Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
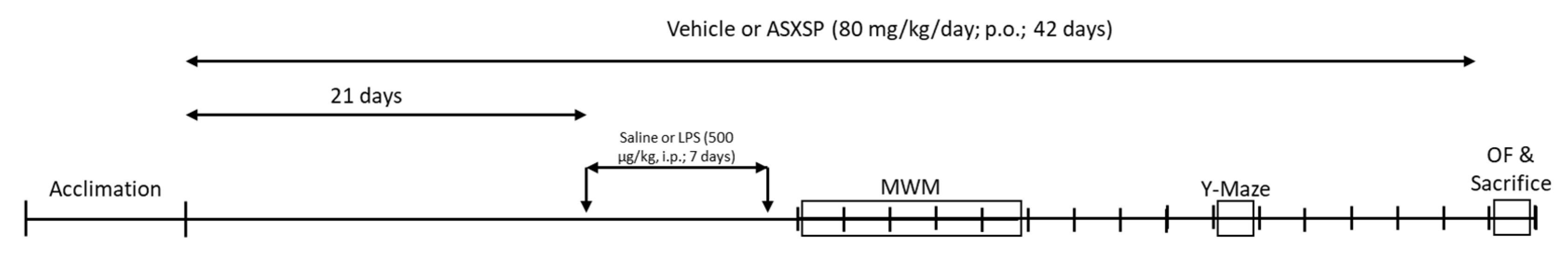
2.2. Protocol
2.3. Behavioral Evaluations
2.3.1. Morris Water Maze Test
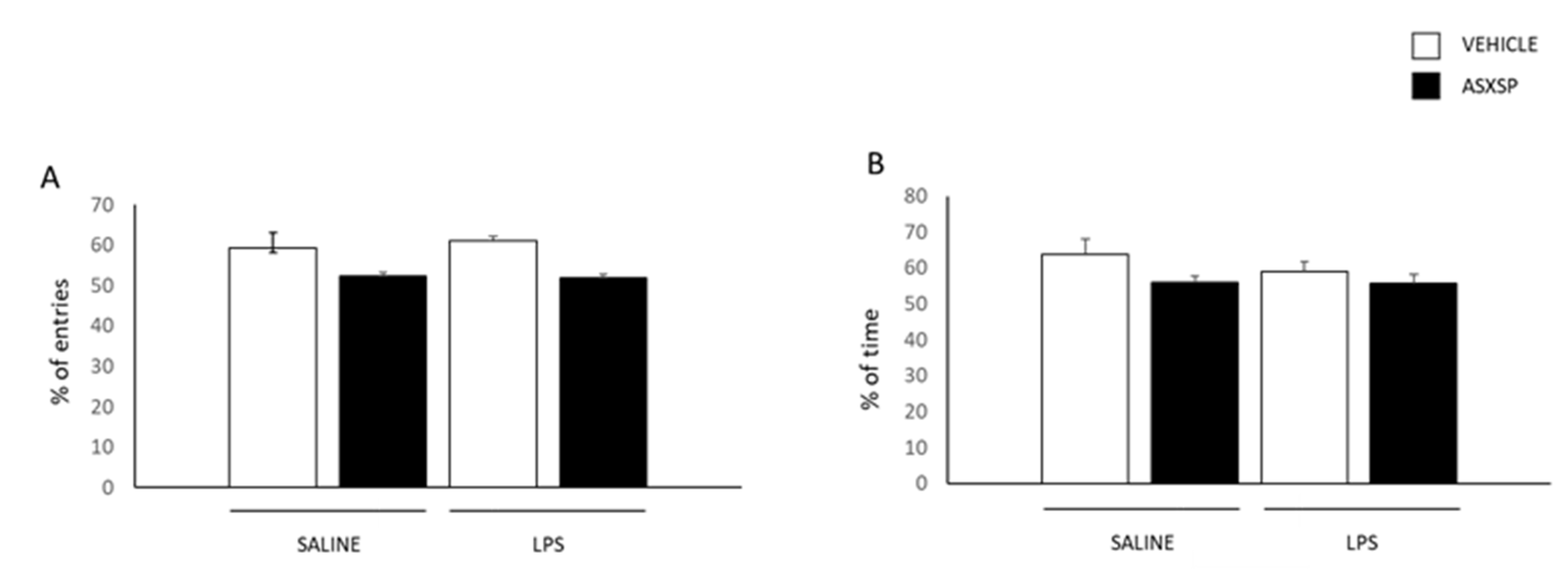
2.3.2. Y-Maze Test
2.4. Sacrifice, Tissue Extraction, and Processing
2.5. Statistical Analysis
3. Results
3.1. Preventive Treatment with ASXSP Ameliorates Long-Term, but Not Short-Term Memory Alterations Associated with the Exposure to LPS
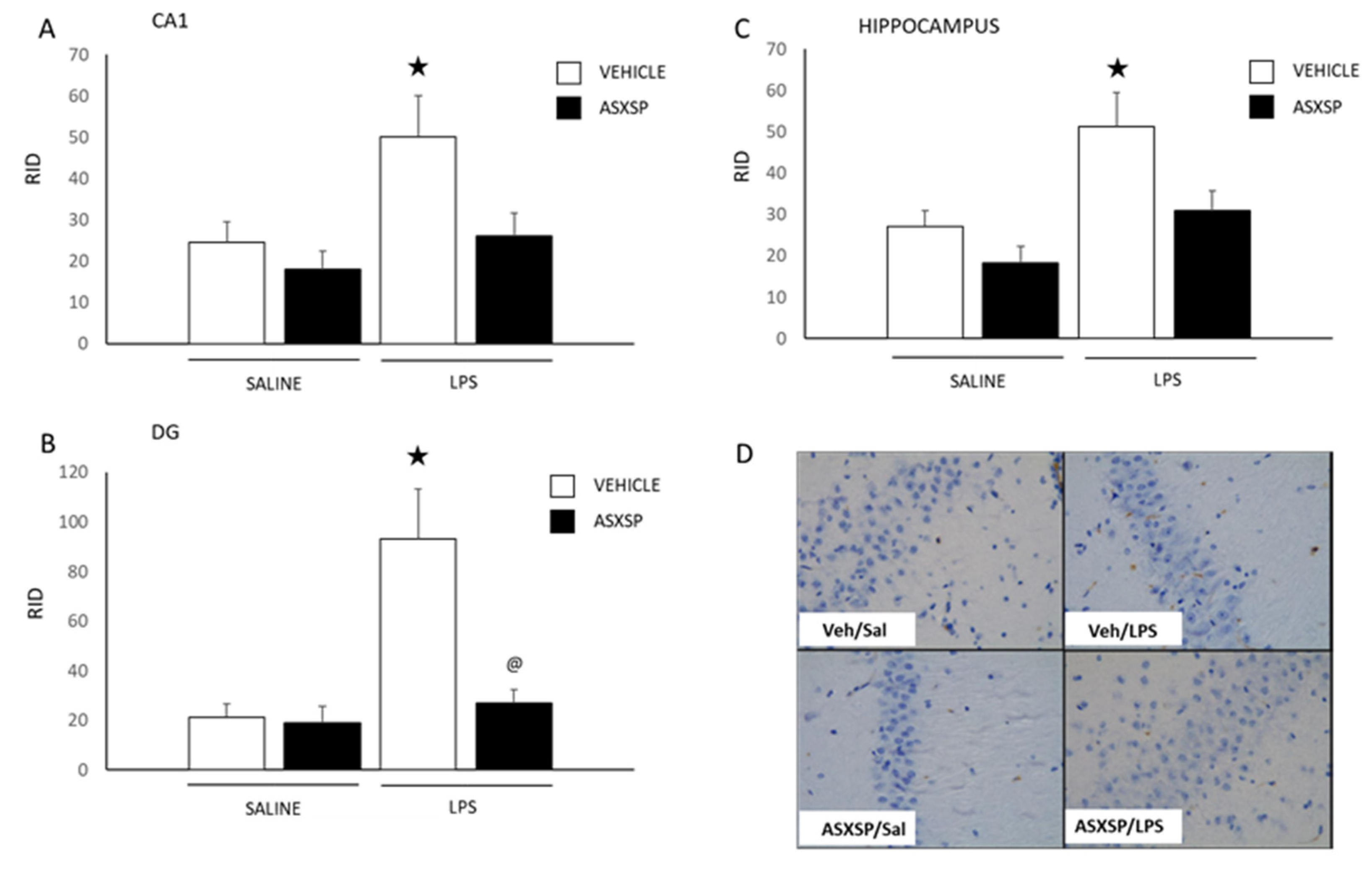
3.2. ASXSP Modulates LPS-Induced Microglial Cell Activation in the Hippocampus and mPFC
3.3. Exposure to LPS and ASXSP Modulates Gut Microbiota Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. GHE: Life Expectancy and Healthy Life Expectancy; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in Aging and Age-related Neurodegenerative Diseases. Ageing Res. Rev. 2022, 28, 101636. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Poddar, M.K.; Chakraborty, A.; Banerjee, S. Neurodegeneration: Diagnosis, Prevention, and Therapy. Oxidoreductase 2021. [CrossRef]
- Gutierrez, A.; Vitorica, J. Toward a New Concept of Alzheimer’s Disease Models: A Perspective from Neuroinflammation. J. Alzheimer’s Dis. 2018, 64, S329–S338. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.H.; Lei, L.; Gao, D.P.; Tong, J.H.; Wang, Y.; Yang, J.J. Neural network disturbance in the medial prefrontal cortex might contribute to cognitive impairments induced by neuroinflammation. Brain Behav. Immun. 2020, 89, 133–144. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Haney, M.S.; Smith, B.A.H.; Sun, J.; Iram, T.; Bonanno, L.; Li, L.; Lee, D.P.; Morgens, D.W.; Yang, A.C.; et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 2019, 568, 187–192. [Google Scholar] [CrossRef]
- Triviño, J.J.; von Bernhardi, R. The effect of aged microglia on synaptic impairment and its relevance in neurodegenerative diseases. Neurochem. Int. 2021, 144, 104982. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Wärnberg, J.; Gomez-Martinez, S.; Romeo, J.; Díaz, L.-E.; Marcos, A. Nutrition, inflammation, and cognitive function. Ann. N. Y. Acad. Sci. 2009, 1153, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.; Pellerin, L. Nutritional Impact on Metabolic Homeostasis and Brain Health. Front. Neurosci. 2022, 15, 767405. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitel, T.; de Cabo, R.; Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef] [PubMed]
- Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Bin Emran, T.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, S.; Simal-Gandara, J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs 2021, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B. Xanthophyllomyces dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Serrano, G.A.; Nishida, Y.; Wood, V. Natural astaxanthin improves blood flow and fights high blood pressure. Agro. Food Ind. Hi Tech. 2014, 25, 8–12. [Google Scholar]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef]
- Lin, S.F.; Chen, Y.C.; Chen, R.N.; Chen, L.C.; Ho, H.O.; Tsung, Y.H.; Sheu, M.T.; Liu, D.Z. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef] [PubMed]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J. Cell Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Nazem, A.; Sankowski, R.; Bacher, M.; Al-Abed, Y. Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 74. [Google Scholar] [CrossRef]
- Tanaka, S.; Ide, M.; Shibutani, T.; Ohtaki, H.; Numazawa, S.; Shioda, S.; Yoshida, T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 2006, 83, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-H.; Ai, W.-M.; Lei, D.-L.; Luo, X.-G.; Yan, X.-X.; Li, Z. Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience 2012, 209, 161–170. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, L.; Wilson, B.; Wu, X.; Qian, L.; Granholm, A.-C.; Crews, F.T.; Hong, J.-S. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology 2008, 29, 864–870. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Wass, C.; Archer, T.; Pålsson, E.; Fejgin, K.; Alexandersson, A.; Klamer, D.; Engel, J.A.; Svensson, L. Phencyclidine affects memory in a nitric oxide-dependent manner: Working and reference memory. Behav. Brain Res. 2006, 174, 49–55. [Google Scholar] [CrossRef]
- Gibaldi, M.; Perrier, D. Pharmacokinetics; Dekker, M., Ed.; Michigan University: Ann Arbor, MI, USA, 1975; ISBN 0824762649/9780824762643. [Google Scholar]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre- and Early Post-treatment with Arthrospira platensis (Spirulina) Extract Impedes Lipopolysaccharide-triggered Neuroinflammation in Microglia. Front. Pharmacol. 2019, 12, 724993. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Beaman, J.L.; Guthrie, J.; Ehmann, A.; Benson, K.F. Antioxidant and anti-inflammatory properties of an aqueous cyanophyta extract derived from Arthrospira platensis: Contribution to bioactivities by the non-phycocyanin aqueous fraction. J. Med. Food 2015, 18, 535–541. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Yvanka de Soysa, T.; Therrien, M.; Walker, A.C.; Stevens, B. Redefining microglia states: Lessons and limits of human and mouse models to study microglia states in neurodegenerative diseases. Semin. Immunol. 2022, 60, 101651. [Google Scholar] [CrossRef]
- Yao, Y.; Han, D.D.; Zhang, T.; Yang, Z. Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytother. Res. 2010, 24, 136–140. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial prefrontal cortex role in recognition memory in rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Jaehne, E.J.; Corrigan, F.; Toben, C.; Jawahar, M.C.; Baune, B.T. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol. Biochem. Behav. 2015, 135, 136–144. [Google Scholar] [CrossRef]
- Engeland, C.G.; Nielsen, D.V.; Kavaliers, M.; Ossenkopp, K.P. Locomotor activity changes following lipopolysaccharide treat ment in mice: A multivariate assessment of behavioral tolerance. Physiol. Behav. 2001, 72, 481–491. [Google Scholar] [CrossRef]
- Ke, Y.; Bu, S.; Ma, H.; Gao, L.; Cai, Y.; Zhang, Y.; Zhou, W. Preventive and Therapeutic Effects of Astaxanthin on Depressive-Like Behaviors in High-Fat Diet and Streptozotocin-Treated Rats. Front. Pharmacol. 2020, 10, 1621. [Google Scholar] [CrossRef] [PubMed]



| p Values (Non-Corrected) | Relative Abundance (Mean per Group) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASXSP/Sal vs. ASXSP/LPS | ASXSP/Sal vs. Veh/LPS | Veh/LPS vs. ASXSP/LPS | Veh/Sal vs. ASXSP/LPS | Veh/Sal vs. ASXSP/Sal | Veh/Sal vs. Veh/LPS | Veh/Sal | Veh/LPS | ASXSP/Sal | ASXSP/LPS | ||
| Prevotellaceae | 0.01 a | 0.60 | 0.02 a | 0.13 | 0.10 | 0.29 | 0.06% | 0.02% b | 0.02% b | 2.84% b | |
| Tannerallaceae | 0.06 | 0.55 | 0.26 | 0.55 | 0.29 | 0.41 | 0.37% | 0.55% | 0.69% | 0.31% | |
| Akkermansiaceae | 0.13 | 0.55 | 0.07 | 0.60 | 0.45 | 0.29 | 0.81% | 0.42% | 0.33% | 1.10% | |
| Lachnospiraceae | 0.17 | 0.26 | 0.55 | 1.00 | 0.33 | 0.94 | 57.69% | 58.40% | 63.02% | 56.72% | |
| Bacteriodaceae | 0.23 | 0.60 | 0.29 | 0.05 a | 0.55 | 0.50 | 0.95% b | 1.35% | 1.27% | 3.17% b | |
| Peptostreptococaceae | 0.41 | 0.94 | 0.23 | 0.65 | 0.88 | 0.94 | 0.95% | 1.15% | 1.29% | 0.66% | |
| Clostridiaceae | 0.41 | 0.33 | 1.00 | 0.71 | 0.60 | 0.50 | 0.18% | 0.19% | 0.12% | 1.02% | |
| Rikenellaceae | 0.55 | 0.41 | 0.94 | 0.55 | 0.82 | 0.71 | 8.53% | 7.77% | 9.35% | 8.07% | |
| Muribaculaceae | 0.65 | 0.41 | 0.45 | 0.45 | 0.36 | 0.76 | 17.44% | 15.86% | 13.69% | 13.62% | |
| Ruminococcaceae | 0.65 | 0.20 | 0.29 | 0.76 | 0.41 | 0.33 | 1.56% | 1.75% | 1.27% | 1.45% | |
| Cyclobacteriaceae | 0.65 | 0.06 | 0.20 | 0.45 | 0.65 | 0.03 | 1.04% b | 0.10% b | 0.27% | 0.73% | |
| Oscillospiraceae | 0.76 | 0.11 | 0.03 a | 0.94 | 0.71 | 0.11 | 6.23% | 8.45% b | 6.12% | 5.81% b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.; Pusceddu, M.M.; Teichenné, J.; Negra, T.; Connolly, A.; Escoté, X.; Torrell Galceran, H.; Cereto Massagué, A.; Samarra Mestre, I.; del Pino Rius, A.; et al. Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats. Nutrients 2023, 15, 2854. https://doi.org/10.3390/nu15132854
Martin M, Pusceddu MM, Teichenné J, Negra T, Connolly A, Escoté X, Torrell Galceran H, Cereto Massagué A, Samarra Mestre I, del Pino Rius A, et al. Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats. Nutrients. 2023; 15(13):2854. https://doi.org/10.3390/nu15132854
Chicago/Turabian StyleMartin, Miquel, Matteo M. Pusceddu, Joan Teichenné, Teresa Negra, Alan Connolly, Xavier Escoté, Helena Torrell Galceran, Adrià Cereto Massagué, Iris Samarra Mestre, Antoni del Pino Rius, and et al. 2023. "Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats" Nutrients 15, no. 13: 2854. https://doi.org/10.3390/nu15132854
APA StyleMartin, M., Pusceddu, M. M., Teichenné, J., Negra, T., Connolly, A., Escoté, X., Torrell Galceran, H., Cereto Massagué, A., Samarra Mestre, I., del Pino Rius, A., Romero-Gimenez, J., Egea, C., Alcaide-Hidalgo, J. M., & del Bas, J. M. (2023). Preventive Treatment with Astaxanthin Microencapsulated with Spirulina Powder, Administered in a Dose Range Equivalent to Human Consumption, Prevents LPS-Induced Cognitive Impairment in Rats. Nutrients, 15(13), 2854. https://doi.org/10.3390/nu15132854








