0.047 |
| Total sugar (%E) | 5.9 ± 0.1 | 4.9 (2.4, 8.1) | 6.2 ± 0.3 | 5.1 (2.9, 8.9) | 5.9 ± 0.1 | 4.9 (2.3, 8.1) | 0.060 | |
| Added sugar intake (g/d) | 4.9 ± 0.2 | 0.0 (0.0, 3.1) | 5.2 ± 0.7 | 0.4 (0.0, 6.2) | 4.9 ± 0.2 | 0.0 (0.0, 3.1) | 0.008 | |
| Added sugar (%E) | 1.0 ± 0.0 | 0.0 (0.0, 0.7) | 1.0 ± 0.1 | 0.1 (0.0, 1.3) | 1.0 ± 0.0 | 0.0 (0.0, 0.7) | 0.006 | |
| ≥60 | Total sugar intake (g/d) | 26.1 ± 0.4 | 20.3 (9.6, 36.3) | 28.6 ± 1.4 | 22.7 (13.2, 36.5) | 25.9 ± 0.4 | 19.8 (9.4, 36.3) | 0.004 |
| Total sugar (%E) | 5.9 ± 0.1 | 4.9 (2.3, 8.3) | 6.7 ± 0.3 | 6.1 (3.5, 8.9) | 5.9 ± 0.1 | 4.8 (2.3, 8.2) | 0.630 | |
| Added sugar intake (g/d) | 4.2 ± 0.2 | 0.0 (0.0, 2.5) | 4.9 ± 0.9 | 0.3 (0.0, 3.1) | 4.1 ± 0.2 | 0.0 (0.0, 2.4) | 0.868 | |
| Added sugar (%E) | 0.9 ± 0.0 | 0.0 (0.0, 0.6) | 1.0 ± 0.2 | 0.1 (0.0, 0.7) | 0.9 ± 0.0 | 0.0 (0.0, 0.6) | 0.766 | |
| Gender | ||||||||
| Male | Total sugar intake (g/d) | 29.7 ± 0.3 | 22.6 (10.8, 41.4) | 33.2 ± 1.7 | 28.1 (16.2, 46.0) | 29.6 ± 0.3 | 22.5 (10.7, 41.2) | <0.001 |
| Total sugar (%E 1) | 5.8 ± 0.1 | 4.8 (2.3, 8.0) | 6.8 ± 0.3 | 5.8 (3.6, 9.2) | 5.7 ± 0.1 | 4.7 (2.3, 7.9) | <0.001 | |
| Added sugar intake (g/d) | 6.2 ± 0.2 | 0.0 (0.0, 4.1) | 6.3 ± 1.1 | 0.8 (0.0, 6.3) | 6.2 ± 0.2 | 0.0 (0.0, 4.0) | 0.005 | |
| Added sugar (%E) | 1.2 ± 0.0 | 0.0 (0.0, 0.8) | 1.2 ± 0.2 | 0.2 (0.0, 1.6) | 1.2 ± 0.0 | 0.0 (0.0, 0.8) | 0.003 | |
| Female | Total sugar intake (g/d) | 26.8 ± 0.3 | 21.4 (10.5, 37.2) | 25.3 ± 1.3 | 20.7 (10.2, 32.8) | 26.8 ± 0.3 | 21.4 (10.5, 37.4) | 0.228 |
| Total sugar (%E) | 6.3 ± 0.1 | 5.3 (2.6, 8.7) | 6.2 ± 0.3 | 5.6 (2.7, 8.5) | 6.3 ± 0.1 | 5.3 (2.6, 8.7) | 0.754 | |
| Added sugar intake (g/d) | 3.9 ± 0.1 | 0.0 (0.0, 3.1) | 3.6 ± 0.5 | 0.0 (0.0, 2.4) | 3.9 ± 0.1 | 0.0 (0.0, 3.1) | 0.800 | |
| Added sugar (%E) | 0.9 ± 0.0 | 0.0 (0.0, 0.7) | 0.8 ± 0.1 | 0.0 (0.0, 0.7) | 0.9 ± 0.0 | 0.0 (0.0, 0.7) | 0.874 | |
| BMI (kg/m2) | ||||||||
| <24.0 | Total sugar intake (g/d) | 27.2 ± 0.3 | 20.6 (9.9, 37.4) | 27.3 ± 1.5 | 20.2 (12.9, 38.4) | 27.2 ± 0.3 | 20.6 (9.9, 37.4) | 0.242 |
| Total sugar (%E 1) | 5.9 ± 0.1 | 4.8 (2.3, 8.2) | 6.4 ± 0.3 | 5.2 (3.4, 9.3) | 5.9 ± 0.1 | 4.8 (2.3, 8.2) | 0.011 | |
| Added sugar intake (g/d) | 4.8 ± 0.2 | 0.0 (0.0, 3.3) | 3.9 ± 0.7 | 0.0 (0.0, 3.0) | 4.8 ± 0.2 | 0.0 (0.0, 3.3) | 0.746 | |
| Added sugar (%E) | 1.0 ± 0.0 | 0.0 (0.0, 0.7) | 0.9 ± 0.1 | 0.0 (0.0, 0.8) | 1.0 ± 0.0 | 0.0 (0.0, 0.7) | 0.641 | |
| 24.0–27.9 | Total sugar intake (g/d) | 29.5 ± 0.4 | 23.6 (11.8, 40.9) | 31.6 ± 1.9 | 24.6 (14.4, 41.1) | 29.4 ± 0.4 | 23.6 (11.7, 40.9) | 0.290 |
| Total sugar (%E) | 6.2 ± 0.1 | 5.3 (2.7, 8.6) | 6.8 ± 0.3 | 6.1 (3.3, 8.8) | 6.2 ± 0.1 | 5.3 (2.6, 8.6) | 0.026 | |
| Added sugar intake (g/d) | 5.2 ± 0.2 | 0.2 (0.0, 3.9) | 6.1 ± 1.2 | 0.8 (0.0, 5.6) | 5.2 ± 0.2 | 0.1 (0.0, 3.7) | 0.024 | |
| Added sugar (%E) | 1.0 ± 0.0 | 0.0 (0.0, 0.9) | 1.2 ± 0.2 | 0.2 (0.0, 1.3) | 1.0 ± 0.0 | 0.0 (0.0, 0.8) | 0.016 | |
| ≥28.0 | Total sugar intake (g/d) | 30.1 ± 0.6 | 25.0 (12.6, 41.4) | 27.7 ± 1.9 | 23.0 (11.9, 37.8) | 30.3 ± 0.7 | 25.3 (12.7, 41.5) | 0.328 |
| Total sugar (%E) | 6.5 ± 0.1 | 5.7 (2.9, 8.9) | 6.3 ± 0.4 | 6.0 (2.9, 8.7) | 6.5 ± 0.1 | 5.6 (2.9, 8.9) | 0.967 | |
| Added sugar intake (g/d) | 5.3 ± 0.4 | 0.2 (0.0, 4.1) | 4.1 ± 1.0 | 0.1 (0.0, 2.3) | 5.4 ± 0.4 | 0.2 (0.0, 4.6) | 0.348 | |
| Added sugar (%E) | 1.1 ± 0.1 | 0.0 (0.0, 1.0) | 0.8 ± 0.2 | 0.0 (0.0, 0.6) | 1.1 ± 0.1 | 0.0 (0.0, 1.0) | 0.314 | |
| Variable | Estimated Coefficient | SE 1 | p-Value | OR 1 (95%CI 1) |
|---|---|---|---|---|
| Total sugar intake (per 1000 kcal) | ||||
| Model 1 | 0.010 | 0.004 | 0.004 | 1.010 (1.003, 1.017) |
| Model 2 | 0.008 | 0.004 | 0.022 | 1.008 (1.001, 1.016) |
| Added sugar intake (per 1000 kcal) | ||||
| Model 1 | 0.004 | 0.007 | 0.525 | 1.004 (0.991, 1.018) |
| Model 2 | 0.004 | 0.007 | 0.601 | 1.004 (0.990, 1.018) |
| Variable | B | SE 1 | p-Value | OR 1 (95%CI 1) |
|---|---|---|---|---|
| BMI < 24.0 kg/m2 | ||||
| Total sugar intake (per 1000 kcal) | ||||
| Model 1 | 0.013 | 0.006 | 0.025 | 1.013 (1.002, 1.025) |
| Model 2 | 0.012 | 0.006 | <0.05 * | 1.012 (1.000, 1.024) |
| Added sugar intake (per 1000 kcal) | ||||
| Model 1 | −0.002 | 0.013 | 0.885 | 0.998 (0.973, 1.024) |
| Model 2 | −0.002 | 0.014 | 0.904 | 0.998 (0.972, 1.025) |
| 24.0 kg/m2 ≤ BMI < 28.0 kg/m2 | ||||
| Total sugar intake (per 1000 kcal) | ||||
| Model 1 | 0.009 | 0.005 | 0.102 | 1.009 (0.998, 1.020) |
| Model 2 | 0.008 | 0.006 | 0.151 | 1.008 (0.997, 1.019) |
| Added sugar intake (per 1000 kcal) | ||||
| Model 1 | 0.010 | 0.010 | 0.274 | 1.010 (0.992, 1.029) |
| Model 2 | 0.010 | 0.010 | 0.323 | 1.010 (0.991, 1.029) |
| BMI > 28.0 kg/m2 | ||||
| Total sugar intake (per 1000 kcal) | ||||
| Model 1 | −0.004 | 0.009 | 0.680 | 0.996 (0.978, 1.014) |
| Model 2 | −0.007 | 0.009 | 0.468 | 0.993 (0.975, 1.012) |
| Added sugar intake (per 1000 kcal) | ||||
| Model 1 | −0.013 | 0.022 | 0.561 | 0.987 (0.946, 1.030) |
| Model 2 | −0.017 | 0.022 | 0.429 | 0.983 (0.941, 1.026) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cheng, J.; Wan, L.; Chen, W. Associations between Total and Added Sugar Intake and Diabetes among Chinese Adults: The Role of Body Mass Index. Nutrients 2023, 15, 3274. https://doi.org/10.3390/nu15143274
Liu Y, Cheng J, Wan L, Chen W. Associations between Total and Added Sugar Intake and Diabetes among Chinese Adults: The Role of Body Mass Index. Nutrients. 2023; 15(14):3274. https://doi.org/10.3390/nu15143274
Chicago/Turabian StyleLiu, Yan, Jing Cheng, Lijin Wan, and Wei Chen. 2023. "Associations between Total and Added Sugar Intake and Diabetes among Chinese Adults: The Role of Body Mass Index" Nutrients 15, no. 14: 3274. https://doi.org/10.3390/nu15143274
APA StyleLiu, Y., Cheng, J., Wan, L., & Chen, W. (2023). Associations between Total and Added Sugar Intake and Diabetes among Chinese Adults: The Role of Body Mass Index. Nutrients, 15(14), 3274. https://doi.org/10.3390/nu15143274





