Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clinical Studies
2.3. Analysis of Urine Coffee Metabolites
2.4. Analysis of Blood Components, Body Composition and Fatty Liver Index
2.5. Alcohol Intake Measurement
2.6. Data Analysis and Presentation
3. Results
3.1. Study Cohort Characteristics
3.2. Urinary Coffee Metabolite Profile
3.3. Coffee Metabolites and NAFLD Severity
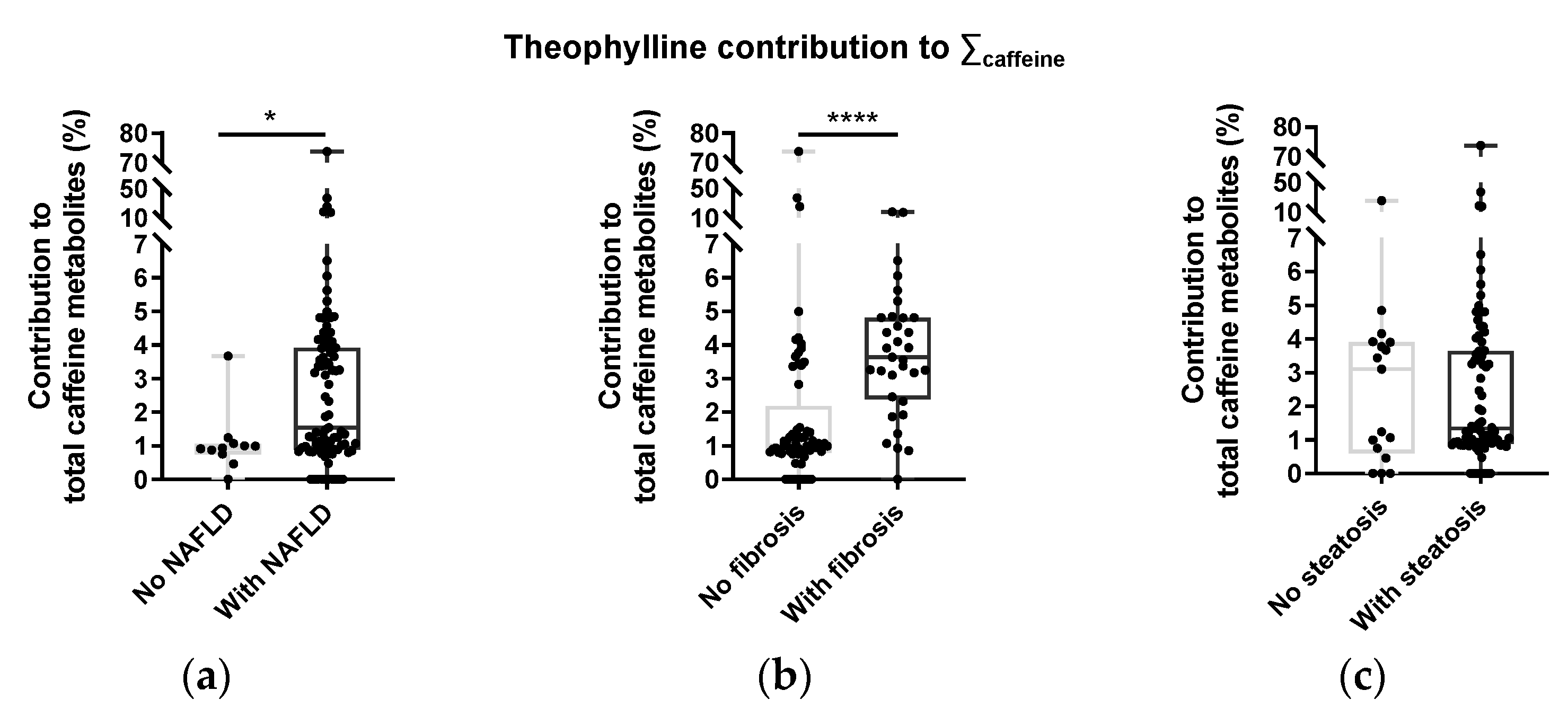
3.4. Caffeine Metabolite Profiles and NAFLD Status
4. Discussion
4.1. Characterizing Coffee Consumption by 24 h Urine Analysis versus Self-Reporting
4.2. Coffee Intake and NAFLD Parameters
4.3. Mechanisms by Which Coffee Metabolites May Protect against NAFLD
4.4. Caffeine Metabolite Profiles and CYP2E1
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Analyte | Q1 | Q3 | CE | CXP | DP |
|---|---|---|---|---|---|
| Caffeine | 195.2 | 138.0 | 27 | 8 | 71 |
| Paraxanthine | 181.1 | 124.1 | 27 | 10 | 90 |
| Theobromine | 181.1 | 138.1 | 25 | 10 | 66 |
| Theophylline | 181.1 | 124.1 | 27 | 10 | 90 |
| 13C3-Caffeine | 198.2 | 140.2 | 27 | 10 | 61 |
| Theobromine-d6 | 187.3 | 144.2 | 25 | 10 | 91 |
| Trigonelline | 138.1 | 94.3 | 29 | 6 | 86 |
| p-Coumaric acid | 165.2 | 147.3 | 15 | 12 | 61 |
| trans-Caffeic acid | 181.2 | 135.2 | 27 | 8 | 56 |
| 13C3-Catechin | 294.3 | 139.8 | 21 | 24 | 51 |
References
- Hodge, A.; Lim, S.; Goh, E.; Wong, O.; Marsh, P.; Knight, V.; Sievert, W.; de Courten, B. Coffee intake is associated with a lower liver stiffness in patients with non-alcoholic fatty liver disease, hepatitis C, and hepatitis B. Nutrients 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012, 55, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, X.F.; Yang, W.Z.; Wang, H.; Zhao, H.; Yang, F.; Yang, Y.; Li, J.; Lv, X.W. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int. Immunopharmacol. 2015, 25, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; White, D.; Kanwal, F.; Ramsey, D.; Mittal, S.; Tavakoli-Tabasi, S.; Kuzniarek, J.; El-Serag, H.B. Coffee and caffeine are associated with decreased risk of advanced hepatic fibrosis among patients with hepatitis C. Clin. Gastroenterol. Hepatol. 2015, 13, 1521–1531. [Google Scholar] [CrossRef]
- Shen, H.F.; Rodriguez, A.C.; Shiani, A.; Lipka, S.; Shahzad, G.; Kumar, A.; Mustacchia, P. Association between caffeine consumption and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Ther. Adv. Gastroenterol. 2016, 9, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.L.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.F.; Schwarzschild, M.A.; et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef]
- Wang, H.; Guan, W.J.; Yang, W.Z.; Wang, Q.; Zhao, H.; Yang, F.; Lv, X.W.; Li, J. Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS ONE 2014, 9, e92482. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Csoke, B.; Pacher, P.; Hasko, G. Adenosine signalling in diabetes mellitus-pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2015, 11, 228–241. [Google Scholar] [CrossRef]
- Csoka, B.; Toro, G.; Vindeirinho, J.; Varga, Z.V.; Koscso, B.; Nemeth, Z.H.; Kokai, E.; Antonioli, L.; Suleiman, M.; Marchetti, P.; et al. A(2A) adenosine receptors control pancreatic dysfunction in high-fat-diet-induced obesity. Faseb J. 2017, 31, 4985–4997. [Google Scholar] [CrossRef]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef]
- Brandt, A.; Nier, A.; Jin, C.J.; Baumann, A.; Jung, F.; Ribas, V.; Garcia-Ruiz, C.; Fernandez-Checa, J.C.; Bergheim, I. Consumption of decaffeinated coffee protects against the development of early non-alcoholic steatohepatitis: Role of intestinal barrier function. Redox Biol. 2019, 21, 101092. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Lembo, V.; D’Argenio, G.; Vitaglione, P.; Rossi, A.; Guarino, M.; Caporaso, N.; Morisco, F. Decaffeinated coffee consumption induces expression of tight junction proteins in high fat diet fed rats. Funct. Foods Health Dis. 2016, 6, 602–611. [Google Scholar]
- Shi, A.M.; Li, T.; Zheng, Y.; Song, Y.H.; Wang, H.T.; Wang, N.; Dong, L.; Shi, H.T. Chlorogenic acid improves NAFLD by regulating gut microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018, 121, 283–296. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Petrovic, D.; Younes, S.E.; Pruijm, M.; Ponte, B.; Ackermann, D.; Ehret, G.; Ansermot, N.; Mohaupt, M.; Paccaud, F.; Vogt, B.; et al. Relation of 24-hour urinary caffeine and caffeine metabolite excretions with self-reported consumption of coffee and other caffeinated beverages in the general population. Nutr. Metab. 2016, 13, 81. [Google Scholar] [CrossRef]
- Vanderlee, L.; Reid, J.L.; White, C.M.; Acton, R.B.; Kirkpatrick, S.I.; Pao, C.L.; Rybak, M.E.; Hammond, D. Evaluation of a 24-hour caffeine intake assessment compared with urinary biomarkers of caffeine intake among young adults in Canada. J. Acad. Nutr. Diet. 2018, 118, 2245–2253.E1. [Google Scholar] [CrossRef]
- Martinez-Lopez, S.; Sarria, B.; Baeza, G.; Mateos, R.; Bravo-Clemente, L. Pharmacokinetics of caffeine and its metabolites in plasma and urine after consuming a soluble green/roasted coffee blend by healthy subjects. Food Res. Int. 2014, 64, 125–133. [Google Scholar] [CrossRef]
- Modi, A.A.; Feld, J.J.; Park, Y.; Kleiner, D.E.; Everhart, J.E.; Liang, T.J.; Hoofnagle, J.H. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 2010, 51, 201–209. [Google Scholar] [CrossRef]
- Mendes, V.M.; Coelho, M.; Tome, A.R.; Cunha, R.A.; Manadas, B. Validation of an LC-MS/MS method for the quantification of caffeine and theobromine using non-matched matrix calibration curve. Molecules 2019, 24, 2863. [Google Scholar] [CrossRef]
- Travassos, M.; Santana, I.; Baldeiras, I.; Tsolaki, M.; Gkatzima, O.; Genc, S.; Yener, G.G.; Simonsen, A.; Hasselbalch, S.G.; Kapaki, E.; et al. Does caffeine consumption modify cerebrospinal fluid amyloid-beta levels in patients with alzheimer’s disease? J. Alzheimers Dis. 2015, 47, 1069–1078. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. Bmc Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Marandola, L.; Romaggioli, S.; Bascetta, S.; Chiesa, C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 17107–17114. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Zheng, Y.; Yue, F.; Russell, R.D.; Zeng, Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res. Clin. Pract. 2014, 106, 312–318. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Kim, J.H.; Heo, J.S.; Baek, K.S.; Kim, S.Y.; Baek, K.H.; Kim, K.E.; Sheen, Y.H. Zonulin level, a marker of intestinal permeability, is increased in association with liver enzymes in young adolescents. Clin. Chim. Acta 2018, 481, 218–224. [Google Scholar] [CrossRef]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef]
- Rybak, M.E.; Pao, C.I.; Pfeiffer, C.M. Determination of urine caffeine and its metabolites by use of high-performance liquid chromatography-tandem mass spectrometry: Estimating dietary caffeine exposure and metabolic phenotyping in population studies. Anal. Bioanal. Chem. 2014, 406, 771–784. [Google Scholar] [CrossRef]
- Lang, R.; Yagar, E.F.; Eggers, R.; Hofmann, T. Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J. Agric. Food Chem. 2008, 56, 11114–11121. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, J.; Niederstaetter, L.; Neuditschko, B.; Bileck, A.; Slany, A.; Janker, L.; Feuerstein, M.L.; Langbauer, C.; Gotsmy, M.; Zanghellini, J.; et al. Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun. 2021, 12, 5993. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Fillatre, Y.; Martin, J.F.; Lyan, B.; Pujos-Guillot, E.; Fezeu, L.; Hercberg, S.; Comte, B.; Galan, P.; Touvier, M.; et al. New biomarkers of coffee consumption identified by the non-targeted metabolomic profiling of cohort study subjects. PLoS ONE 2014, 9, e93474. [Google Scholar] [CrossRef]
- Anty, R.; Marjoux, S.; Iannelli, A.; Patouraux, S.; Schneck, A.S.; Bonnafous, S.; Gire, C.; Amzolini, A.; Ben-Amor, I.; Saint-Paul, M.C.; et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J. Hepatol. 2012, 57, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puscion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of various factors on caffeine content in coffee brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Wetzel, C.R.; Grand, A.N. Caffeine content in coffee as influenced by grinding and brewing techniques. Food Res. Int. 1996, 29, 785–789. [Google Scholar] [CrossRef]
- Bambha, K.; Wilson, L.A.; Unalp, A.; Loomba, R.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Bass, N.M.; Nonalcoholic, S. Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014, 34, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Notarnicola, M.; Cisternino, A.M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Rotolo, O.; Zinzi, I.; Leandro, G.; Correale, M.; et al. Coffee intake and liver steatosis: A population study in a mediterranean area. Nutrients 2018, 10, 89. [Google Scholar] [CrossRef]
- Kositamongkol, C.; Kanchanasurakit, S.; Auttamalang, C.; Inchai, N.; Kabkaew, T.; Kitpark, S.; Chaiyakunapruk, N.; Duangjai, A.; Saokaew, S.; Phisalprapa, P. Coffee consumption and non-alcoholic fatty liver disease: An umbrella review and a systematic review and meta-analysis. Front. Pharmacol. 2021, 12, 786596. [Google Scholar] [CrossRef]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of coffee consumption on non-alcoholic fatty liver disease incidence, prevalence and risk of significant liver fibrosis: Systematic review with meta-analysis of observational studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lu, F.B.; Hu, Y.B.; Xu, L.M.; Zheng, M.H.; Hu, E.D. A systematic review and a dose-response meta-analysis of coffee dose and nonalcoholic fatty liver disease. Clin. Nutr. 2019, 38, 2552–2557. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Coffee consumption and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, E8–E12. [Google Scholar] [CrossRef]
- Xiao, Q.; Sinha, R.; Graubard, B.I.; Freedman, N.D. Inverse associations of total and decaffeinated coffee with liver enzyme levels in national health and nutrition examination survey 1999–2010. Hepatology 2014, 60, 2091–2098. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Morton, C.; Udaltsova, N.; Friedman, G.D. Coffee, cirrhosis, and transaminase enzymes. Arch. Intern. Med. 2006, 166, 1190–1195. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Gastaldelli, A. Nonalcoholic fatty liver disease and type 2 diabetes: Common pathophysiologic mechanisms. Curr. Diabetes Rep. 2015, 15, 34. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Qadri, S.; Ahlholm, N.; Porthan, K.; Mannisto, V.; Sammalkorpi, H.; Penttila, A.K.; Hakkarainen, A.; Lehtimaki, T.E.; Gaggini, M.; et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 526–535. [Google Scholar] [CrossRef]
- Trico, D.; Caprio, S.; Umano, G.R.; Pierpont, B.; Nouws, J.; Galderisi, A.; Kim, G.; Mata, M.M.; Santoro, N. Metabolic features of nonalcoholic fatty liver (NAFL) in obese adolescents: Findings from a multiethnic cohort. Hepatology 2018, 68, 1376–1390. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Baeckhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.J.; Lee, F.Y.; Wang, S.S.; Hsin, I.F.; Lin, T.Y.; Huang, H.C.; Chang, C.C.; Chuang, C.L.; Ho, H.L.; Lin, H.C.; et al. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 2015, 61, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Ko, H.J. Plasma concentrations of zonulin are elevated in obese men with fatty liver disease. Diabetes Metab. Syndr. Obes.-Targets Ther. 2018, 11, 149–157. [Google Scholar] [CrossRef]
- Hendy, O.M.; Elsabaawy, M.M.; Aref, M.M.; Khalaf, F.M.; Oda, A.M.A.; El Shazly, H.M. Evaluation of circulating zonulin as a potential marker in the pathogenesis of nonalcoholic fatty liver disease. Apmis 2017, 125, 607–613. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 2013, 368, 20120431. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Hartman, J.H.; Fromenty, B. Role of mitochondrial cytochrome P450 2E1 in healthy and diseased liver. Cells 2022, 11, 288. [Google Scholar] [CrossRef]
- Lieber, C.S.; Leo, M.A.; Mak, K.M.; Xu, Y.Q.; Cao, Q.; Ren, C.L.; Ponomarenko, A.; DeCarli, L.M. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004, 79, 502–509. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.H.; Jang, S.; Gonzalez, F.J.; Song, B.J. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef]
- Ma, H.L.; Chen, S.D.; Zheng, K.I.; Yu, Y.; Wang, X.X.; Tang, L.J.; Li, G.; Rios, R.S.; Huang, O.Y.; Zheng, X.Y.; et al. TA allele of rs2070673 in the CYP2E1 gene is associated with lobular inflammation and nonalcoholic steatohepatitis in patients with biopsy-proven nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.M.; Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 395–398. [Google Scholar] [CrossRef] [PubMed]
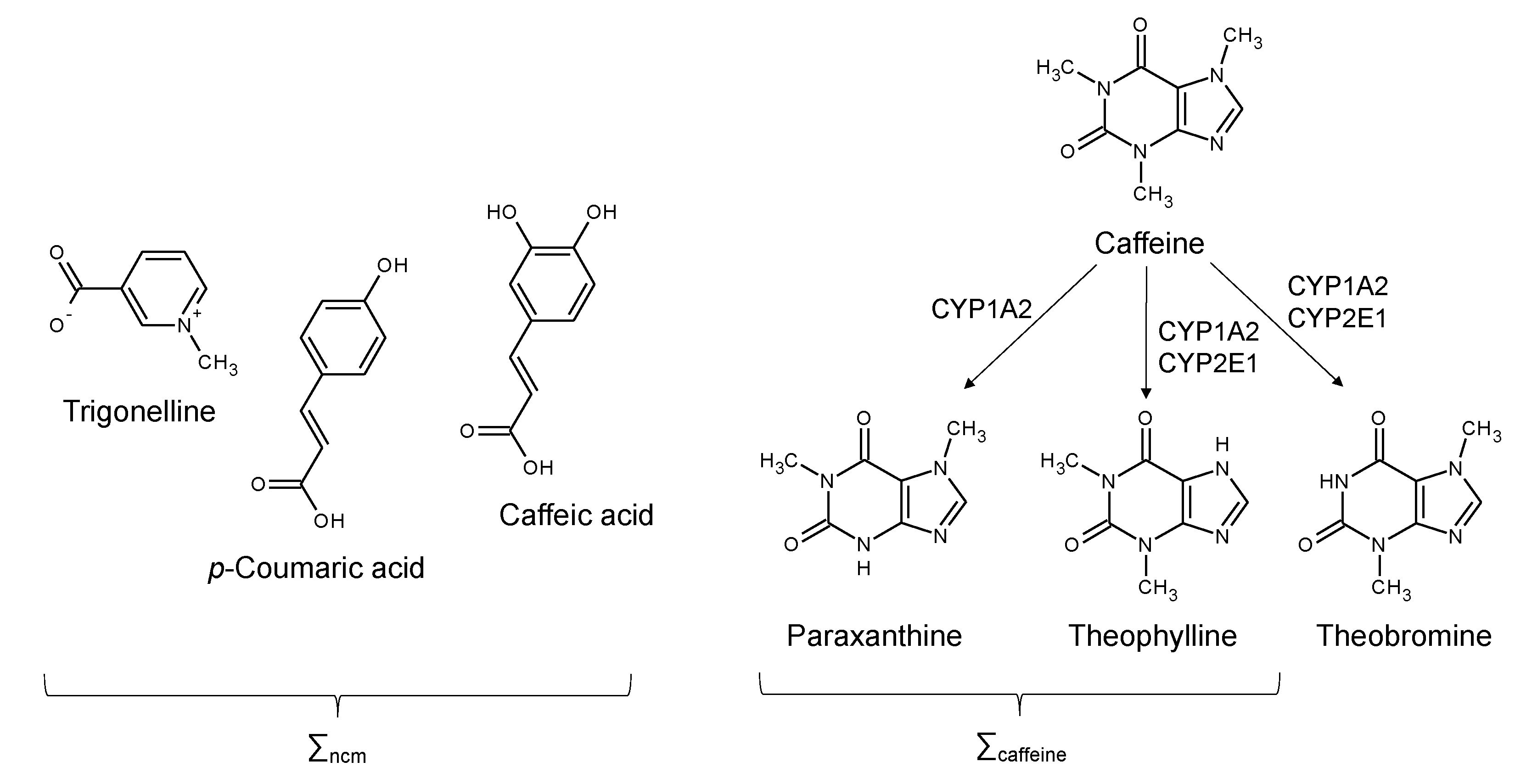


| Parameters | Values | |
|---|---|---|
| Total (n = 156) | With Urine Data (n = 98) | |
| Age (yr) | 59 ± 9 | 60 ± 6 |
| Sex distribution (F/M) | 76/80 | 52/46 |
| Type 2 Diabetes incidence & duration (yr) | 135/156 (87%), 11 ± 7 | 98/98 (100%), 11 ± 6 |
| Body mass index (BMI, kg·m−2) | 29 ± 5 | 30 ± 4 |
| Aspartate aminotransferase (AST, U·L−1) | 23 ± 6 | 22 ± 4 |
| Alanine aminotransferase (ALT, U·L−1) | 27 ± 12 | 26 ± 7 |
| γ-glutamyltransferase (GGT, U·L−1) | 31 ± 21 | 31 ± 14 |
| Fibrosis (transient elastography, kPa) | 5.7 ± 2.9 | 6.1 ± 2.1 |
| Steatosis (controlled attenuation parameter, dB·m−1) | 283 ± 56 | 290 ± 44 |
| Fatty liver index (FLI) score | 60 ± 26 | 63 ± 20 |
| Total Cholesterol (mg·dL−1) | 180 ± 39 | 178 ± 32 |
| HDL-Cholesterol (mg·dL−1) | 51 ± 11 | 49 ± 9 |
| LDL-Cholesterol (mg·dL−1) | 121 ± 32 | 120 ± 27 |
| Triglyceride (mg·dL−1) | 141 ± 66 | 149 ± 53 |
| Glucose (mg·dL−1) | 145 ± 41 | 153 ± 31 |
| Insulin (µU·mL−1) | 11 ± 10 | 11 ± 7 |
| Zonulin (ng·mL−1) | 46 ± 10 | 48 ± 7 |
| Alcohol intake (alcohol units per week) | 8 ± 14 | 10 ± 16 |
| Liver Parameter | Correlation with Σcaffeine | Correlation with Σncm |
|---|---|---|
| FLI | −0.1783 (p = 0.0789) | −0.2266 * (p = 0.0249) |
| Fibrosis (kPa) | −0.1090 (p = 0.2852) | −0.0646 (p = 0.5271) |
| Steatosis (CAP, db·m−1) | −0.0734 (p = 0.4728) | −0.0860 (p = 0.3996) |
| Zonulin (ng·mL−1) | −0.0804 (p = 0.4510) | 0.06918 (p = 0.5171) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.; Patarrão, R.S.; Sousa-Lima, I.; Ribeiro, R.T.; Meneses, M.J.; Andrade, R.; Mendes, V.M.; Manadas, B.; Raposo, J.F.; Macedo, M.P.; et al. Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes. Nutrients 2023, 15, 4. https://doi.org/10.3390/nu15010004
Coelho M, Patarrão RS, Sousa-Lima I, Ribeiro RT, Meneses MJ, Andrade R, Mendes VM, Manadas B, Raposo JF, Macedo MP, et al. Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes. Nutrients. 2023; 15(1):4. https://doi.org/10.3390/nu15010004
Chicago/Turabian StyleCoelho, Margarida, Rita S. Patarrão, Inês Sousa-Lima, Rogério T. Ribeiro, Maria João Meneses, Rita Andrade, Vera M. Mendes, Bruno Manadas, João Filipe Raposo, M. Paula Macedo, and et al. 2023. "Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes" Nutrients 15, no. 1: 4. https://doi.org/10.3390/nu15010004
APA StyleCoelho, M., Patarrão, R. S., Sousa-Lima, I., Ribeiro, R. T., Meneses, M. J., Andrade, R., Mendes, V. M., Manadas, B., Raposo, J. F., Macedo, M. P., & Jones, J. G. (2023). Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes. Nutrients, 15(1), 4. https://doi.org/10.3390/nu15010004










