Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Registration and Reporting of Systematic Review
2.2. Research Questions
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Study Selection
2.6. Data Extraction
2.7. Risk of Bias
2.8. Statistical Analysis
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Risk of Bias
3.4. Effect of Zinc Alone on Risk of Malaria Parasitaemia
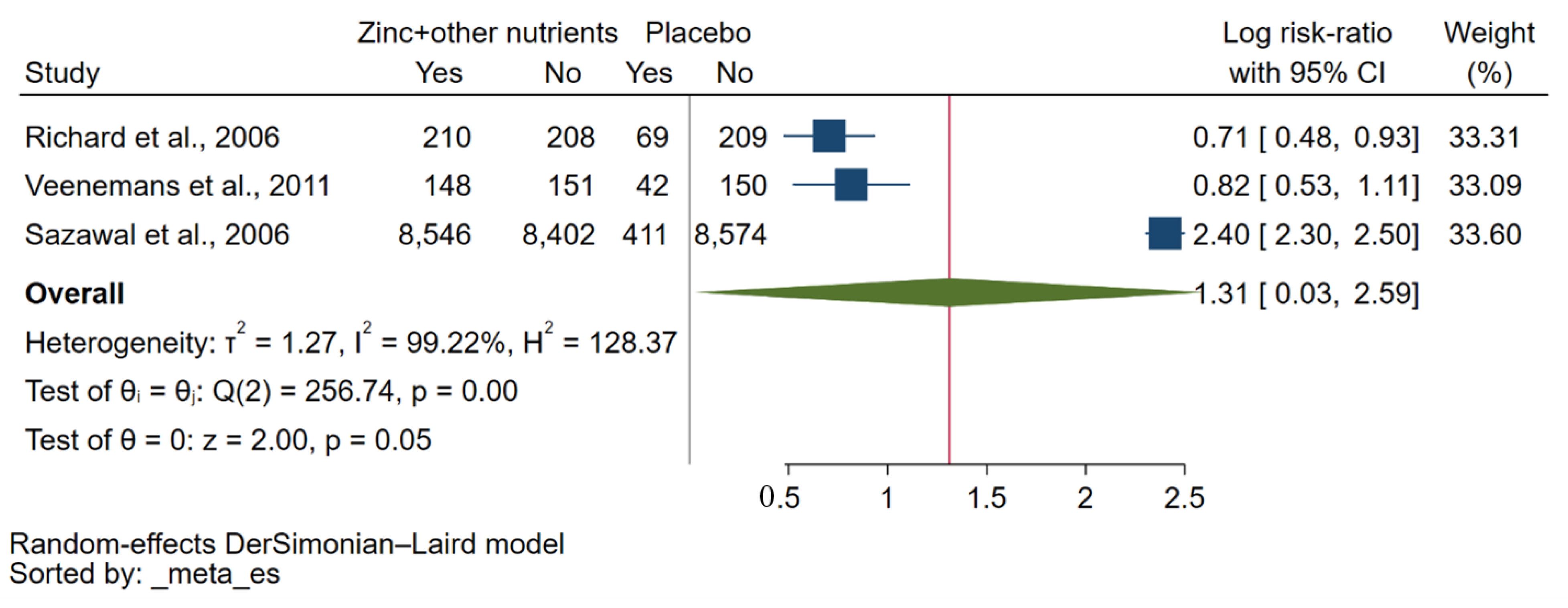
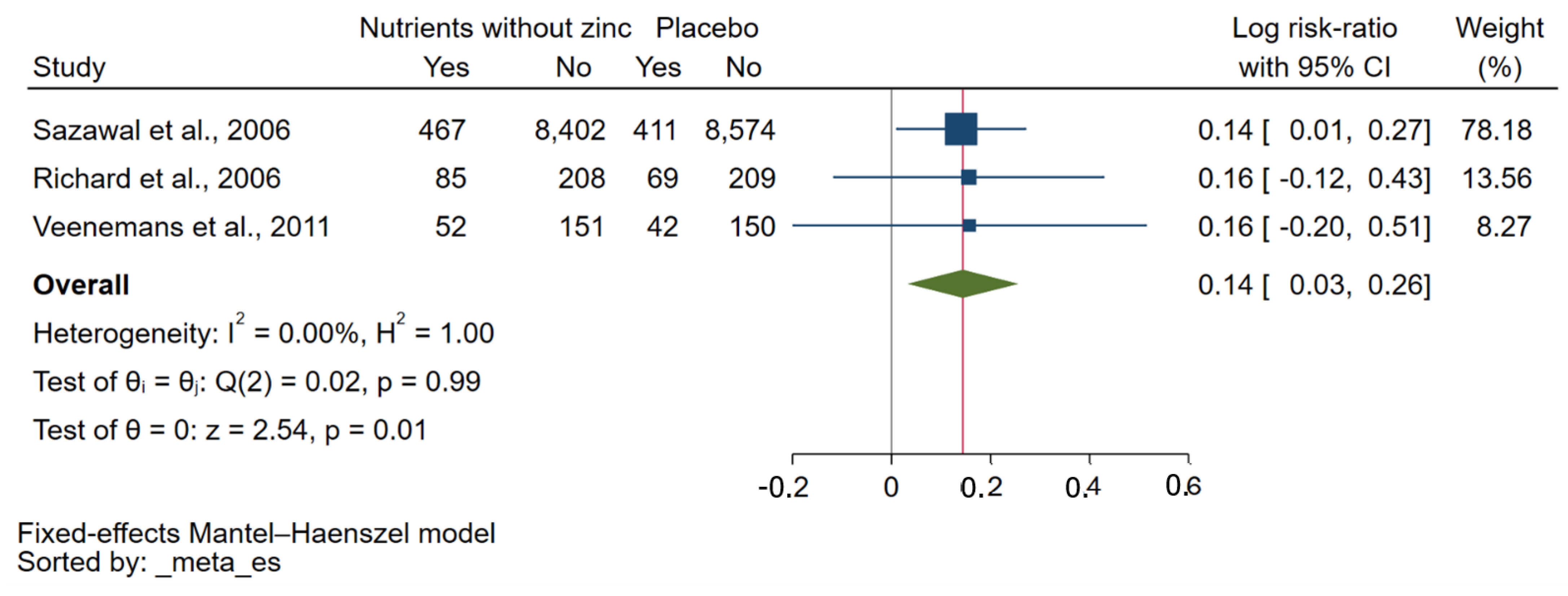
3.5. Effect of Zinc Plus Other Micronutrients on Risk of Malaria Parasitaemia
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sukkanon, C.; Masangkay, F.R.; Mala, W.; Kotepui, K.U.; Wilairatana, P.; Chareonviriyaphap, T.; Kotepui, M. Prevalence of Plasmodium spp. in Anopheles mosquitoes in Thailand: A systematic review and meta-analysis. Parasites Vectors 2022, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Correction to: Plasmodium-a brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthr. 2021, 40, 3. [Google Scholar] [CrossRef] [PubMed]
- Imboumy-Limoukou, R.K.; Lendongo-Wombo, J.B.; Nguimbyangue-Apangome, A.F.; Biteghe Bi Essone, J.C.; Mounioko, F.; Oyegue-Libagui, L.S.; Ngoungou, B.E.; Lekana-Douki, J.-B. Severe malaria in Gabon: Epidemiological, clinical and laboratory features in Amissa Bongo Hospital of Franceville. Malar. J. 2023, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Tannous, S.; Ghanem, E. A bite to fight: Front-line innate immune defenses against malaria parasites. Pathog. Glob. Health 2018, 112, 1–12. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, K.; Tongren, J.E.; Riley, E.M. The war between the malaria parasite and the immune system: Immunity, immunoregulation and immunopathology. Clin. Exp. Immunol. 2003, 133, 145–152. [Google Scholar] [CrossRef]
- Millington, O.R.; Gibson, V.B.; Rush, C.M.; Zinselmeyer, B.H.; Phillips, R.S.; Garside, P.; Brewer, J.M. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog. 2007, 3, 1380–1387. [Google Scholar] [CrossRef]
- Ocana-Morgner, C.; Mota, M.M.; Rodriguez, A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 2003, 197, 143–151. [Google Scholar] [CrossRef]
- Percario, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.; Goncalves, A.C.; Laurindo, P.S.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef]
- Vasquez, M.; Zuniga, M.; Rodriguez, A. Oxidative stress and pathogenesis in malaria. Front. Cell. Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Alfawaz, W.; Almutlaq, M.; Alzeer, H.; Alwashmi, Y.; Aljuraiban, G.S.; Alsaid, M.; Alnashmi, S. The relation between dietary zinc and immune status in saudi adults. Heliyon 2023, 9, e15042. [Google Scholar] [CrossRef]
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Vega-Cabello, V.; Caballero, F.F.; Lana, A.; Arias-Fernandez, L.; Banegas, J.R.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Struijk, A.E. Association of zinc intake with risk of impaired physical function and frailty among older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 2015–2022. [Google Scholar] [CrossRef]
- Abdulkareem, B.O.; Adam, A.O.; Ahmed, A.O.; Mariam, A.A.; Samuel, U.U. Malaria-induced anaemia and serum micronutrients in asymptomatic Plasmodium falciparum infected patients. J. Parasit. Dis. 2017, 41, 1093–1097. [Google Scholar] [CrossRef]
- Saad, A.A.; Doka, Y.A.; Osman, S.M.; Magzoub, M.; Ali, N.I.; Adam, I. Zinc, copper and C-reactive protein in children with severe Plasmodium falciparum malaria in an area of unstable malaria transmission in eastern Sudan. J. Trop. Pediatr. 2013, 59, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; MacLeod, W.B.; Krebs, N.F.; Westcott, J.L.; Fawzi, W.W.; Premji, Z.G.; Mwanakasale, V.; Simon, J.L.; Yeboah-Antwi, K.; Hamer, D.H. Plasma zinc concentrations are depressed during the acute phase response in children with falciparum malaria. J. Nutr. 2005, 135, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Ekeh, F.N.; Ekechukwu, N.E.; Chukwuma, C.F.; Aguzie, I.O.N.; Ohanu, C.M.; Ebido, C.; Oluah, S.N. Mixed vitamin C and zinc diet supplements co-administered with artemether drug improved haematological profile and survival of mice infected with Plasmodium berghei. Food Sci. Hum. Wellness 2019, 8, 275–282. [Google Scholar] [CrossRef]
- Zinc against Plasmodium Study Group. Effect of zinc on the treatment of Plasmodium falciparum malaria in children: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 805–812. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane, Chichester (UK). 2022. Available online: www.training.cochrane.org/handbook (accessed on 1 June 2023).
- Becquey, E.; Ouédraogo, C.T.; Hess, S.Y.; Rouamba, N.; Prince, L.; Ouédraogo, J.-B.; Vosti, A.S.; Brown, K.H. Comparison of preventive and therapeutic zinc supplementation in young children in Burkina Faso: A cluster-randomized, community-based trial. J. Nutr. 2016, 146, 2058. [Google Scholar] [CrossRef]
- Darling, A.M.; Mugusi, F.M.; Etheredge, A.J.; Gunaratna, N.S.; Abioye, A.I.; Aboud, S.; Duggan, C.; Mongi, R.; Spiegelman, D.; Roberts, D.; et al. Vitamin A and zinc supplementation among pregnant women to prevent placental malaria: A randomized, double-blind, placebo-controlled trial in Tanzania. Am. J. Trop. Med. Hyg. 2017, 96, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Becher, H.; Van Zweeden, A.B.; Ye, Y.; Diallo, D.A.; Konate, A.T.; Gbangou, A.; Kouyate, B.; Garenne, M. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: Randomised double blind placebo controlled trial. Br. Med. J. 2001, 322, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyei, S.; Newton, S.; Mahama, E.; Febir, L.G.; Ali, M.; Adjei, K.; Tchum, K.; Alhassan, L.; Moleah, T.; Tanumihardjo, A.S. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr. J. 2013, 12, 131. [Google Scholar] [CrossRef]
- Richard, S.A.; Zavaleta, N.; Caulfield, L.E.; Black, R.E.; Witzig, R.S.; Shankar, A.H. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 75, 126–132. [Google Scholar] [CrossRef]
- Saaka, M.; Oosthuizen, J.; Beatty, S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East Afr. J. Public Health 2009, 6, 55–62. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Shankar, A.H.; West, K.P.; Rare, L.; Bannon, D.; Adiguma, T.; Tielsch, J.; Wu, L.; Baisor, M.; Tamja, S.; Paino, J.; et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: A randomized trial in preschool children in Papua New Guinea. Am. J. Trop. Med. Hyg. 2000, 62, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Veenemans, J.; Milligan, P.; Prentice, A.M.; Schouten, L.R.A.; Inja, N.; van der Heijden, A.C.; de Boer, L.C.C.; Jansen, E.J.S.; Koopmans, A.E.; Enthoven, W.T.M.; et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: A randomised trial. PLoS Med. 2011, 8, e1001125. [Google Scholar] [CrossRef] [PubMed]
- Zeba, A.N.; Sorgho, H.; Rouamba, N.; Zongo, I.; Rouamba, J.; Guiguemdé, R.T.; Hamer, D.H.; Mokhtar, N.; Ouedraogo, J.-B. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: A randomized double blind trial. Nutr. J. 2008, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Ding, L.-R.; Zhuang, C.; Zhou, Y.-H. Effects of zinc supplementation on the incidence of mortality in preschool children: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e79998. [Google Scholar] [CrossRef]
- Aamer, I.; Junior, J.; Sohni, D.; Bhutta, Z.A. Preventive zinc supplementation for children, and the effect of additional iron: A systematic review and meta-analysis. BMJ Open 2014, 4, e004647. [Google Scholar]
- Imdad, A.; Rogner, J.; Sherwani, R.N.; Sidhu, J.; Regan, A.; Haykal, M.R.; Tsistinas, O.; Smith, A.; Chan, X.H.S.; Mayo-Wilson, E.; et al. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years. Cochrane Database Syst. Rev. 2023, 3, Cd009384. [Google Scholar] [PubMed]
- Yakoob, M.Y.; Theodoratou, E.; Jabeen, A.; Imdad, A.; Eisele, T.P.; Ferguson, J.; Jhass, A.; Rudan, I.; Campbell, H.; E Black, R.; et al. Preventive zinc supplementation in developing countries: Impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011, 11 (Suppl. 3), S23. [Google Scholar] [CrossRef]
- Ohashi, W.; Fukada, T. Contribution of Zinc and Zinc Transporters in the Pathogenesis of Inflammatory Bowel Diseases. J. Immunol. Res. 2019, 2019, 8396878. [Google Scholar] [CrossRef]
- Hassan, A.; Sada, K.K.; Ketheeswaran, S.; Dubey, A.K.; Bhat, M.S. Role of Zinc in Mucosal Health and Disease: A Review of Physiological, Biochemical, and Molecular Processes. Cureus 2020, 12, e8197. [Google Scholar] [CrossRef]



| Author (Year) | Country | Study Period | Participant (N) | Age Range | Qualitative Synthesis | Plasmodium Species | Methods for Malaria | Zinc Measurement |
|---|---|---|---|---|---|---|---|---|
| Becquey et al., 2016 [26] | Burkina Faso | 2010–2012 | Children (7641) | 6–30 months | Incidences of malaria were not different across study groups. (p = 0.48) | NS | Microscopy/RDT | Coupled plasma optical emission spectrophotometry (VWR International) |
| Darling et al., 2017 [27] | Tanzania | 2010–2013 | Pregnant women (2005) | NS | 1. Participants who received zinc had a lower risk of histopathology-positive placental malaria than those who did not receive zinc; risk ratio (RR = 0.64), 2. There was no significant tendency for risk of PCR-positive malaria between participants who received zinc and those who did not. | P. falciparum | Placental malaria: histopathology (PCR) | NS |
| Müller et al., 2001 [28] | Burkina Faso | 1999 | Children (709) | 6–31 months | No evidence for zinc supplementation being effective against P. falciparum malaria. | P. falciparum/P. malariae/P. ovale | Microscopy | Flame atomic absorption spectrometry (Perkin-Elmer 1100 B, Germany) |
| Owusu-Agyei et al., 2013 [29] | Ghana | 2009 | Children (200) | 6–24 months | The number of children who were diagnosed with uncomplicated malaria in the intervention group was 27% significantly lower compared with the children in the control group (p = 0.03). | NS | Microscopy | Atomic Absorption Spectrophotometer AA-6300 (P/N 206-51800) Kyoto, Japan. |
| Richard et al., 2006 [30] | Peru | 1998 | Children (855) | 0.5–15 years | Zinc and iron plus zinc did not statistically significantly affect P. vivax incidence in all children (p > 0.36). | P. falciparum/P. vivax | Microscopy | Atomic absorption spectrophotometry |
| Saaka et al., 2009 [31] | Ghana | 2005–2006 | Pregnant women | 16–44 years | At 34-36 weeks gestation, malaria parasitaemia, though not significant, was less frequent in the iron–zinc-supplemented group compared to the iron group (adjusted OR 0.60; 95% CI: 0.25–1.44). | P. falciparum | Microscopy | Flame atomic absorption spectrophotometry |
| Sazawal et al., 2006 [32] | Tanzania | 2002–2003 | Children (32,155) | 1–35 months | No qualitative data. | P. falciparum | Microscopy | NS |
| Shankar et al., 2000 [33] | Papua New Guinea | 1995 | Children (274) | 6–60 months | No differences were observed between groups for parasite prevalence. There was a tendency toward a higher prevalence of P. falciparum malaria in the zinc group (42%) compared to the placebo group (29%) (p = 0.050). | P. falciparum/P. vivax/P. malariae | Microscopy | Inductively coupled plasma analysis |
| Veenemans et al., 2011 [34] | Tanzania | 2008–2009 | Children (612) | 6–60 months | There was no evidence that multi-nutrients influenced the effect of zinc (or vice versa). Neither zinc nor multi-nutrients influenced malaria rates. | P. falciparum/other Plasmodium spp. | Microscopy/RDT | Inductively coupled plasma-mass spectrometry (Varian 820-MS) |
| Zeba et al., 2008 [35] | Burkina Faso | NS | Children (148) | 6–72 months | A significant decrease in the prevalence of malaria in the supplemented group (34%) compared to the placebo group (3.5%) was observed (p < 0.001). | P. falciparum | Microscopy | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotepui, M.; Wilairatana, P.; Mala, W.; Kotepui, K.U.; Masangkay, F.R.; Wangdi, K. Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2023, 15, 2855. https://doi.org/10.3390/nu15132855
Kotepui M, Wilairatana P, Mala W, Kotepui KU, Masangkay FR, Wangdi K. Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2023; 15(13):2855. https://doi.org/10.3390/nu15132855
Chicago/Turabian StyleKotepui, Manas, Polrat Wilairatana, Wanida Mala, Kwuntida Uthaisar Kotepui, Frederick Ramirez Masangkay, and Kinley Wangdi. 2023. "Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 15, no. 13: 2855. https://doi.org/10.3390/nu15132855
APA StyleKotepui, M., Wilairatana, P., Mala, W., Kotepui, K. U., Masangkay, F. R., & Wangdi, K. (2023). Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 15(13), 2855. https://doi.org/10.3390/nu15132855







