Abstract
Zinc (Zn2+) is the second most abundant necessary trace element in the human body, exerting a critical role in many physiological processes such as cellular proliferation, transcription, apoptosis, growth, immunity, and wound healing. It is an essential catalyst ion for many enzymes and transcription factors. The maintenance of Zn2+ homeostasis is essential for the central nervous system, in which Zn2+ is abundantly distributed and accumulates in presynaptic vesicles. Synaptic Zn2+ is necessary for neural transmission, playing a pivotal role in neurogenesis, cognition, memory, and learning. Emerging data suggest that disruption of Zn2+ homeostasis is associated with several central nervous system disorders including Alzheimer’s disease, depression, Parkinson’s disease, multiple sclerosis, schizophrenia, epilepsy, and traumatic brain injury. Here, we reviewed the correlation between Zn2+ and these central nervous system disorders. The potential mechanisms were also included. We hope that this review can provide new clues for the prevention and treatment of nervous system disorders.
1. Introduction
Zinc (Zn2+), the second most abundant necessary trace element in the human body, is an indispensable co-factor of more than 300 enzymes and 2000 transcription factors. Evidence suggests that about 10% of all proteins need to bind to Zn2+ to function properly and their expression levels are also regulated by Zn2+ []. It plays an important role in many physiological processes, including DNA repair, transcription, protein synthesis, apoptosis, proliferation, wound healing, and immune response [,]. Zn2+ was found to be involved in rapid ligand exchange reactions as a second messenger in several signal transduction pathways like calcium [] (Figure 1). Thus, Zn2+ deficiency can lead to many disorders, including retarded growth and brain development, immune dysfunction, delayed wound healing, learning disabilities, and olfactory and taste disorders [,,,].
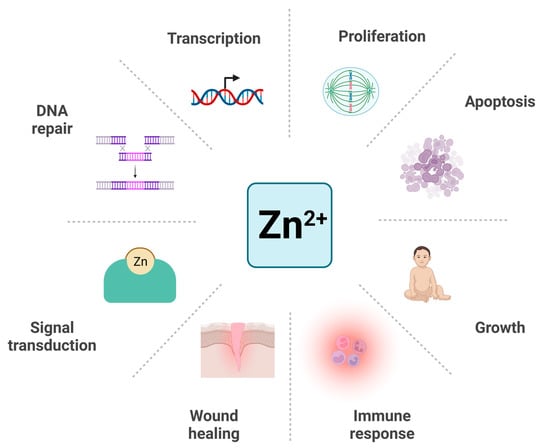
Figure 1.
The involvement of Zn2+ in the physiological processes. Zn2+ participates in many physiological processes including DNA repair, transcription, proliferation, apoptosis, growth, immune response, wound healing, and signal transduction. Created with BioRender.com. Adapted from “Circular Diagram with 8 Sections (Layout)”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 5 February 2023).
In normal conditions, Zn2+ content in the human body should be kept around 2–3 grams, less than 50 mg/kg, and over 90% of Zn2+ exists in cells of bones and muscles [,]. The absorption of Zn2+ is determined by intake rather than the host’s Zn2+ status []. Nowadays, Zn2+ deficiency remains a global public health problem to be addressed. According to World Health Organization (WHO), 0.8 million (1.4%) deaths worldwide are due to Zn2+ deficiency [], thus appropriate Zn2+ supplementation is vital for the prevention and treatment of certain diseases caused by Zn2+ deficiency []. In addition to the exogenous supplementation, Zn2+ also needs to be tightly regulated at a physiological homeostatic level in vivo by a variety of proteins involving Zn2+ transporters and binders, especially in the central nervous system (CNS) [,]. The maintenance of Zn2+ homeostasis is significant for the normal functions of CNS []. Given the crucial role of Zn2+ in CNS, the disruption of Zn2+ homeostasis is correlated to several CNS diseases as a contributing factor [,]. The relationship between Zn2+ and CNS disorders is complex. Although there is extensive evidence on this topic, the results are conflicting. This review provides an overview of the latest preclinical and clinical data on the role of Zn2+ in the pathophysiology of seven central nervous system disorders, aiming to draw a link between altered extracellular/intracellular Zn2+ levels and targets which can be influenced by Zn2+ homeostasis. Also, the potential of Zn2+-based intervention as a therapeutic strategy for these disorders was also discussed in this review.
2. Zn2+ in the CNS
Zn2+ is a significant element required by CNS throughout life. The brain is the organ with the highest Zn2+ concentration of about 150 µmol/L in the human body, in which Zn2+ accumulates in the cerebral cortex, olfactory cortex, thalamus, hippocampus and amygdala []. It plays a critical role in learning, memory, synaptic plasticity, and neurogenesis [].
Most of the brain Zn2+ (80–95%) binds to proteins including Zn2+ metalloenzymes and metalloproteins, while about 20% of the brain Zn2+ is in its free ionic form, presenting in the presynaptic vesicles of glutamatergic nerve terminals []. When glutamatergic neurons are excited, Zn2+ within vesicles is released into synaptic clefts along with glutamate [], activating many receptors such as NMDA (N-methyl-d-aspartate), GABAA (γ-aminobutyric acid type A), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors, glycine receptors and voltage-gated ion channels, thus influences synaptic plasticity and transmission [,,]. Studies suggest that excitatory cortico-amygdala synapses need Zn2+ to induce long-term potentiation (LTP) and form auditory fear memory []. Also, Zn2+ is necessary for the LTP induction of mossy fibers for information storage []. Significantly, Zn2+ is involved in somatosensory processing and precision perception of stimuli [,]. Glutamatergic Zn2+-enriched neurons (ZENs) are present in the dorsal cochlear nucleus and olfactory bulb, indicating that Zn2+ is involved in auditory and olfactory stimuli response [].
Zn2+ is a crucial factor for several steps of neurogenesis including proliferation, migration, differentiation, and survival in both developmental and adult stages. Zn2+ deficiency results in impaired neuronal proliferation, differentiation, and activation of apoptotic pathways []. Indeed, evidence shows that the hippocampus is probably the most susceptible region to Zn2+ deficiency [,], which reduces progenitor cell number and neuronal differentiation, thus causing irreversible impairment of learning and memory capacity during early development [,].
As a redox-inert metal, Zn2+ exhibits strong antioxidant properties by a series of molecules and enzymes, thus playing a crucial role in resisting oxidative stress []. However, excess Zn2+ can cause neurotoxicity mainly through oxidative stress generation and is a risk factor for stroke, epilepsy, and ischemia []. Studies have shown that under detrimental stimuli, Zn2+ is released in large quantities from presynaptic terminals and enters postsynaptic neurons [,]. Significantly increased intracellular free Zn2+ enhances the level of neuronal NADPH oxidase subunit, which is involved in the generation of reactive oxygen species (ROS) [,].
Therefore, as a special class of neurotransmitter that cannot be metabolized or synthesized, Zn2+ needs to be tightly regulated to prevent neuronal death associated with Zn2+ dysregulation. In the brain, Zn2+ homeostasis is mainly maintained by three families of proteins, metallothioneins (MT), Zn2+ transporters (ZnT), and Zrt-, Irt-like proteins (ZIP) []. They work together to modulate Zn2+ signaling spatially and temporally.
MT is the major binding protein to buffer cytoplasmic Zn2+. It is a cysteine-rich peptide consisting of 61 amino acids. The 20 cysteine residues of MT are the binding sites of several divalent metals including Zn2+, copper (Cu2+), mercury (Hg2+) and cadmium (Cd3+) []. Each MT can bind to 7 Zn2+ atoms by metal-thiolate clusters as a reservoir []. The expression of MTs is induced by a high level of Zn2+. There are four isotypes of MTs, of which MT-1 and MT-2 are generally present in all cells. MT-3 is found mainly in the CNS, especially the glutamatergic neurons containing Zn2+, and MT-4 mainly exists in stratified epithelial cells [,]. MTs regulate and detoxify the intracellular heavy metals and are involved in metal absorption through the intestinal mucosa. They have specific roles in the immune system and transcription []. Evidence suggests that MTs have redox properties, exerting a critical role in the protection against oxidative stress [].
ZnTs regulate intracellular Zn2+ by efflux and transport into intracellular compartments from the cytosol. There are currently 10 members of the ZnT family (ZnT1–ZnT10), which are designated SLC30. At present, most structural information about Zn2+ transporters is obtained from analysis and prediction studies on their prokaryotic homologs. Based on the crystal structure of their Escherichia coli homolog YiiP, ZnTs are predicted to have six transmembrane domains with cytosolic NH2 and COOH termini []. The expression of ZnTs is closely related to the fluctuations in Zn2+ concentration. Except for ZnT1, ZnTs are located in intracellular compartments and act as Zn2+/H+ antiporters. ZnT1 is found on the cell membrane of neurons and glia, transporting Zn2+ out of the cells, thus it is implicated in protection from neurotoxic Zn2+ surges during pathological conditions []. The expression of ZnT1 is upregulated by the increase of intracellular Zn2+ triggered by nitric oxide during cerebral ischemia. ZnT3 is the neuron-specific isoform found on synaptic vesicles and the only route by which Zn2+ can be transported into vesicles []. As the most extensively researched ZnT, ZnT3 is significant for brain Zn2+-related research. ZnT3-knockout (KO) mice lack synaptic Zn2+, and thus are a vital model for studying its role in pathological conditions of CNS. ZnT3 KO mice showed age-dependent cognitive impairment and increased susceptibility to seizures [,]. In addition, ZnT3 mRNA levels have been proven to decrease with age and in postmortem brain tissues of Alzheimer’s patients [,]. ZnT2, ZnT4, ZnT5, ZnT6 and ZnT10 are located in several regions of the brain. Among them, ZnT2, ZnT4 and ZnT10 transport Zn2+ into lysosomes, endosomes, and secretory vesicles, while ZnT5 and ZnT6 are involved in the transport of Zn2+ into Golgi apparatus and trans-Golgi network []. ZnT9 is expressed in the nucleus and cytoplasm of the cerebellum [].
In contrast to ZnTs, ZIPs encoded by SLC39 genes transport Zn2+ from extracellular space and intracellular compartments into the cytoplasm to increase cytoplasmic Zn2+ level []. ZIP family constitutes 14 members (ZIP1–ZIP14), which are widely distributed in brain and peripheral tissues. ZIP transporters have been predicted to have eight transmembrane domains with extracellular NH2 and COOH termini []. Evidence suggests that the expression and location of ZIPs can also be regulated by the Zn2+ level []. Zn2+. Except for ZIP5, the expression of cell surface ZIPs generally increases under Zn2+ deficiency []. In addition to Zn2+, ZIPs can also transport other metals including iron (Fe2+), Cu2+, Cd3+ and manganese (Mn2+) [,]. Defects of ZIPs have been shown to be associated with several neurological diseases. ZIP8 mutations have been linked to cerebellar atrophy syndrome []. ZIP12, abundantly expressed in the brain, plays a crucial role in neurite outgrowth and tubulin polymerization [].
3. Zn2+ and CNS Diseases
A growing body of evidence suggests that disruption of Zn2+ homeostasis is associated with the pathogenesis of a variety of CNS disorders, including Alzheimer’s disease, depression, Parkinson’s disease, multiple sclerosis, schizophrenia, epilepsy and traumatic brain injury. In this review, the roles of Zn2+ in these disorders were introduced. The main preclinical and clinical findings about the involvement of Zn2+ in several central nervous system disorders are summarized in Table 1.

Table 1.
Summary of the main preclinical and clinical findings about the involvement of Zn2+ in several central nervous system disorders.
3.1. Zn2+ and Alzheimer’s Disease
As a multifactorial chronic neurodegenerative disorder, Alzheimer’s disease (AD) is the most common cause of dementia, accounting for more than half of dementia cases. Age is considered the main risk factor for sporadic cases, which account for 95% of all AD cases. While other cases mostly have a genetic inheritance, called familial Alzheimer’s disease (FAD). FAD can be caused by mutations of several genes including presenilin 1 (PSEN1), presenilin 2 (PSEN2) and amyloid precursor protein (APP) []. AD is pathologically characterized by the deposition of β-amyloid (Aβ) plaques and hyperphosphorylated tau proteins (p-tau). Currently, the primary pathogenic factor of AD is still controversial, and the overall failure of drug discovery based on the Aβ and tau hypothesis implies that a reappraisal of the AD model is urgent. Given the critical neurological effects and the rich content of Zn2+ in the neocortex, the research on the role of Zn2+ in the pathogenesis of AD has advanced rapidly since Burnet et al. first described Zn2+ as a potential pathogenic factor of dementia [].
Of all the studies on Zn2+ in the pathogenesis of AD, the involvement of Zn2+ in the deposition of Aβ was the most extensively researched. The imbalance between Aβ production and clearance is thought to be a likely drive of AD, in which Zn2+ plays a significant role []. Aβ, a peptide composed of 39–43 amino acids, is the cleavage product of amyloid precursor protein (APP) catalyzed by β-secretase and γ-secretase in sequence. Zn2+ has been shown to be involved in the synthesis and processing of APP to affect the production of Aβ. Some Zn2+-containing transcription factors such as p53 and NF-κB participate in the APP synthesis [,]. Zn2+ also modulates the expression levels and activity of α, β and γ-secretase. A high concentration of Zn2+ enhances the expression levels of β and γ-secretase while decreasing that of α-secretase []. Several Zn2+-binding sites have been found on APP, by which Zn2+ can influence the digestive efficiency of the 3 secretases through conformational changes []. There is a complex interplay between Zn2+ and Aβ. Zn2+ can interact with Aβ to regulate the polymerization of Aβ into different forms []. At low concentrations, by selective precipitation of aggregation intermediates, Zn2+ performs a protective effect on resisting the neurotoxicity of Aβ. While at high concentrations, Zn2+ binding increases the fibrillar Aβ aggregation []. Results from both in vitro and in vivo studies have shown that Zn2+-amyloid interactions promote the precipitation of Aβ fibrils, in which Zn2+ accumulates in large quantities [,]. Lee et al. have demonstrated that transgenic Tg2576 mice (mice overexpressing human APP) crossed with ZnT3 KO mice had markedly reduced plaque load, providing further evidence that Zn2+ contributes to Aβ aggregation []. By sequestrating Zn2+, Aβ depletes the Zn2+-mediated glutamatergic neurotransmission, leading to the neurotoxicity correlated with glutamatergic overdrive []. Metabotropic ZnR (GPR39) and TrkB receptors are also affected, which in turn perturb LTP []. Accumulated Aβ can incorporate into membranes to form unregulated amyloid channels, initiating a continuous inward flow of Ca2+ [] (Figure 2). The consequent disruption of calcium homeostasis might be the primary cause of Aβ neurotoxicity [].
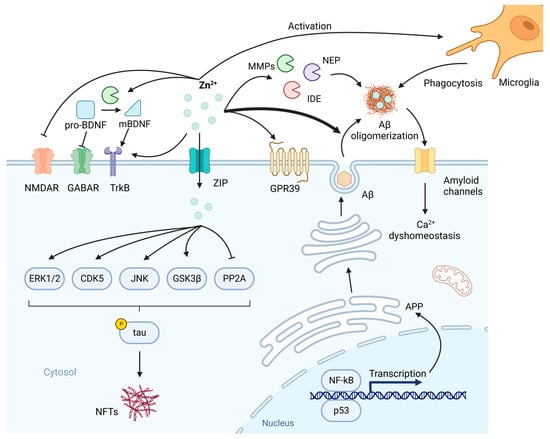
Figure 2.
The involvement of Zn2+ in the pathogenesis of Alzheimer’s disease (AD). Zn2+-containing transcription factors NF-κB and p53 participate in the synthesis of amyloid precursor protein (APP) and subsequent β-amyloid (Aβ). After production, Aβ is secreted upon neuronal activity and aggregated by Zn2+. In normal conditions, Zn2+ inhibits N-methyl-d-aspartate receptor (NMDAR) to regulate synaptic neurotransmission. Also, Zn2+ is involved in the normal memory events such as long-term potentiation (LTP) by TrkB and GPR39. brain-derived neurotrophic factor (BDNF)-TrkB axis needs to be modulated by Zn2+ because Zn2+-dependent MMPs are indispensable to the transformation of pro-BDNF to mBDNF, while pro-BDNF inhibits GABAergic transmission. In addition, the clearance of Aβ is affected by Zn2+. Zn2+ metalloproteinases including matrix metalloproteinases (MMPs), neprilysin (NEP) and insulin-degrading enzyme (IDE) are involved in the degradation of Aβ, while Zn2+-loaded Aβ oligomers have increased resistance to proteolysis. Microglia can be activated by Zn2+ to enhance the phagocytosis of Aβ. Due to the sequestration of Zn2+ by Aβ, these normal neurobiological activities in which Zn2+ plays a crucial role are disrupted. Aβ can incorporate into membranes to form amyloid channels, as a result, an inward flow of Ca2+ is initiated, leading to Ca2+ dyshomeostasis. On the other hand, intracellular Zn2+ can lead to phosphorylation of tau by activating extracellular signal-regulated protein kinase 1/2 (ERK1/2), cyclin-dependent kinase 5 (CDK5), c-Jun N-terminal kinase (JNK), glycogen synthase kinase 3β (GSK3β) and inhibiting protein phosphatase 2A (PP2A). Created with BioRender.com. Adapted from “Drosophila Toll Pathway”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 9 February 2023).
Accumulating evidence implies that enhanced Aβ:metal interactions aggravate oxidative stress by uncontrolled production of ROS []. Specifically, the abundant Fe3+ and Cu2+ in the vicinity of Aβ provide the fuel for the generation of H2O2 and downstream ROS by Fenton and Haber-Weiss chemistry, affecting lipid peroxidation and the formation of DNA and protein adducts []. The conformational changes of Aβ induced by Zn2+ may exert a protective role by suppressing the interaction of oxidizing metals with Aβ and the ROS generation [].
In addition, Zn2+ is involved in the clearance of Aβ. The sequestration of Zn2+ by Aβ affects the catalytic activity of several Zn2+ metalloproteinases which can degrade Aβ, including matrix metalloproteinases (MMP), neprilysin (NEP) and insulin-degrading enzyme (IDE) [] (Figure 2). The Aβ-Zn2+ complex has increased resistance to proteolysis [], and S100A6 from astrocytes can promote its clearance by sequestrating Zn2+ []. Whereas Zn2+ can also activate microglia to enhance the phagocytosis of Aβ [] (Figure 2).
Neurotrophic signaling is also defective in AD. Zn2+ is critical to the modulation of neurotrophic signaling in AD, especially the brain-derived neurotrophic factor (BDNF)-TrkB axis []. BDNF level is associated with the severity of AD-related cognitive decline [,]. Zn2+-dependent MMPs (mainly MMP-2 and 9) are involved in the transformation of pro-BDNF to mBDNF [] (Figure 2). It was observed that Zn2+ supplementation counteracted the decline in BDNF level through MMP activation in 3xTg-AD mice []. Pro-BDNF was found to inhibit GABAergic transmission [], and this along with the impaired maturation of BDNF may provide a mechanism for neuronal death and altered neuronal excitability [,].
Aβ pathology is regarded as a biomarker of Zn2+ dyshomeostasis []. Due to the excessive interaction between Zn2+ and Aβ, extracellular and intracellular Zn2+ homeostasis are both impaired [] (Figure 2). There may be 2 stages of Zn2+ dyshomeostasis in AD []: Slow turnover of synaptic Zn2+ in the early stage is vital for the formation of Aβ deposition. Reuptake of released Zn2+ after the glutamatergic transmission is an energy-dependent process, thus decreased mitochondrial energy during aging leads to increased average extracellular Zn2+ level, promoting Aβ aggregation []. Increased intracellular Zn2+ can only be observed in advanced AD, possibly because of the inhibitory effect on Zn2+ export of 4-hydroxynonenal, which is a peroxidation product generated by Aβ-Cu2+ complexes and elevated in AD [].
Hyperphosphorylation of tau and the formation of neurofibrillary tangles (NFT) is another contributing factor to neuronal disorder in AD. It has been found that Zn2+ triggers tau phosphorylation by activation of glycogen synthase kinase 3β (GSK3β), cyclin-dependent kinase 5 (CDK5), extracellular signal-regulated protein kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) []. Recent evidence suggests that Zn2+ can inhibit protein phosphatase 2A (PP2A) via the Src-dependent pathway, leading to the hyperphosphorylation of tau [] (Figure 2). In addition to phosphorylation, it was observed that Zn2+ can promote tau aggregation by direct interaction with tau []. Huang et al. have demonstrated that Zn2+ binding can also directly enhance tau toxicity using a drosophila tauopathy model []. The hyperphosphorylation of tau may be associated with the elevated Zn2+ level during advanced AD [].
Many studies showed that Zn2+ homeostasis can affect Aβ/tau pathology and is the potential therapeutic target. Based on these findings, there are two main strategies for Zn2+-targeting therapy, including Zn2+ supplementation and modulation []. The role of Zn2+ supplementation in AD is not fully understood. Nutritional Zn2+ deficiency commonly occurred in the advanced age of AD. Low dietary Zn2+ has been found to increase the volume of amyloid plaques in APP/PS1 mice []. Zn2+ supplementation in 3xTg-AD mice was described to significantly delay memory deficits and reduce both Aβ and tau pathology []. On the contrary, it was found that Zn2+-enriched diets could impair spatial memory in Tg2576 and TgCRND8 transgenic models []. Also, in addition to spatial learning and memory impairment, Zn2+ treatment increased Aβ deposition in APP/PS1 mice []. Deficits in biochemistry and behavior triggered by tau were observed to be exacerbated by Zn2+ supplementation in a tau mouse model []. A meta-analysis indicated no evidence that Zn2+ supplementation can lower the risk of AD [].
Zn2+ ionophores including clioquinol (CQ, 5-chloro-7-iodoquinolin-8-ol) and PBT2 (5,7-dichloro-2-[(dimethylamino)methyl]quinolin-8-ol) have shown positive results in Aβ reduction and cognition promotion in clinical trials [,]. These two metal-protein-attenuating compounds (MPACs) have been shown to promote the uptake of Zn2+ into cells with an impact on several relevant neurochemical pathways, thus normalizing the distribution of Zn2+ and Zn2+-dependent signaling pathways [,]. These findings further illustrated the critical role of Zn2+ homeostasis in AD and the complex interaction between them (Figure 2).
3.2. Zn2+ and Depression
Depression is a common mental disorder associated with high morbidity and mortality. A growing body of evidence suggesting that there is a correlation between Zn2+ and depression has sparked enormous interest. The link between Zn2+ deficiency and depression or depression-like symptoms has been observed in both animal models and clinical trials. Dietary Zn2+ deficiency was found to cause depression-like behaviors in rats []. Serum Zn2+ levels are markedly lower in major depressed patients compared to normal controls and there is a negative correlation between serum Zn2+ and the severity of depression []. It was reported that treatment-resistant depressed patients have lower Zn2+ levels than treatment-non-resistant patients []. A systemic review showed that Zn2+ significantly lowered depressive symptom scores of patients suffering from depression as an adjunct to antidepressant drug treatment []. Another recent meta-analysis revealed the role of Zn2+ supplementation in the improvement of depression status as a monotherapy []. In addition, postpartum Zn2+ supplementation was demonstrated to markedly improve maternal blood Zn2+ status with a positive effect on reducing the risk of postpartum depression [].
At present, the underlying neurobiological mechanism of the association between depression and low Zn2+ levels is still unclear, and many pathways may be involved. Zn2+ has been shown to be implicated in abnormal endocrine pathways in the pathogenesis of depression. It was reported that the hypothalamic-pituitary–adrenal (HPA) system is disrupted in about 50% of depressed patients [,,]. The increase in serum corticosterone has been demonstrated in Zn2+-deficient mice and rats [,]. It may be that the chronically increased serum glucocorticoid rather than insufficient chelatable Zn2+ is associated with the increase in depression-like behavior []. Repeated corticosterone injections were also found to increase depression-like behavior in rats and mice [,]. Increased glucocorticoid under Zn2+ deficiency may be involved in the change in the excitability of glutamatergic neurons. The excessive excitation of glutamatergic neurons in the hippocampus observed in Zn2+-deficient rats after exposure to acute stress may be due to aberrant glucocorticoid secretion, and contribute to stress susceptibility and increased depression-like behavior []. It is believed that corticosterone secretion during Zn2+ deficiency may be involved in the blocking of glutamate transporter, causing excess glutamate accumulation [].
Zn2+ may exert antidepressant action through neurotransmission. Chronic treatment with citalopram and fluoxetine, which are selective serotonin reuptake inhibitors (SSRI), can significantly enhance the serum Zn2+ level [,]. The antidepressant-like effect of Zn2+ in the forced swim test (FST) was demonstrated to be markedly abolished by serotonin synthesis inhibitors or serotonin receptor antagonists []. The density of 5-HT1A and 5-HT2A receptors was enhanced by chronic Zn2+ administration in the hippocampus and frontal cortex, respectively []. The anti-immobility effect of Zn2+ was partially abolished in 5-HT1A autoreceptor KO mice []. Relevant studies have shown that Zn2+ can regulate serotonin signaling by the allosteric modulation of 5-HT receptors [,,]. Zn2+ potentiates agonist binding to 5-HT1A receptor at sub-micromolar concentrations (10 μM) while exerting an inhibitory effect at sub-millimolar concentrations (500 μM) []. Additionally, the binding of the antagonist to the 5-HT1A receptor is inhibited by Zn2+ []. The oligomerization state between GalR1-5-HT1A-GPR39 is regulated by Zn2+ concentration and this may affect depressive behavior []. Zn2+ has been shown to disrupt the heterodimerization of 5-HT1A and GalR1 [], which may be a novel therapeutic target of depression [].
Previous studies have also shown that glutamatergic signaling is crucial in the pathology and treatment of depression. In the limbic system, Zn2+ typically acts as an inhibitory modulator of the NMDA receptor []. N-methyl-d-aspartic acid (NMDA) administration was shown to antagonize the antidepressant-like activity of Zn2+ []. Coadministration of ineffectively low doses of NMDA antagonists and ineffective doses of Zn2+ became effective in FST []. A NMDA receptor agonist was observed to block the antidepressant-like effect of both Zn2+ and magnesium (Mg) []. The affinity of glycine to glycine/NMDA receptors was demonstrated to be decreased by chronic Zn2+ administration []. Increased levels of GluN2A and GluN2B in the rat hippocampus were found following Zn2+ restriction []. Also, the role of the AMPA receptor in antidepressant-like effects of Zn2+ has been shown. An antagonist of the AMPA receptor abolished the antidepressant-like activity of Zn2+ while an AMPA receptor potentiator presented a synergistic effect with Zn2+ in the FST [].
Emerging evidence also suggests the involvement of metabotropic mGluRs in depression. Several antagonists of group I and group II mGluRs have exhibited antidepressant-like effects in the FST and mouse tail suspension test (TST) [,]. Zn2+ has been demonstrated to be an antagonist of group I and group II mGluRs (Figure 3), and this is a potential mechanism of antidepressant-like effects of Zn2+ [].
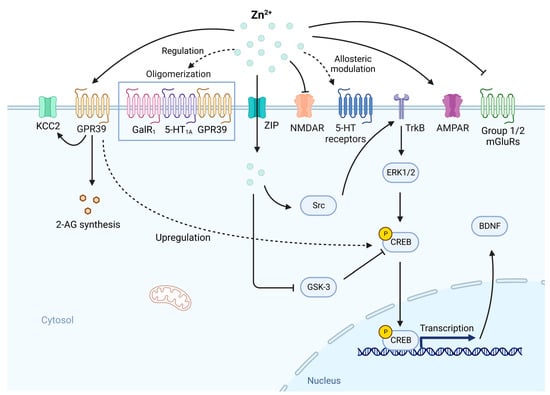
Figure 3.
The involvement of Zn2+ deficiency in the abnormal neurotransmission and neurotropic signaling in depression. In normal conditions, the activities of glutamatergic receptors including NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) need to be regulated by Zn2+. The activation of GPR39 by Zn2+ is essential for endocannabinoid 2-arachidonoylglycerol (2-AG) synthesis and the upregulation of potassium chloride co-transporter 2 (KCC2). GPR39 activation can upregulate cAMP response element binding protein (CREB) expression, thus increase BDNF expression. Zn2+ is involved in the allosteric modulation of 5-HT receptors and regulation of the oligomerization state between GalR1-5-HTA1-GPR39. Zn2+ is also an antagonist of groupⅠand group Ⅱ mGluRs, which are linked to depression. While intracellular Zn2+ participates in the modulation of neurotropic signaling, which is crucial in depression. Zn2+ can transactivate TrkB and downstream ERK1/2 and CREB via Src family kinase, on the other hand, inhibit glycogen synthase kinase-3 (GSK-3), which inhibits CREB activity, leading to the expression of BDNF. Under Zn2+ deficiency, these activities associated with depression are disrupted, contributing to the development of depression. Created with BioRender.com. Adapted from “Drosophila Toll Pathway”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 17 February 2023).
Zn2+ may act as a neurotransmitter through another metabotropic receptor, GPR39 []. GPR39 KO mice exhibited depressive-like behavior in FST and TST []. A decreased level of GPR39 was observed in the hippocampus and frontal cortex of Zn2+-deficient rats and mice []. Suicide victims also show a lower level of hippocampal and cortical GPR39 []. In the study by Omar and Tash, a joint administration of fluoxetine and Zn2+ significantly increased the hippocampal protein level of GPR39 []. There are data suggesting that GPR39 is also involved in glutamatergic neurotransmission. Zn2+ was found to upregulate the activity of potassium chloride co-transporter 2 (KCC2) by a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent process via GPR39 [] (Figure 3). KCC2 is a transporter that plays a crucial role in the maintenance of neuronal chloride gradient, enabling the generation of inhibitory currents within postsynaptic neurons through GABAA receptors []. By the enhanced activity of KCC2, Zn2+ can provide protection from high-level stimulation and resultant excitotoxicity []. The activation of GPR39 by Zn2+ is needed for the synthesis of endocannabinoid 2-arachidonoylglycerol (2-AG) (Figure 3), which exerts an inhibitory effect on glutamate release []. Notably, monoamine-based antidepressants had no effectiveness in GPR39 KO mice, indicating that GPR39 is indispensable for the effects of drugs targeting the 5-HT system [].
Neurotrophic signaling also plays an indispensable role in depression. Two meta-analyses showed that BDNF level decreases in depressed subjects and BDNF level markedly increases after antidepressant treatment []. Peripheral BDNF administration produced antidepressant-like responses at both behavioral and cellular levels and increased hippocampal neurogenesis []. BDNF may be regarded as a crucial biomarker of major depressive disorder (MDD) []. Chronic treatment with Zn2+ was observed to increase BDNF levels in the hippocampus and cortex in rats [,], implying the potential involvement of Zn2+ in the depression via BDNF system. Zn2+ has been demonstrated to transactivate TrkB and its downstream signaling pathways, including extracellular signal-regulated kinase (ERK1/2), cAMP response element binding protein (CREB), and phospholipase C-γ (PLC-γ) by increasing the activity of Src family kinase independent of BDNF [,] (Figure 3). This finding is in accordance with increased cortical ERK phosphorylation after Zn2+ treatment in a study by Franco et al. []. In addition, data suggest that the BDNF pathway depends on the GPR39. In GPR39 KO mice, the CREB and BDNF levels were decreased in the hippocampus []. Acute administration of TC-G 1008 (a GPR39 agonist) was found to increase hippocampal GPR39 and BDNF levels []. Activation of GPR39 seems to cause CREB over-expression via cAMP responsive element (CRE)-mediated transcription and thus up-regulates the BDNF expression []. Another potential mechanism involving CREB/BDNF pathway may be the antagonism of glycogen synthase kinase-3 (GSK-3). Zn2+ can suppress the phosphorylation activity of GSK-3, which inhibits the activity of CREB and is negatively regulated by BDNF [] (Figure 3). Thus, Zn2+ can enhance BDNF function via GSK-3 inhibition.
A growing body of evidence also suggests the involvement of neuronal precursor cells in Zn2+ deficiency-induced depression. About a 50% decrease in proliferating cells was observed in the subgranular zone (SGZ) and granular cell layer of the dentate gyrus in the rats treated with a Zn2+-restricted diet []. Similar findings were also demonstrated in Zn2+-deficient mice []. There was also a significant increase in the number of TUNEL-labeled cells in the SGZ, which is relevant to p53-dependent apoptotic mechanisms []. On the other hand, the number of dividing cells in the hilus and dentate gyrus of the hippocampus was reported to markedly increase by chronic antidepressant treatment []. Many imaging studies have identified reductions in hippocampal volume in patients with depression []. In addition to proliferation, Zn2+ deficiency also impairs neuronal differentiation, and this may be partially mediated by changes in TGF-β signaling [].
The antioxidative activity of Zn2+ may be involved in its antidepressant effects. Increased ROS and lipid peroxidation and decreased superoxide dismutase (SOD) levels were found in depressed patients [,]. Eight weeks of antidepressant treatment has been shown to increase SOD activity and decrease nitric oxide (NO) levels []. There is evidence showing that the antidepressant properties of Zn2+ are related to the involvement of the L-arginine-nitric oxide (NO) pathway, in which Zn2+ acts as an inhibitor of nitric oxide synthase (NOS) []. Malathion is a toxic organophosphate that could cause depressant-like behavior and oxidative damage in rodents [,], while the depressant-like effect and a series of oxidative damage caused by malathion were attenuated by Zn2+ []. The total glutathione level in the hippocampus and cerebral cortex was significantly increased accompanied by an antidepressant-like effect by a chronic Zn2+ treatment in rats, indicating an enhancement of antioxidant buffering capacity over time []. It was reported that Zn2+ supplementation reversed lithium-induced reductions in catalase and glutathione-s-transferase (GST) activities in the cerebellum, relieving the oxidative stress caused by lithium toxicity [].
Extensive evidence suggests that MDD is accompanied by the activation of an inflammatory response with changes in inflammatory markers []. Increased inflammatory activity was found in rat models of depression treated by bilateral olfactory bulbectomy [] or chronic mild stress (CMS) []. Depressed patients have been shown to have an increased number of monocytes, neutrophils and T-lymphocytes and increased generation of proinflammatory cytokines including IL-1, IL-6 and TNF-α [,]. Additionally, the indoleamine 2,3-dioxygenase (IDO) activated by proinflammatory cytokines metabolizes tryptophan into quinolinic acid, which is a NMDA receptor agonist involved in depression []. An acute phase (AP) response with alterations in AP proteins is also involved in depression, while one characteristic of acute phase response is reduced serum Zn2+ level []. It is thought that the Zn2+ reduction is due to Zn2+ sequestration by up-regulation of metallothionein in the liver, bone marrow and thymus, which is induced by increased proinflammatory cytokines [,]. Furthermore, IL-6-induced increase in Zip14 also plays a role in the mechanism of hypozincemia in acute phase response []. A significant negative correlation between serum IL-6 and Zn2+ levels was also reported []. In addition, the concentration of albumin, the major Zn2+ binding protein in serum, is significantly reduced in MDD patients and there is a markedly positive correlation between serum albumin and Zn2+ level [,], indicating the potential role of the carrier protein of Zn2+ in hypozincemia during depression. These findings indicate that inflammatory response is linked to a crucial Zn2+ redistribution [].
Besides, there is a negative correlation between neopterin and Zn2+ levels, suggesting that hypozincemia is also associated with the activation of cell-mediated immunity, which is related to increased cytokines such as IL-6 []. Zn2+ plays a significant role in cell-mediated immunity, and the availability of Zn2+ is indispensable for the normal functions of T cells and B cells [,]. Low Zn2+ level was shown to be correlated with a decrease in the CD4+/CD8+ T cell ratio [,]. Cytokines secreted by type 1 helper T (Th1) cells including IFN-γ and IL-2 are decreased under Zn2+ deficiency, suggesting an impairment of Th1 function [,], while one function of IFN-γ is to inhibit the development of the Th17 cells [], which play a significant role in autoimmune diseases. The study by Chen et al. described an imbalance of Th17/Treg ratio in MDD patients []. Zn2+ was also reported to inhibit Th17 development through attenuation of STAT3 activation []. The suppression of the proliferation and IL-17 production of stimulated human T cells by zinc aspartate provides further evidence of the relationship between Zn2+ and Th17 cells []. In addition, a significantly increased subset of B cells producing autoantibodies was found in MDD patients []. These findings suggest that there is an impaired cell-mediated immune function accompanied by a tendency towards autoimmune activity in depression [].
3.3. Zn2+ and Parkinson’s Disease
As the second most common neurodegenerative disorder after AD, Parkinson’s disease (PD) is a long-term CNS disorder affecting the motor system. The symptoms of PD progress slowly over time, with the most common and usually the first symptom of PD being bradykinesia, which is followed by other characteristic symptoms including tremors, muscle rigidity, postural instability and hypokinesia []. In addition to motor symptoms, non-motor symptoms such as psychiatric symptoms, fatigue, impaired sense of smell, sleep problems and symptoms associated with autonomous systems are also common in PD []. At present, the etiology of PD is not fully understood and 75% of cases are known as idiopathic PD, besides, more than 90% of PD cases are sporadic, whereas genetic factor only accounts for 5–10% of cases []. A wealth of evidence has implied the potential involvement of Zn2+ in the pathogenesis of PD. Multiple studies found a decreased circulating Zn2+ level in PD patients [,,,], whereas there is evidence showing a normal or even increased Zn2+ level []. Furthermore, recent meta-analysis studies showed that the Zn2+ levels in serum, plasma, and cerebrospinal fluid (CSF) are reduced in PD patients [,,]. In a drosophila model with a mutant Parkin, which is involved in familiar PD, Zn2+ supplementation improved both lifespan and motor abilities []. Quiroga et al. reported a case of PD with low Zn2+ and vitamin C levels whose movement disorder was rapidly resolved after the replacement of Zn2+ and vitamin C, highlighting the correlation between PD and Zn2+ deficiency [].
It is thought that the Zn2+ deficiency found in PD patients is attributed to the antioxidative properties of Zn2+ []. Oxidative stress is thought to play a crucial role in the pathogenesis of PD []. Many markers of oxidative stress, such as nucleic acid oxidation [], lipid peroxidation [] and protein nitration [] have been observed in dopaminergic brain regions. In rats with PD induced by rotenone, Zn2+ supplementation decreased lipid peroxidation and cell death, indicating that the neuroprotective role of Zn2+ may be mediated by its antioxidant effects []. Loss of ATP13A2 (PARK9), a lysosomal type 5 P-type ATPase which is correlated with early-onset PD, was shown to lead to Zn2+ dyshomeostasis, impaired mitochondrial function and altered ROS metabolism [], providing further evidence of the association between Zn2+ and oxidative stress in PD.
PD is pathologically characterized by the loss of dopaminergic neurons within substantia nigra pars compacta, subsequently leading to decreased dopamine secretion. Evidence suggests that excessive Zn2+ is linked to dopaminergic neurodegeneration. Post-mortem studies showed Zn2+ depositions in the substantia nigra of idiopathic PD patients []. In mouse models of PD induced with MPTP, the accumulation of Zn2+ was observed in degenerating dopaminergic neurons []. It was also demonstrated that the Zn2+ level increased in all structures located along the dopaminergic pathway in the 6-OHDA-induced parkinsonian brain []. In addition, Zn2+ chelation attenuated the neuronal death induced by MPP+, which is used to model PD []. These findings collectively suggest that the cytosolic labile accumulation of Zn2+ contributes to the death of dopaminergic neurons in PD.
Another characteristic feature of PD is the Lewy body, an insoluble aggregate composed of α-synuclein. It was reported that the accumulation of α-synuclein in dopaminergic neurons caused apoptosis, which required endogenous production of dopamine and was mediated by ROS, while non-dopaminergic neurons were not affected, providing a possible explanation for the selective loss of dopaminergic neurons in PD []. The increase of α-synuclein expression by METH was found to be reversed by pretreatment with Zn2+Cl2 []. Additionally, intracellular Zn2+ dyshomeostasis induced by PARK9 loss of function was demonstrated to cause accumulation of α-synuclein [].
3.4. Zn2+ and Multiple Sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disorder of CNS characterized by immune cell infiltration and myeline sheath damage. T cells with autoimmune activity can become sensitive to endogenous myelin to initiate the demyelinating process of the myelinated structures, leading to impairment of the communication of the brain and remaining body parts, as a result, MS patients suffer from coordination difficulties, muscle weakness and even irreversible neurological damage [].
Currently, the exact etiology of MS is not clear, it is thought that several factors including infection, genetics, immunology, and environment contribute to the disorder []. Accumulating evidence suggests that the disruption of Zn2+ homeostasis is associated with the pathogenesis of MS. Several studies have shown that MS patients have both decreased serum Zn2+ levels [,,] and reduced Zn2+ intake [], while others showed no significant differences in serum Zn2+ level between healthy controls and MS patients [], or even higher plasma Zn2+ concentration in MS patients []. However, two recent meta-analysis studies revealed lower circulating Zn2+ levels in MS [,]. It was reported that erythrocyte Zn2+ levels in patients with active disease exhibited a significant decrease during the early stages of exacerbation and a gradual increase with the recovery from the attack []. These findings suggest the potential role of Zn2+ dyshomeostasis during MS.
In terms of the mechanism, abnormal synaptic release and intracellular accumulation of Zn2+ are believed to be involved in multiple steps of MS including the activation of MMP-9 and disruption of the blood-brain barrier (BBB) and subsequent infiltration of immune cells []. Clioquinol (CQ) and ZnT3 gene deletion were shown to significantly suppress EAE (an animal model of MS)-associated clinical features and neuropathological changes, as well as inhibit MMP-9 activation, BBB disruption and immune cell infiltration, implying the involvement of synaptic Zn2+ in myelin damage of spinal cord white matter [,]. Likewise, similar results were also found in a recent study using 1H10, a novel Zn2+ chelator [].
Besides, activation of NADPH mediated by Zn2+ plays a role in the microglial activation and oligodendrocyte death, which act as mediators in the MS. Evidence suggests that Zn2+ alone can induce NADPH oxidase activation and subsequent ROS generation, which have been demonstrated to significantly contribute to the MS pathogenesis []. Apocynin, an NADPH oxidase assembly inhibitor, was reported to decrease clinical symptoms of EAE and MOG-induced proliferation, morphology transformation and pro-inflammatory cytokine release of cultured microglia []. In terms of oligodendrocyte death, the peroxynitrite produced through NADPH and iNOS is thought to be a candidate. Peroxynitrite was reported to promote Zn2+ release from intracellular stores, resulting in sequential activation of ERK1/2 and 12-lipoxygenase (12-LOX), ROS production and eventual oligodendroglia death. Meanwhile, toxicity induced by peroxynitrite could be blocked completely by N,N,N′,N′-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN), a Zn2+ chelator. In addition, it was observed that AMPA-mediated Ca2+-dependent Zn2+ accumulation participates in the excitotoxic injury of oligodendrocytes. The mechanism underlying this may be associated with Zn2+-induced ROS production, ERK1/2 and PARP-1 activation, leading to cell death [].
However, zinc aspartate was shown to inhibit proliferation and cytokine production in stimulated human T cells and mouse splenocytes in vitro, and reduce the clinical severity of EAE [,]. Furthermore, high concentrations of Zn2+ were observed to impair the metabolic fitness and differentiation of Th1 cells, a significant participant of MS, and decrease Th1 autoimmune inflammation []. Based on present findings, it seems to be paradoxical that both Zn2+ excess and deficiency are correlated with MS. One interpretation of this is the specific local Zn2+ requirements, to be specific, MS is a complex disease accompanied by both demyelination and remyelination processes, although Zn2+ excess has been shown to cause white matter damage, the following remyelination phase may require Zn2+ influx to proceed []. Current studies underline the complex interrelationship between Zn2+ and MS and provide novel insight into Zn2+ as a potential therapeutic target of MS, while the related molecular mechanisms need to be further investigated.
3.5. Zn2+ and Schizophrenia
Schizophrenia (SCZ) is a severe psychotic disorder with both neurodevelopmental and degenerative pathologies affecting approximately 1% of the population, and its typical symptoms can be divided into positive, negative, and cognitive symptoms [,]. The underlying causes of the SCZ phenotype involve the interactions of prenatal Zn2+ deficiency with genetic risk factors []. Moreover, fetal Zn2+ deficiency has been shown to be linked to maternal exposure to infectious agents, and evidence suggests that maternal infection is a key risk factor for the development of SCZ in offspring []. Given the crucial role of Zn2+ in the immune system and neurodevelopment, prenatal Zn2+ deficiency associated with inflammation causes deleterious effects in the fetal brain. Maternal immune activation induced by LPS can be utilized as a model of SCZ, and prenatal Zn2+ supplementation was observed to attenuate the behavioral impairments [], as well as alleviate the increased pro-inflammatory mediator expression, microglial and astrocyte density in the prefrontal cortex (PFC) of male offspring prenatally exposed to LPS [].
Besides, compared with the control group, a 30–50% decrease in brain Zn2+ content was found in postmortem brain tissue of early onset SCZ patients []. Several studies have revealed reduced serum Zn2+ levels in SCZ patients [,,]. Two recent meta-analysis studies also showed significantly lower serum and blood Zn2+ concentrations in SCZ patients [,]. The lower Zn2+ level in SCZ can lead to NMDA hyperactivity and possibly psychotic symptoms []. It was demonstrated that a combination of zinc sulfate and risperidone was effective in the improvement of SCZ symptoms and aggression risk, indicating the potential of Zn2+ as an adjuvant agent of SCZ management []. Another study further highlighted the possibility of Zn2+ as a therapeutic agent for SCZ management []. These findings illustrate the participation of Zn2+ dyshomeostasis in the pathogenesis of SCZ.
In addition, abnormalities of intracellular Zn2+ due to the dysfunction of molecules transporting Zn2+ are also involved in SCZ. One missense SNP of SLC39A8 (ZIP8 gene), rs13107325, was reported to be strongly associated with SCZ []. Whereas the potential mechanism by which this missense variant confers the risk of SCZ is not clear. Using a knock-in mouse model, in which a threonine was introduced at the 393rd amino acid of mouse SLC39A8 to correspond to rs13107325 (p.Ala391Thr) of human SLC39A8, Li et al. demonstrated dysregulation of Zn2+ level in the blood and brain and significantly reduced cortical dendritic spine density in the SLC39A8-p.393T knock-in mice, providing a reasonable explanation of the biological effects of rs13107325 in SCZ, as it is believed that dysfunction of dendritic spines may play a critical role in SCZ []. Furthermore, it was reported that this missense variant resulted in increased innate immune response and glutamate receptor hypofunction, which was partly associated with decreased surface expression of glutamate receptor subunits, further illustrating the role of rs13107325 []. In addition, mRNA for SLC39A12 (ZIP12 gene) was found to increase in the dorsolateral prefrontal cortex of SCZ patients []. Emerging data suggest that polymorphisms of SLC30A3, the gene encoding ZnT3, is also linked to a higher risk of schizophrenia []. Of note, genetic associations are emerging in other gene families related to Zn2+, such as the ZNF family, in which Zn2+ acts as a structural cofactor []. Among this family, ZNF804A is a typical example, and it has been demonstrated to be correlated with several psychiatric disorders including SCZ []. Current research provides novel insight into the involvement of Zn2+ and possible interventions in SCZ, while it is obvious that a better understanding is needed.
3.6. Zn2+ and Epilepsy
Epilepsy is a common neurological disorder affecting 65 million people worldwide []. It is characterized by recurrent and spontaneous seizures. Currently, antiepileptic drug (AED) therapy is the treatment of choice for epilepsy, although most patients can attain remission using AED, more than one-third of patients have resistance []. The available drugs can relieve symptoms rather than improve the underlying state or progression []. Therefore, the development of novel medication is still urgent. An imbalance between neuronal excitation and inhibition has been established as a potential explanation of epilepsy [], however, the mechanisms underlying epilepsy development and progression are still unclear, further limiting the search for novel drugs. Zn2+ is able to exert effects that either increase or decrease neuronal excitability, implying the possibility of Zn2+ involvement in the pathogenesis of epilepsy.
Although there is evidence from both preclinical and clinical studies corroborating the association between Zn2+ and epilepsy, the results are conflicting. Several studies reported a decreased serum Zn2+ level in children with epilepsy [,,]. However, a meta-analysis found a significantly higher serum Zn2+ concentration in nontreated patients with epilepsy []. Also, this study revealed a significantly lower serum Zn2+ level in epileptic patients receiving valproate monotherapy compared to epileptic patients without anticonvulsant therapy, which was consistent with the findings of another study reporting a markedly decreased serum concentration in epileptic patients after valproic acid treatment []. Moreover, a medium dose of Zn2+ has been demonstrated to reduce the severity of pilocarpine-induced limbic seizures either as a monotherapy or in combination with valproic acid in a rat model of epilepsy, while a high dose of Zn2+ exacerbated that, confirmed a dose-dependent effect of Zn2+ []. A double-blind, placebo-controlled trial found that 31% of children with refractory epilepsy who received Zn2+ treatment for six months showed significant improvement, compared with only 4.5% of patients treated with a placebo []. These findings suggest the critical role of Zn2+ in epilepsy and the potential add-on effect of Zn2+ supplementation in AED treatment.
The association between Zn2+ and epilepsy is complex and poorly understood. Evidence suggests that inflammation may act as both a consequence and the cause of epileptic seizures. Brain inflammation can be caused by seizures, and chronic inflammation can be aggravated by recurring seizures []. Studies have demonstrated that neuroinflammation with increased pro-inflammatory cytokines, such as IL-1β, lead to seizure-evoked excitotoxicity [,]. It was reported that consumption of antioxidants [], mitochondrial dysfunction and activated intrinsic pathway components [] may also be involved in epilepsy. Furthermore, the study by Baraka et al. [] showed that an appropriate dose of Zn2+ can mitigate seizures by suppressing increased IL-1β levels, oxidative stress, and apoptotic activity, which was consistent with another recent study by Mehmet et al. [], providing further evidence.
The involvement of proteins regulating Zn2+ homeostasis were also reported in epilepsy. Using animals lacking genes encoding specific proteins regulating Zn2+ homeostasis is a useful method to clarify the role of these proteins in epilepsy. Qian et al. [] showed increased susceptibility to seizures induced by kainic acid (KA) in mice lacking ZnT3. Likewise, a lower seizure threshold to KA was found in ZIP-1,3-null mutants. In addition, there was a significant difference in lethality caused by seizures, with 17% for mutants compared to 4% for the control group following a 15 mg/kg KA injection. Also, this study found that the decrease of Zn2+ uptake either by knockout of ZIP-1,3 transporters or abolishment of synaptic Zn2+ by knockout of ZnT3 exerts a protective role in neurodegeneration after prolonged seizures [].
In conclusion, as the pathways linking Zn2+ and the regulation of epilepsy are not fully understood, further research is needed to assess the efficacy of Zn2+-based therapy for epilepsy.
3.7. Zn2+ and Traumatic Brain Injury
Traumatic brain injury (TBI) is a cerebral pathology that has become a severe public health and socioeconomic issue in the modern world. It affects 64–79 million people worldwide annually []. TBI patients can develop many impairments in communication, sensory processing, and cognition. In addition, behavioral problems such as anxiety and depression can also be found in TBI patients [].
The role of Zn2+ in TBI has been studied over the past three decades. Emerging evidence suggests that Zn2+ seems to have both neurotoxic and neuroprotective roles in TBI []. Thus, there have been many studies examining the potential benefits of Zn2+ chelation or Zn2+ supplementation.
Following TBI, excessive Zn2+ releases from presynaptic vesicles acutely, and the inappropriate accumulation of intracellular Zn2+ in postsynaptic neurons leads to excitotoxicity and cell death []. Therefore, the possible application of Zn2+ chelators has been investigated. Calcium disodium ethylenediaminetetraacetate (CaEDTA) has been demonstrated to upregulate several neuroprotective genes and reduce apoptotic cell death after TBI []. Zhao et al. [] reported that Zn2+ translocation and autophagy were inhibited by TPEN following TBI.
Although Zn2+ exerts a deadly role in acute neuronal damage after injury, it is crucial for longer-term reparative processes including neurogenesis and gene expression []. In a study by McClain et al. [], decreased serum Zn2+ levels and increased urine Zn2+ excretion which was proportional to the severity of injury were found. In addition, moderate Zn2+ deficiency has been observed to increase cell death after TBI []. Furthermore, a recent multicenter prospective study [] reported an association between serum Zn2+ deficiency and a higher incidence of disability and long- and short-term mortality in TBI patients with intracranial injury. On the other hand, it was shown that although Zn2+ chelation could provide short-term histological neuroprotection, it failed to improve neurobehavioral outcomes, including spatial memory deficits, after TBI []. Another study revealed significantly decreased BrdU, Ki67 and DCX positive cells following CQ treatment, indicating that neurogenesis induced by TBI is suppressed by Zn2+ chelation [], further stressing the potential role of free Zn2+ in the repair of TBI.
As a result, there has been a recent trend to investigate the efficacy of appropriately timed Zn2+ supplementation in the improvement of outcomes following TBI. Evidence suggests that Zn2+ supplementation is effective in improving cognitive impairment and depression in animal models []. Also, it was shown that Zn2+ supplementation before injury could provide more protection from depression and anxiety associated with TBI than using Zn2+ solely as a treatment, indicating the possible use of chronic Zn2+ supplementation for populations at risk for TBI []. Similarly, in a double-blinded controlled trial, 68 patients with severe brain injury were randomly assigned to either a standard Zn2+ group or Zn2+-supplemented group. One month after injury, the standard Zn2+ group had a mortality rate of 26%, compared with only 12% in the Zn2+-supplemented group [].
Although the Zn2+ supplementation has been demonstrated to be effective in improving outcomes associated with TBI in current studies, future research is needed to determine the optimal timing and dosage of Zn2+ intervention to provide behavioral resiliency to TBI as a treatment.
4. Conclusions
As described above, current findings have consolidated that Zn2+ exerts a critical role in the initiation and progress of the pathological features of multiple CNS disorders. Zn2+ dyshomeostasis acts as an intersection of the pathogenesis and symptoms of these disorders. Therefore, the maintenance of Zn2+ homeostasis is crucial as any substantial alterations may lead to deleterious outcomes. As the acceleration of the appreciation for the relevance of Zn2+ in the pathogenesis of CNS disorders, restoration of Zn2+ homeostasis has been regarded as a potential therapeutic target. Although Zn2+ supplementation has shown some beneficial effects in the improvement of symptoms and pathological characteristics, considering the neurotoxicity of excess Zn2+ and the heterogeneity of neurological disorders, the dose of Zn2+ supplementation should be under strict control. Zn2+ chelation is another significant aspect of the intervention of CNS disorders, especially AD, nevertheless it also shows some side effects due to the chelation of other essential divalent metal ions, highlighting the timing of intervention. Thus, understanding how Zn2+ level changes over the course of the disorders is the key to optimize a Zn2+-based targeted therapy. In conclusion, a better comprehension of the underlying mechanisms linking Zn2+ and these CNS disorders is urgent and may contribute to the acceptance of Zn2+ as a key point in the CNS disorders. We hope that this review can provide new clues for the prevention and treatment of nervous system disorders.
Author Contributions
Writing—original draft preparation, B.W.; writing—review & editing, T.F.; supervision, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Haase, H. Zinc homeostasis and immunity. Trends Immunol. 2007, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, T.V. Transition metals in control of gene expression. Science 1993, 261, 715–725. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Hambidge, M. Human zinc deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef]
- Sandstead, H.H. Subclinical zinc deficiency impairs human brain function. J. Trace Elem. Med. Biol. 2012, 26, 70–73. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Zinc, Carnosine, and Neurodegenerative Diseases. Nutrients 2018, 10, 147. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and Skin Disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef]
- Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; Henkin, R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R398–R408. [Google Scholar] [CrossRef]
- Solomons, N.W. Update on zinc biology. Ann. Nutr. Metab. 2013, 62 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- World Health Organization. Zinc Supplementation and Growth in Children: Biological, Behavioural and Contextual Rationale. Available online: http://www.who.int/elena/bbc/zinc_stunting/en/ (accessed on 15 November 2022).
- Heintschel, M.; Heuberger, R. The Potential Role of Zinc Supplementation on Pressure Injury Healing in Older Adults: A Review of the Literature. Wounds-Compend. Clin. Res. Pract. 2017, 29, 56–61. [Google Scholar]
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef]
- Nuttall, J.R.; Oteiza, P.I. Zinc and the aging brain. Genes Nutr. 2014, 9, 379. [Google Scholar] [CrossRef]
- Koh, J.Y. Zinc and disease of the brain. Mol. Neurobiol. 2001, 24, 99–106. [Google Scholar] [CrossRef]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Singh, K.; Avasthi, K.; Kim, J.J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr. 2021, 60, 55–64. [Google Scholar] [CrossRef]
- Smart, T.G.; Hosie, A.M.; Miller, P.S. Zn2+ ions: Modulators of excitatory and inhibitory synaptic activity. Neuroscientist 2004, 10, 432–442. [Google Scholar] [CrossRef]
- Westbrook, G.L.; Mayer, M.L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 1987, 328, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Yin, H.Z.; Carriedo, S.G.; Rao, S.S.; Weiss, J.H. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. USA 1999, 96, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Kodirov, S.A.; Takizawa, S.; Joseph, J.; Kandel, E.R.; Shumyatsky, G.P.; Bolshakov, V.Y. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 15218–15223. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nakamura, M.; Fujii, H.; Tamano, H. Synaptic Zn2+ homeostasis and its significance. Metallomics 2013, 5, 417–423. [Google Scholar] [CrossRef]
- Patrick, W.H.; Dyck, R.H. Signaling by Synaptic Zinc is Required for Whisker-Mediated, Fine Texture Discrimination. Neuroscience 2018, 369, 242–247. [Google Scholar] [CrossRef]
- Barr, C.A.; Burdette, S.C. The zinc paradigm for metalloneurochemistry. Essays Biochem. 2017, 61, 225–235. [Google Scholar]
- Takeda, A.; Tamano, H.; Tochigi, M.; Oku, N. Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem. Int. 2005, 46, 221–225. [Google Scholar] [CrossRef]
- Choi, B.Y.; Hong, D.K.; Jeong, J.H.; Lee, B.E.; Koh, J.Y.; Suh, S.W. Zinc transporter 3 modulates cell proliferation and neuronal differentiation in the adult hippocampus. Stem Cells 2020, 38, 994–1006. [Google Scholar] [CrossRef]
- Keller, K.A.; Grider, A.; Coffield, J.A. Age-dependent influence of dietary zinc restriction on short-term memory in male rats. Physiol. Behav. 2001, 72, 339–348. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Tonder, N.; Johansen, F.F.; Frederickson, C.J.; Zimmer, J.; Diemer, N.H. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 1990, 109, 247–252. [Google Scholar] [CrossRef]
- Li, Y.; Hough, C.J.; Suh, S.W.; Sarvey, J.M.; Frederickson, C.J. Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J. Neurophysiol. 2001, 86, 2597–2604. [Google Scholar] [CrossRef]
- Suh, S.W.; Thompson, R.B.; Frederickson, C.J. Loss of vesicular zinc and appearance of perikaryal zinc after seizures induced by pilocarpine. Neuroreport 2001, 12, 1523–1525. [Google Scholar] [CrossRef]
- Li, M.S.; Adesina, S.E.; Ellis, C.L.; Gooch, J.L.; Hoover, R.S.; Williams, C.R. NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am. J. Physiol.-Cell Physiol. 2017, 312, C47–C55. [Google Scholar] [CrossRef]
- Choi, S.; Hong, D.K.; Choi, B.Y.; Suh, S.W. Zinc in the Brain: Friend or Foe? Int. J. Mol. Sci. 2020, 21, 8941. [Google Scholar] [CrossRef]
- Maret, W.; Krezel, A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007, 13, 371–375. [Google Scholar] [CrossRef]
- Quaife, C.J.; Findley, S.D.; Erickson, J.C.; Froelick, G.J.; Kelly, E.J.; Zambrowicz, B.P.; Palmiter, R.D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 1994, 33, 7250–7259. [Google Scholar] [CrossRef]
- Manso, Y.; Adlard, P.A.; Carrasco, J.; Vasak, M.; Hidalgo, J. Metallothionein and brain inflammation. J. Biol. Inorg. Chem. 2011, 16, 1103–1113. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Shyam, S.A.; Manisenthil, K.K.; Senthil, K.M.; Ganesh, G.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef]
- Sato, M.; Kondoh, M. Recent studies on metallothionein: Protection against toxicity of heavy metals and oxygen free radicals. Tohoku J. Exp. Med. 2002, 196, 9–22. [Google Scholar] [CrossRef]
- Thingholm, T.E.; Ronnstrand, L.; Rosenberg, P.A. Why and how to investigate the role of protein phosphorylation in ZIP and ZnT zinc transporter activity and regulation. Cell. Mol. Life Sci. 2020, 77, 3085–3102. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. Embo J. 1995, 14, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Robbins, C.A.; Wenzel, H.J.; Schwartzkroin, P.A.; Palmiter, R.D. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000, 39, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Olesen, R.H.; Hyde, T.M.; Kleinman, J.E.; Smidt, K.; Rungby, J.; Larsen, A. Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain. Transl. Psychiatr. 2016, 6, e838. [Google Scholar] [CrossRef]
- Beyer, N.; Coulson, D.T.; Heggarty, S.; Ravid, R.; Irvine, G.B.; Hellemans, J.; Johnston, J.A. ZnT3 mRNA levels are reduced in Alzheimer’s disease post-mortem brain. Mol. Neurodegener. 2009, 4, 53. [Google Scholar] [CrossRef]
- McAllister, B.B.; Dyck, R.H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef]
- Wang, F.; Dufner-Beattie, J.; Kim, B.E.; Petris, M.J.; Andrews, G.; Eide, D.J. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 2004, 279, 24631–24639. [Google Scholar] [CrossRef]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Kury, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef]
- Baum, L.; Chan, I.H.; Cheung, S.K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tan, L.; Wang, H.F.; Ma, J.; Liu, J.; Tan, M.S.; Sun, J.H.; Zhu, X.C.; Jiang, T.; Yu, J.T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef]
- Corona, C.; Masciopinto, F.; Silvestri, E.; Viscovo, A.D.; Lattanzio, R.; Sorda, R.L.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91. [Google Scholar] [CrossRef]
- Loef, M.; von Stillfried, N.; Walach, H. Zinc diet and Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2012, 15, 2–12. [Google Scholar] [CrossRef]
- Maes, M.; D’Haese, P.C.; Scharpe, S.; D’Hondt, P.; Cosyns, P.; De Broe, M.E. Hypozincemia in depression. J. Affect. Disord. 1994, 31, 135–140. [Google Scholar] [CrossRef]
- Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. The efficacy of zinc supplementation in depression: Systematic review of randomised controlled trials. J. Affect. Disord. 2012, 136, e31–e39. [Google Scholar] [CrossRef]
- Yosaee, S.; Clark, C.; Keshtkaran, Z.; Ashourpour, M.; Keshani, P.; Soltani, S. Zinc in depression: From development to treatment: A comparative/dose response meta-analysis of observational studies and randomized controlled trials. Gen. Hosp. Psychiatry 2022, 74, 110–117. [Google Scholar] [CrossRef]
- Aoki, C.; Imai, K.; Owaki, T.; Kobayashi-Nakano, T.; Ushida, T.; Iitani, Y.; Nakamura, N.; Kajiyama, H.; Kotani, T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Med. Lith. 2022, 58, 731. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.Y.; Zhong, X.; Wei, M.J. Decreased circulating Zinc levels in Parkinson’s disease: A meta-analysis study. Sci. Rep. 2017, 7, 3902. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, X.; Ge, H.; Wang, T.; Wang, Y.; Li, W. Association Between Serum Zinc Levels and the Risk of Parkinson’s Disease: A Meta-Analysis. Biol. Trace Elem. Res. 2017, 179, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Adani, G.; Filippini, T.; Michalke, B.; Vinceti, M. Selenium and Other Trace Elements in the Etiology of Parkinson’s Disease: A Systematic Review and Meta-Analysis of Case-Control Studies. Neuroepidemiology 2020, 54, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Schaffner, W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol. Chem. 2010, 391, 513–518. [Google Scholar] [CrossRef]
- Bredholt, M.; Frederiksen, J.L. Zinc in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Asn Neuro 2016, 8, 1759091416651511. [Google Scholar] [CrossRef]
- Nirooei, E.; Kashani, S.; Owrangi, S.; Malekpour, F.; Niknam, M.; Moazzen, F.; Nowrouzi-Sohrabi, P.; Farzinmehr, S.; Akbari, H. Blood Trace Element Status in Multiple Sclerosis: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 200, 13–26. [Google Scholar] [CrossRef]
- Stoye, D.; Schubert, C.; Goihl, A.; Guttek, K.; Reinhold, A.; Brocke, S.; Grungreiff, K.; Reinhold, D. Zinc aspartate suppresses T cell activation in vitro and relapsing experimental autoimmune encephalomyelitis in SJL/J mice. Biometals 2012, 25, 529–539. [Google Scholar] [CrossRef]
- Schubert, C.; Guttek, K.; Grungreiff, K.; Thielitz, A.; Buhling, F.; Reinhold, A.; Brocke, S.; Reinhold, D. Oral zinc aspartate treats experimental autoimmune encephalomyelitis. Biometals 2014, 27, 1249–1262. [Google Scholar] [CrossRef]
- Joe, P.; Petrilli, M.; Malaspina, D.; Weissman, J. Zinc in schizophrenia: A meta-analysis. Gen. Hosp. Psych. 2018, 53, 19–24. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Mahmoudi, M.; Shahrokhi, S.; Mojarrad, M.; Dastmardi, M.; Mirbeyk, M.; Rezaei, N. Trace elements in schizophrenia: A systematic review and meta-analysis of 39 studies (N = 5151 participants). Nutr. Rev. 2020, 78, 278–303. [Google Scholar] [CrossRef]
- Mortazavi, M.; Farzin, D.; Zarhghami, M.; Hosseini, S.H.; Mansoori, P.; Nateghi, G. Efficacy of Zinc Sulfate as an Add-on Therapy to Risperidone Versus Risperidone Alone in Patients with Schizophrenia: A Double-Blind Randomized Placebo-Controlled Trial. Iran. J. Psychiatry Behav. Sci. 2015, 9, e853. [Google Scholar] [CrossRef]
- Onaolapo, O.J.; Ademakinwa, O.Q.; Olalekan, T.O.; Onaolapo, A.Y. Ketamine-induced behavioural and brain oxidative changes in mice: An assessment of possible beneficial effects of zinc as mono- or adjunct therapy. Psychopharmacology 2017, 234, 2707–2725. [Google Scholar] [CrossRef]
- Alizadeh, F.; Davoodian, N.; Kazemi, H.; Ghasemi-Kasman, M.; Shaerzadeh, F. Prenatal zinc supplementation attenuates lipopolysaccharide-induced behavioral impairments in maternal immune activation model. Behav. Brain Res. 2020, 377, 112247. [Google Scholar] [CrossRef]
- Wojciak, R.W.; Mojs, E.; Stanislawska-Kubiak, M.; Samborski, W. The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res. 2013, 104, 40–44. [Google Scholar] [CrossRef]
- Seven, M.; Basaran, S.Y.; Cengiz, M.; Unal, S.; Yuksel, A. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. 2013, 104, 35–39. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Mahmoudi, M.; Meysamie, A.; Gharedaghi, M.; Zamponi, G.W.; Rezaei, N. Possible role of trace elements in epilepsy and febrile seizures: A meta-analysis. Nutr. Rev. 2015, 73, 760–779. [Google Scholar] [CrossRef]
- Baraka, A.M.; Hassab El Nabi, W.; El Ghotni, S. Investigating the role of zinc in a rat model of epilepsy. CNS Neurosci. Ther. 2012, 18, 327–333. [Google Scholar] [CrossRef]
- Saad, K.; El-Houfey, A.A.; Abd El-Hamed, M.A.; El-Asheer, O.M.; Al-Atram, A.A.; Tawfeek, M.S.K. A randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc in children with intractable epilepsy. Funct. Neurol. 2015, 30, 181–185. [Google Scholar] [CrossRef]
- Kirazlar, M.; Erdogan, M.A.; Erbas, O. Anti-seizure effect of zinc on PTZ-induced epilepsy in rat model. Bratisl. Lek. Listy 2022, 123, 648–652. [Google Scholar] [CrossRef]
- McClain, C.J.; Twyman, D.L.; Ott, L.G.; Rapp, R.P.; Tibbs, P.A.; Norton, J.A.; Kasarskis, E.J.; Dempsey, R.J.; Young, B. Serum and urine zinc response in head-injured patients. J. Neurosurg. 1986, 64, 224–230. [Google Scholar] [CrossRef]
- Cope, E.C.; Morris, D.R.; Scrimgeour, A.G.; Levenson, C.W. Use of zinc as a treatment for traumatic brain injury in the rat: Effects on cognitive and behavioral outcomes. Neurorehabilit. Neural Repair 2012, 26, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Ott, L.; Kasarskis, E.; Rapp, R.; Moles, K.; Dempsey, R.J.; Tibbs, P.A.; Kryscio, R.; McClain, C. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J. Neurotrauma 1996, 13, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Lill, C.M.; Tanzi, R.E. The genetics of Alzheimer disease: Back to the future. Neuron 2010, 68, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Burnet, F.M. A possible role of zinc in the pathology of dementia. Lancet 1981, 1, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. Embo Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Grilli, M.; Goffi, F.; Memo, M.; Spano, P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 1996, 271, 15002–15007. [Google Scholar] [CrossRef]
- Cuesta, A.; Zambrano, A.; Royo, M.; Pascual, A. The tumour suppressor p53 regulates the expression of amyloid precursor protein (APP). Biochem. J. 2009, 418, 643–650. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, H.; Zhao, J. Multifunctional roles of zinc in Alzheimer’s disease. Neurotoxicology 2020, 80, 112–123. [Google Scholar] [CrossRef]
- Dahms, S.O.; Konnig, I.; Roeser, D.; Guhrs, K.H.; Mayer, M.C.; Kaden, D.; Multhaup, G.; Than, M.E. Metal binding dictates conformation and function of the amyloid precursor protein (APP) E2 domain. J. Mol. Biol. 2012, 416, 438–452. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado, S.A.; Vissoci, R.E. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Cristovao, J.S.; Santos, R.; Gomes, C.M. Metals and Neuronal Metal Binding Proteins Implicated in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2016, 2016, 9812178. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Religa, D.; Strozyk, D.; Cherny, R.A.; Volitakis, I.; Haroutunian, V.; Winblad, B.; Naslund, J.; Bush, A.I. Elevated cortical zinc in Alzheimer disease. Neurology 2006, 67, 69–75. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cole, T.B.; Palmiter, R.D.; Suh, S.W.; Koh, J.Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7705–7710. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Kawahara, M.; Mizuno, D.; Koyama, H.; Konoha, K.; Ohkawara, S.; Sadakane, Y. Disruption of zinc homeostasis and the pathogenesis of senile dementia. Metallomics 2014, 6, 209–219. [Google Scholar] [CrossRef]
- Arispe, N.; Diaz, J.C.; Simakova, O. Abeta ion channels. Prospects for treating Alzheimer’s disease with Abeta channel blockers. Biochim. Biophys Acta 2007, 1768, 1952–1965. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, F.; Liu, Y.; Lu, N. Zinc and its effects on oxidative stress in Alzheimer’s disease. Neurol. Sci. 2014, 35, 923–928. [Google Scholar] [CrossRef]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Goldstein, L.E.; Nunomura, A.; Smith, M.A.; Lim, J.T.; Atwood, C.S.; Huang, X.; Farrag, Y.W.; Perry, G.; Bush, A.I. Evidence that the beta-amyloid plaques of Alzheimer’s disease represent the redox-silencing and entombment of abeta by zinc. J. Biol. Chem. 2000, 275, 19439–19442. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Pettingell, W.J.; Paradis, M.D.; Tanzi, R.E. Modulation of A beta adhesiveness and secretase site cleavage by zinc. J. Biol. Chem. 1994, 269, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Wang, C.Y.; Wang, T.; Li, Y.C.; Wang, Z.Y. Glial S100A6 Degrades beta-amyloid Aggregation through Targeting Competition with Zinc Ions. Aging Dis. 2019, 10, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Segawa, S.; Matsuo, T.; Nakamura, S.; Kikkawa, Y.; Nishida, K.; Nagasawa, K. Microglial zinc uptake via zinc transporters induces ATP release and the activation of microglia. Glia 2011, 59, 1933–1945. [Google Scholar] [CrossRef]
- Hwang, J.J.; Park, M.H.; Choi, S.Y.; Koh, J.Y. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J. Biol. Chem. 2005, 280, 11995–12001. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016, 86, 735–741. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef]
- Riffault, B.; Medina, I.; Dumon, C.; Thalman, C.; Ferrand, N.; Friedel, P.; Gaiarsa, J.L.; Porcher, C. Pro-brain-derived neurotrophic factor inhibits GABAergic neurotransmission by activating endocytosis and repression of GABAA receptors. J. Neurosci. 2014, 34, 13516–13534. [Google Scholar] [CrossRef]
- Busche, M.A.; Konnerth, A. Neuronal hyperactivity—A key defect in Alzheimer’s disease? Bioessays 2015, 37, 624–632. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I. The essential elements of Alzheimer’s disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Datki, Z.; Galik-Olah, Z.; Janosi-Mozes, E.; Szegedi, V.; Kalman, J.; Hunya, A.G.; Fulop, L.; Tamano, H.; Takeda, A.; Adlard, P.A.; et al. Alzheimer risk factors age and female sex induce cortical Abeta aggregation by raising extracellular zinc. Mol. Psychiatr. 2020, 25, 2728–2741. [Google Scholar] [CrossRef]
- Smith, J.L.; Xiong, S.; Lovell, M.A. 4-Hydroxynonenal disrupts zinc export in primary rat cortical cells. Neurotoxicology 2006, 27, 1–5. [Google Scholar] [CrossRef]
- Xiong, Y.; Jing, X.P.; Zhou, X.W.; Wang, X.L.; Yang, Y.; Sun, X.Y.; Qiu, M.; Cao, F.Y.; Lu, Y.M.; Liu, R.; et al. Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol. Aging 2013, 34, 745–756. [Google Scholar] [CrossRef]
- Moreira, G.G.; Cristovao, J.S.; Torres, V.M.; Carapeto, A.P.; Rodrigues, M.S.; Landrieu, I.; Cordeiro, C.; Gomes, C.M. Zinc Binding to Tau Influences Aggregation Kinetics and Oligomer Distribution. Int. J. Mol. Sci. 2019, 20, 5979. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Cao, Y.; Lang, M.; Lu, B.; Zhou, B. Zinc binding directly regulates tau toxicity independent of tau hyperphosphorylation. Cell Rep. 2014, 8, 831–842. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Bush, A.I.; Bach, G.; Smidt, K.; Larsen, A.; Rungby, J.; Lund, S.; Doering, P.; Danscher, G. Amyloid plaques arise from zinc-enriched cortical layers in APP/PS1 transgenic mice and are paradoxically enlarged with dietary zinc deficiency. Neuroscience 2007, 150, 357–369. [Google Scholar] [CrossRef]
- Linkous, D.H.; Adlard, P.A.; Wanschura, P.B.; Conko, K.M.; Flinn, J.M. The effects of enhanced zinc on spatial memory and plaque formation in transgenic mice. J. Alzheimers Dis. 2009, 18, 565–579. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, T.; Zheng, W.; Zhao, B.L.; Danscher, G.; Chen, Y.H.; Wang, Z.Y. Zinc overload enhances APP cleavage and Abeta deposition in the Alzheimer mouse brain. PLoS ONE 2010, 5, e15349. [Google Scholar] [CrossRef]
- Craven, K.M.; Kochen, W.R.; Hernandez, C.M.; Flinn, J.M. Zinc Exacerbates Tau Pathology in a Tau Mouse Model. J. Alzheimers Dis. 2018, 64, 617–630. [Google Scholar] [CrossRef]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Ritchie, C.W.; Bush, A.I.; Mackinnon, A.; Macfarlane, S.; Mastwyk, M.; MacGregor, L.; Kiers, L.; Cherny, R.; Li, Q.X.; Tammer, A.; et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003, 60, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Du, T.; Laughton, K.M.; Volitakis, I.; Sharples, R.A.; Xilinas, M.E.; Hoke, D.E.; Holsinger, R.M.; Evin, G.; Cherny, R.A.; et al. Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J. Biol. Chem. 2006, 281, 17670–17680. [Google Scholar] [CrossRef] [PubMed]
- Tassabehji, N.M.; Corniola, R.S.; Alshingiti, A.; Levenson, C.W. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008, 95, 365–369. [Google Scholar] [CrossRef]
- Siwek, M.; Dudek, D.; Schlegel-Zawadzka, M.; Morawska, A.; Piekoszewski, W.; Opoka, W.; Zieba, A.; Pilc, A.; Popik, P.; Nowak, G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J. Affect. Disord. 2010, 126, 447–452. [Google Scholar] [CrossRef]
- Carroll, B.J.; Feinberg, M.; Greden, J.F.; Tarika, J.; Albala, A.A.; Haskett, R.F.; James, N.M.; Kronfol, Z.; Lohr, N.; Steiner, M.; et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 1981, 38, 15–22. [Google Scholar] [CrossRef]
- Holsboer, F. The dexamethasone suppression test in depressed patients: Clinical and biochemical aspects. J. Steroid Biochem. Mol. Biol. 1983, 19, 251–257. [Google Scholar] [CrossRef]
- Arana, G.W.; Baldessarini, R.J.; Ornsteen, M. The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Commentary and review. Arch. Gen. Psychiatry 1985, 42, 1193–1204. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H.; Kan, F.; Itoh, H.; Oku, N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav. Brain Res. 2007, 177, 1–6. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H.; Kan, F.; Hanajima, T.; Yamada, K.; Oku, N. Enhancement of social isolation-induced aggressive behavior of young mice by zinc deficiency. Life Sci. 2008, 82, 909–914. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H.; Ogawa, T.; Takada, S.; Ando, M.; Oku, N.; Watanabe, M. Significance of serum glucocorticoid and chelatable zinc in depression and cognition in zinc deficiency. Behav. Brain Res. 2012, 226, 259–264. [Google Scholar] [CrossRef]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Watanabe, M.; Tamano, H.; Kikuchi, T.; Takeda, A. Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem. Int. 2010, 56, 410–416. [Google Scholar] [CrossRef]
- Mlyniec, K. Zinc in the Glutamatergic Theory of Depression. Curr. Neuropharmacol. 2015, 13, 505–513. [Google Scholar] [CrossRef]
- Nowak, G.; Schlegel-Zawadzka, M. Alterations in serum and brain trace element levels after antidepressant treatment: Part I. Zinc. Biol. Trace Elem. Res. 1999, 67, 85–92. [Google Scholar] [CrossRef]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kucma, M.; Mlyniec, K.; Rafalo, A.; Ostachowicz, B.; Lankosz, M.; Nowak, G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav. Brain Res. 2015, 287, 323–330. [Google Scholar] [CrossRef]
- Szewczyk, B.; Poleszak, E.; Wlaz, P.; Wrobel, A.; Blicharska, E.; Cichy, A.; Dybala, M.; Siwek, A.; Pomierny-Chamiolo, L.; Piotrowska, A.; et al. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 323–329. [Google Scholar] [CrossRef]
- Cichy, A.; Sowa-Kucma, M.; Legutko, B.; Pomierny-Chamiolo, L.; Siwek, A.; Piotrowska, A.; Szewczyk, B.; Poleszak, E.; Pilc, A.; Nowak, G. Zinc-induced adaptive changes in NMDA/glutamatergic and serotonergic receptors. Pharmacol. Rep. 2009, 61, 1184–1191. [Google Scholar] [CrossRef]
- Satala, G.; Duszynska, B.; Stachowicz, K.; Rafalo, A.; Pochwat, B.; Luckhart, C.; Albert, P.R.; Daigle, M.; Tanaka, K.F.; Hen, R.; et al. Concentration-Dependent Dual Mode of Zn2+ Action at Serotonin 5-HT1A Receptors: In Vitro and In Vivo Studies. Mol. Neurobiol. 2016, 53, 6869–6881. [Google Scholar] [CrossRef]
- Barrondo, S.; Salles, J. Allosteric modulation of 5-HT(1A) receptors by zinc: Binding studies. Neuropharmacology 2009, 56, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Satala, G.; Duszynska, B.; Lenda, T.; Nowak, G.; Bojarski, A.J. Allosteric Inhibition of Serotonin 5-HT7 Receptors by Zinc Ions. Mol. Neurobiol. 2018, 55, 2897–2910. [Google Scholar] [CrossRef] [PubMed]
- Tena-Campos, M.; Ramon, E.; Borroto-Escuela, D.O.; Fuxe, K.; Garriga, P. The zinc binding receptor GPR39 interacts with 5-HT1A and GalR1 to form dynamic heteroreceptor complexes with signaling diversity. Biochim. Biophys. Acta 2015, 1852, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Tena-Campos, M.; Ramon, E.; Lupala, C.S.; Perez, J.J.; Koch, K.W.; Garriga, P. Zinc Is Involved in Depression by Modulating G Protein-Coupled Receptor Heterodimerization. Mol. Neurobiol. 2016, 53, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Narvaez, M.; Marcellino, D.; Parrado, C.; Narvaez, J.A.; Tarakanov, A.O.; Agnati, L.F.; Diaz-Cabiale, Z.; Fuxe, K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 2010, 393, 767–772. [Google Scholar] [CrossRef]
- Petrilli, M.A.; Kranz, T.M.; Kleinhaus, K.; Joe, P.; Getz, M.; Johnson, P.; Chao, M.V.; Malaspina, D. The Emerging Role for Zinc in Depression and Psychosis. Front. Pharmacol. 2017, 8, 414. [Google Scholar] [CrossRef]
- Szewczyk, B.; Poleszak, E.; Sowa-Kucma, M.; Wrobel, A.; Slotwinski, S.; Listos, J.; Wlaz, P.; Cichy, A.; Siwek, A.; Dybala, M.; et al. The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids 2010, 39, 205–217. [Google Scholar] [CrossRef]
- Poleszak, E.; Szewczyk, B.; Wlaz, A.; Fidecka, S.; Wlaz, P.; Pilc, A.; Nowak, G. D-serine, a selective glycine/N-methyl-D-aspartate receptor agonist, antagonizes the antidepressant-like effects of magnesium and zinc in mice. Pharmacol. Rep. 2008, 60, 996–1000. [Google Scholar]
- Doboszewska, U.; Sowa-Kucma, M.; Mlyniec, K.; Pochwat, B.; Holuj, M.; Ostachowicz, B.; Pilc, A.; Nowak, G.; Szewczyk, B. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 254–263. [Google Scholar] [CrossRef]
- Belozertseva, I.V.; Kos, T.; Popik, P.; Danysz, W.; Bespalov, A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 2007, 17, 172–179. [Google Scholar] [CrossRef]
- Chaki, S.; Yoshikawa, R.; Hirota, S.; Shimazaki, T.; Maeda, M.; Kawashima, N.; Yoshimizu, T.; Yasuhara, A.; Sakagami, K.; Okuyama, S.; et al. MGS0039: A potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 2004, 46, 457–467. [Google Scholar] [CrossRef]
- Szewczyk, B.; Poleszak, E.; Sowa-Kucma, M.; Siwek, M.; Dudek, D.; Ryszewska-Pokrasniewicz, B.; Radziwon-Zaleska, M.; Opoka, W.; Czekaj, J.; Pilc, A.; et al. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol. Rep. 2008, 60, 588–589. [Google Scholar]
- Holst, B.; Egerod, K.L.; Schild, E.; Vickers, S.P.; Cheetham, S.; Gerlach, L.O.; Storjohann, L.; Stidsen, C.E.; Jones, R.; Beck-Sickinger, A.G.; et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007, 148, 13–20. [Google Scholar] [CrossRef]
- Mlyniec, K.; Budziszewska, B.; Holst, B.; Ostachowicz, B.; Nowak, G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 2014, 18, pyu002. [Google Scholar] [CrossRef]
- Mlyniec, K.; Doboszewska, U.; Szewczyk, B.; Sowa-Kucma, M.; Misztak, P.; Piekoszewski, W.; Trela, F.; Ostachowicz, B.; Nowak, G. The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology 2014, 79, 290–297. [Google Scholar] [CrossRef]
- Omar, N.N.; Tash, R.F. Fluoxetine coupled with zinc in a chronic mild stress model of depression: Providing a reservoir for optimum zinc signaling and neuronal remodeling. Pharmacol. Biochem. Behav. 2017, 160, 30–38. [Google Scholar] [CrossRef]
- Saadi, R.A.; He, K.; Hartnett, K.A.; Kandler, K.; Hershfinkel, M.; Aizenman, E. SNARE-dependent upregulation of potassium chloride co-transporter 2 activity after metabotropic zinc receptor activation in rat cortical neurons in vitro. Neuroscience 2012, 210, 38–46. [Google Scholar] [CrossRef]
- Siodlak, D.; Nowak, G.; Mlyniec, K. Interaction between zinc, the GPR39 zinc receptor and the serotonergic system in depression. Brain Res. Bull. 2021, 170, 146–154. [Google Scholar] [CrossRef]
- Perez-Rosello, T.; Anderson, C.T.; Schopfer, F.J.; Zhao, Y.; Gilad, D.; Salvatore, S.R.; Freeman, B.A.; Hershfinkel, M.; Aizenman, E.; Tzounopoulos, T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. 2013, 33, 9259–9272. [Google Scholar] [CrossRef]
- Mlyniec, K.; Gawel, M.; Nowak, G. Study of antidepressant drugs in GPR39 (zinc receptor(−)/(−)) knockout mice, showing no effect of conventional antidepressants, but effectiveness of NMDA antagonists. Behav. Brain Res. 2015, 287, 135–138. [Google Scholar] [CrossRef]
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Duman, R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 2010, 35, 2378–2391. [Google Scholar] [CrossRef] [PubMed]
- Mlyniec, K. Interaction between Zinc, GPR39, BDNF and Neuropeptides in Depression. Curr. Neuropharmacol. 2021, 19, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kucma, M.; Legutko, B.; Szewczyk, B.; Novak, K.; Znojek, P.; Poleszak, E.; Papp, M.; Pilc, A.; Nowak, G. Antidepressant-like activity of zinc: Further behavioral and molecular evidence. J. Neural Transm. 2008, 115, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.L.; Posser, T.; Brocardo, P.S.; Trevisan, R.; Uliano-Silva, M.; Gabilan, N.H.; Santos, A.R.; Leal, R.B.; Rodrigues, A.L.; Farina, M.; et al. Involvement of glutathione, ERK1/2 phosphorylation and BDNF expression in the antidepressant-like effect of zinc in rats. Behav. Brain Res. 2008, 188, 316–323. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Pan, E.; Xiong, Z.Q.; McNamara, J.O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 2008, 57, 546–558. [Google Scholar] [CrossRef]
- Nagappan, G.; Woo, N.H.; Lu, B. Ama “zinc” link between TrkB transactivation and synaptic plasticity. Neuron 2008, 57, 477–479. [Google Scholar] [CrossRef]
- Mlyniec, K.; Starowicz, G.; Gawel, M.; Frackiewicz, E.; Nowak, G. Potential antidepressant-like properties of the TC G-1008, a GPR39 (zinc receptor) agonist. J. Affect. Disord. 2016, 201, 179–184. [Google Scholar] [CrossRef]
- Corniola, R.S.; Tassabehji, N.M.; Hare, J.; Sharma, G.; Levenson, C.W. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008, 1237, 52–61. [Google Scholar] [CrossRef]
- Gao, H.L.; Zheng, W.; Xin, N.; Chi, Z.H.; Wang, Z.Y.; Chen, J.; Wang, Z.Y. Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox. Res. 2009, 16, 416–425. [Google Scholar] [CrossRef]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, L.R.; Marcinowska, A.; Obuchowicz, E. Antiapoptotic and neurotrophic effects of antidepressants: A review of clinical and experimental studies. Brain Res. Bull. 2009, 79, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Gower-Winter, S.D.; Corniola, R.S.; Morgan, T.J.; Levenson, C.W. Zinc deficiency regulates hippocampal gene expression and impairs neuronal differentiation. Nutr. Neurosci. 2013, 16, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.O.; Lin, J.; Calixto, J.B.; Santos, A.R.; Rodrigues, A.L. Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav. Brain Res. 2003, 144, 87–93. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Assini, F.; Franco, J.L.; Pandolfo, P.; Muller, Y.M.; Takahashi, R.N.; Dafre, A.L.; Rodrigues, A.L. Zinc attenuates malathion-induced depressant-like behavior and confers neuroprotection in the rat brain. Toxicol. Sci. 2007, 97, 140–148. [Google Scholar] [CrossRef]
- Dorri, S.A.; Hosseinzadeh, H.; Abnous, K.; Hasani, F.V.; Robati, R.Y.; Razavi, B.M. Involvement of brain-derived neurotrophic factor (BDNF) on malathion induced depressive-like behavior in subacute exposure and protective effects of crocin. Iran. J. Basic Med. Sci. 2015, 18, 958–966. [Google Scholar]
- Bhalla, P.; Chadha, V.D.; Dhar, R.; Dhawan, D.K. Neuroprotective effects of zinc on antioxidant defense system in lithium treated rat brain. Indian J. Exp. Biol. 2007, 45, 954–958. [Google Scholar]
- Swardfager, W.; Herrmann, N.; McIntyre, R.S.; Mazereeuw, G.; Goldberger, K.; Cha, D.S.; Schwartz, Y.; Lanctot, K.L. Potential roles of zinc in the pathophysiology and treatment of major depressive disorder. Neurosci. Biobehav. Rev. 2013, 37, 911–929. [Google Scholar] [CrossRef]
- Kelly, J.P.; Wrynn, A.S.; Leonard, B.E. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Cousins, R.J.; Leinart, A.S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. Faseb J. 1988, 2, 2884–2890. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.J.; Kim, D.H.; Jeong, T.C.; Lee, E.S.; Choi, Y.M.; Jeong, H.G. Cytokine-mediated induction of metallothionein in Hepa-1c1c7 cells by oleanolic acid. Biochem. Biophys. Res. Commun. 2004, 325, 792–797. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef]
- Van Hunsel, F.; Wauters, A.; Vandoolaeghe, E.; Neels, H.; Demedts, P.; Maes, M. Lower total serum protein, albumin, and beta- and gamma-globulin in major and treatment-resistant depression: Effects of antidepressant treatments. Psychiatry Res. 1996, 65, 159–169. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Demedts, P.; Wauters, A.; Neels, H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J. Affect. Disord. 1999, 56, 189–194. [Google Scholar] [CrossRef]
- Prasad, A.S.; Meftah, S.; Abdallah, J.; Kaplan, J.; Brewer, G.J.; Bach, J.F.; Dardenne, M. Serum thymulin in human zinc deficiency. J. Clin. Investig. 1988, 82, 1202–1210. [Google Scholar] [CrossRef]
- Maes, M.; Vandoolaeghe, E.; Neels, H.; Demedts, P.; Wauters, A.; Meltzer, H.Y.; Altamura, C.; Desnyder, R. Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol. Psychiatry. 1997, 42, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.; Grabowski, S.M.; Kaplan, J.; Mathog, R.H. Zinc deficiency: Changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc. Assoc. Am. Phys. 1997, 109, 68–77. [Google Scholar] [PubMed]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar] [CrossRef]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Murakami, M.; et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef]
- Robertson, M.J.; Schacterle, R.S.; Mackin, G.A.; Wilson, S.N.; Bloomingdale, K.L.; Ritz, J.; Komaroff, A.L. Lymphocyte subset differences in patients with chronic fatigue syndrome, multiple sclerosis and major depression. Clin. Exp. Immunol. 2005, 141, 326–332. [Google Scholar] [CrossRef]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Molho, E.S.; Celmins, D.F.; Heckman, S.M.; Dick, R. Subclinical zinc deficiency in Alzheimer’s disease and Parkinson’s disease. Am. J. Alzheimers Dis. Other Dement. 2010, 25, 572–575. [Google Scholar] [CrossRef]
- Zhao, H.W.; Lin, J.; Wang, X.B.; Cheng, X.; Wang, J.Y.; Hu, B.L.; Zhang, Y.; Zhang, X.; Zhu, J.H. Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PLoS ONE 2013, 8, e83060. [Google Scholar] [CrossRef]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Sepe, M.M.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef]
- Hegde, M.L.; Shanmugavelu, P.; Vengamma, B.; Rao, T.S.; Menon, R.B.; Rao, R.V.; Rao, K.S. Serum trace element levels and the complexity of inter-element relations in patients with Parkinson’s disease. J. Trace Elem. Med. Biol. 2004, 18, 163–171. [Google Scholar] [CrossRef]
- Forte, G.; Bocca, B.; Senofonte, O.; Petrucci, F.; Brusa, L.; Stanzione, P.; Zannino, S.; Violante, N.; Alimonti, A.; Sancesario, G. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson’s disease. J. Neural Transm. 2004, 111, 1031–1040. [Google Scholar] [CrossRef]
- Quiroga, M.J.; Carroll, D.W.; Brown, T.M. Ascorbate- and zinc-responsive parkinsonism. Ann. Pharmacother. 2014, 48, 1515–1520. [Google Scholar] [CrossRef]
- Sikora, J.; Ouagazzal, A.M. Synaptic Zinc: An Emerging Player in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4724. [Google Scholar] [CrossRef]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromol. Med. 2014, 16, 217–230. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Good, P.F.; Hsu, A.; Werner, P.; Perl, D.P.; Olanow, C.W. Protein nitration in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1998, 57, 338–342. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Ninsiima, H.I.; Valladares, M.B.; Pieme, C.A. Zinc and linoleic acid pre-treatment attenuates biochemical and histological changes in the midbrain of rats with rotenone-induced Parkinsonism. BMC Neurosci. 2018, 19, 29. [Google Scholar] [CrossRef]
- Park, J.S.; Koentjoro, B.; Veivers, D.; Mackay-Sim, A.; Sue, C.M. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 2014, 23, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Son, H.J.; Choi, J.H.; Cho, E.; Kim, J.; Chung, S.J.; Hwang, O.; Koh, J.Y. Cytosolic labile zinc accumulation in degenerating dopaminergic neurons of mouse brain after MPTP treatment. Brain Res. 2009, 1286, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Tarohda, T.; Ishida, Y.; Kawai, K.; Yamamoto, M.; Amano, R. Regional distributions of manganese, iron, copper, and zinc in the brains of 6-hydroxydopamine-induced parkinsonian rats. Anal. Bioanal. Chem. 2005, 383, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Zhu, J.; Zhang, W.; Shi, C.; Cai, A.L. Mitochondrial inhibitor models of Huntington’s disease and Parkinson’s disease induce zinc accumulation and are attenuated by inhibition of zinc neurotoxicity in vitro or in vivo. Neurodegener. Dis. 2013, 11, 49–58. [Google Scholar] [CrossRef]
- Xu, J.; Kao, S.Y.; Lee, F.J.; Song, W.; Jin, L.W.; Yankner, B.A. Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002, 8, 600–606. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Phansuwan-Pujito, P.; Ebadi, M.; Govitrapong, P. Zinc protects SK-N-SH cells from methamphetamine-induced alpha-synuclein expression. Neurosci. Lett. 2007, 419, 59–63. [Google Scholar] [CrossRef]
- Tsunemi, T.; Krainc, D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet. 2014, 23, 2791–2801. [Google Scholar] [CrossRef]
- Choi, B.Y.; Jung, J.W.; Suh, S.W. The Emerging Role of Zinc in the Pathogenesis of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 2070. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Simao, A.; Oliveira, S.R.; Flauzino, T.; Alfieri, D.F.; de Carvalho, J.P.W.; Kallaur, A.P.; Lozovoy, M.; Kaimen-Maciel, D.R.; Maes, M.; et al. Antioxidant and Anti-inflammatory Diagnostic Biomarkers in Multiple Sclerosis: A Machine Learning Study. Mol. Neurobiol. 2020, 57, 2167–2178. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Uebelhor, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef]
- Matar, A.; Jennani, S.; Abdallah, H.; Mohsen, N.; Borjac, J. Serum Iron and Zinc Levels in Lebanese Multiple Sclerosis Patients. Acta Neurol. Taiwan 2020, 29, 5–11. [Google Scholar]
- Castro, A.; Albuquerque, L.; Melo, M.; D’Almeida, J.; Braga, R.; Assis, R.C.; Marreiro, D.; Matos, W.O.; Maia, C. Relationship between zinc-related nutritional status and the progression of multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 66, 104063. [Google Scholar] [CrossRef]
- Ho, S.Y.; Catalanotto, F.A.; Lisak, R.P.; Dore-Duffy, P. Zinc in multiple sclerosis. II: Correlation with disease activity and elevated plasma membrane-bound zinc in erythrocytes from patients with multiple sclerosis. Ann. Neurol. 1986, 20, 712–715. [Google Scholar] [CrossRef]
- Choi, B.Y.; Jang, B.G.; Kim, J.H.; Seo, J.N.; Wu, G.; Sohn, M.; Chung, T.N.; Suh, S.W. Copper/zinc chelation by clioquinol reduces spinal cord white matter damage and behavioral deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 2013, 54, 382–391. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, I.Y.; Kim, J.H.; Kho, A.R.; Lee, S.H.; Lee, B.E.; Sohn, M.; Koh, J.Y.; Suh, S.W. Zinc transporter 3 (ZnT3) gene deletion reduces spinal cord white matter damage and motor deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 2016, 94, 205–212. [Google Scholar] [CrossRef]
- Choi, B.Y.; Jeong, J.H.; Eom, J.W.; Koh, J.Y.; Kim, Y.H.; Suh, S.W. A Novel Zinc Chelator, 1H10, Ameliorates Experimental Autoimmune Encephalomyelitis by Modulating Zinc Toxicity and AMPK Activation. Int. J. Mol. Sci. 2020, 21, 3375. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kho, A.R.; Kim, I.Y.; Lee, S.H.; Lee, B.E.; Choi, E.; Sohn, M.; Stevenson, M.; Chung, T.N.; et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J. Neuroinflamm. 2015, 12, 104. [Google Scholar] [CrossRef]
- Domercq, M.; Mato, S.; Soria, F.N.; Sanchez-gomez, M.V.; Alberdi, E.; Matute, C. Zn2+ -induced ERK activation mediates PARP-1-dependent ischemic-reoxygenation damage to oligodendrocytes. Glia 2013, 61, 383–393. [Google Scholar] [CrossRef]
- Krone, A.; Fu, Y.; Schreiber, S.; Kotrba, J.; Borde, L.; Notzold, A.; Thurm, C.; Negele, J.; Franz, T.; Stegemann-Koniszewski, S.; et al. Ionic mitigation of CD4(+) T cell metabolic fitness, Th1 central nervous system autoimmunity and Th2 asthmatic airway inflammation by therapeutic zinc. Sci. Rep. 2022, 12, 1943. [Google Scholar] [CrossRef] [PubMed]
- Elitt, C.M.; Fahrni, C.J.; Rosenberg, P.A. Zinc homeostasis and zinc signaling in white matter development and injury. Neurosci. Lett. 2019, 707, 134247. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Puls, I.; Muller, D.J.; Strutz-Seebohm, N.; Gallinat, J. Molecular mechanisms of schizophrenia. Cell Physiol. Biochem. 2007, 20, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Mousaviyan, R.; Davoodian, N.; Alizadeh, F.; Ghasemi-Kasman, M.; Mousavi, S.A.; Shaerzadeh, F.; Kazemi, H. Zinc Supplementation During Pregnancy Alleviates Lipopolysaccharide-Induced Glial Activation and Inflammatory Markers Expression in a Rat Model of Maternal Immune Activation. Biol. Trace Elem. Res. 2021, 199, 4193–4204. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhang, T.; Yao, Y.; Shen, C.; Xue, Y. Association of Serum Trace Elements with Schizophrenia and Effects of Antipsychotic Treatment. Biol. Trace Elem. Res. 2018, 181, 22–30. [Google Scholar] [CrossRef]
- Santa, C.E.; Madrid, K.C.; Arruda, M.; Sussulini, A. Association between trace elements in serum from bipolar disorder and schizophrenia patients considering treatment effects. J. Trace Elem. Med. Biol. 2020, 59, 126467. [Google Scholar] [CrossRef]
- Cai, L.; Chen, T.; Yang, J.; Zhou, K.; Yan, X.; Chen, W.; Sun, L.; Li, L.; Qin, S.; Wang, P.; et al. Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Sci. Rep. 2015, 5, 15013. [Google Scholar] [CrossRef]
- Carrera, N.; Arrojo, M.; Sanjuan, J.; Ramos-Rios, R.; Paz, E.; Suarez-Rama, J.J.; Paramo, M.; Agra, S.; Brenlla, J.; Martinez, S.; et al. Association study of nonsynonymous single nucleotide polymorphisms in schizophrenia. Biol. Psychiatry 2012, 71, 169–177. [Google Scholar] [CrossRef]
- Li, S.; Ma, C.; Li, Y.; Chen, R.; Liu, Y.; Wan, L.P.; Xiong, Q.; Wang, C.; Huo, Y.; Dang, X.; et al. The schizophrenia-associated missense variant rs13107325 regulates dendritic spine density. Transl. Psychiatr. 2022, 12, 361. [Google Scholar] [CrossRef]
- Tseng, W.C.; Reinhart, V.; Lanz, T.A.; Weber, M.L.; Pang, J.; Le, K.X.V.; Bell, R.D.; O’Donnell, P.; Buhl, D.L. Schizophrenia-associated SLC39A8 polymorphism is a loss-of-function allele altering glutamate receptor and innate immune signaling. Transl. Psychiatr. 2021, 11, 136. [Google Scholar] [CrossRef]
- Scarr, E.; Udawela, M.; Greenough, M.A.; Neo, J.; Suk, S.M.; Money, T.T.; Upadhyay, A.; Bush, A.I.; Everall, I.P.; Thomas, E.A.; et al. Increased cortical expression of the zinc transporter SLC39A12 suggests a breakdown in zinc cellular homeostasis as part of the pathophysiology of schizophrenia. Npj Schizophr. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Perez-Becerril, C.; Morris, A.G.; Mortimer, A.; McKenna, P.J.; de Belleroche, J. Allelic variants in the zinc transporter-3 gene, SLC30A3, a candidate gene identified from gene expression studies, show gender-specific association with schizophrenia. Eur. Psychiat. 2014, 29, 172–178. [Google Scholar] [CrossRef]
- Marger, L.; Schubert, C.R.; Bertrand, D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014, 91, 426–435. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, D.; Liang, J.; Bao, Y.P.; Meng, S.Q.; Lu, L.; Shi, J. Association between variants of zinc finger genes and psychiatric disorders: Systematic review and meta-analysis. Schizophr. Res. 2015, 162, 124–137. [Google Scholar] [CrossRef]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Dalic, L.; Cook, M.J. Managing drug-resistant epilepsy: Challenges and solutions. Neuropsychiatr. Dis. Treat. 2016, 12, 2605–2616. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshé, S.L.; Perucca, E.; Engel, J.J.; Löscher, W.; Noebels, J.L.; Pitkänen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef]
- Staley, K. Molecular mechanisms of epilepsy. Nat. Neurosci. 2015, 18, 367–372. [Google Scholar] [CrossRef]
- Saad, K.; Hammad, E.; Hassan, A.F.; Badry, R. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int. J. Neurosci. 2014, 124, 181–186. [Google Scholar] [CrossRef]
- Jia, W.; Song, Y.; Yang, L.; Kong, J.; Boczek, T.; He, Z.; Wang, Y.; Zhang, X.; Hu, H.; Shao, D.; et al. The changes of serum zinc, copper, and selenium levels in epileptic patients: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2020, 13, 1047–1058. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nature reviews. Neurology 2011, 7, 31–40. [Google Scholar] [PubMed]
- Vezzani, A.; Baram, T.Z. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007, 7, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Lee, S.; Kim, T.; Cho, J.; Koh, J. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem. Soc. Trans. 2007, 35, 421–423. [Google Scholar] [CrossRef]
- Qian, J.; Xu, K.; Yoo, J.; Chen, T.T.; Andrews, G.; Noebels, J.L. Knockout of Zn2+ transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 97–104. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Morris, D.R.; Levenson, C.W. Zinc in traumatic brain injury: From neuroprotection to neurotoxicity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 708–711. [Google Scholar] [CrossRef]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. BioFactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Hellmich, H.L.; Frederickson, C.J.; DeWitt, D.S.; Saban, R.; Parsley, M.O.; Stephenson, R.; Velasco, M.; Uchida, T.; Shimamura, M.; Prough, D.S. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci. Lett. 2004, 355, 221–225. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Ma, S.; Zhang, Y.; Liang, P. TPEN Attenuates Neural Autophagy Induced by Synaptically-released Zinc Translocation and Improves Histological Outcomes after Traumatic Brain Injury in Rats. Ann. Clin. Lab. Sci. 2018, 48, 446–452. [Google Scholar]
- Levenson, C.W. Zinc and Traumatic Brain Injury: From Chelation to Supplementation. Med. Sci. 2020, 8, 36. [Google Scholar] [CrossRef]
- Yeiser, E.C.; Vanlandingham, J.W.; Levenson, C.W. Moderate zinc deficiency increases cell death after brain injury in the rat. Nutr. Neurosci. 2002, 5, 345–352. [Google Scholar] [CrossRef]
- Kim, K.H.; Ro, Y.S.; Yoon, H.; Lee, S.G.W.; Jung, E.; Moon, S.B.; Park, G.J.; Shin, S.D. Serum Zinc and Long-Term Prognosis after Acute Traumatic Brain Injury with Intracranial Injury: A Multicenter Prospective Study. J. Clin. Med. 2022, 11, 6496. [Google Scholar] [CrossRef]
- Hellmich, H.L.; Eidson, K.; Cowart, J.; Crookshanks, J.; Boone, D.K.; Shah, S.; Uchida, T.; DeWitt, D.S.; Prough, D.S. Chelation of neurotoxic zinc levels does not improve neurobehavioral outcome after traumatic brain injury. Neurosci. Lett. 2008, 440, 155–159. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kim, H.J.; Lee, B.E.; Kim, I.Y.; Sohn, M.; Suh, S.W. Zinc chelation reduces traumatic brain injury-induced neurogenesis in the subgranular zone of the hippocampal dentate gyrus. J. Trace Elem. Med. Biol. 2014, 28, 474–481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).