The Association of Perceived Vulnerability to Disease with Cognitive Restraint and Compensatory Behaviors
Abstract
1. Introduction
1.1. Obesity as a Disease Cue
1.2. Fear of Fat and Disordered Eating
1.3. The Current Study
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Perceived Vulnerability to Disease Scale (PVD)
2.3.2. Goldfarb Fear of Fat Scale (FOF)
2.3.3. Dutch Eating Behaviors Scale (DEBQ)
2.3.4. Eating Disorder Diagnostic Scale (EDDS)
2.4. Data Analytic Plan
3. Results
3.1. Associations between PVD Subscales, Cognitive Restraint and Compensatory Behaviors
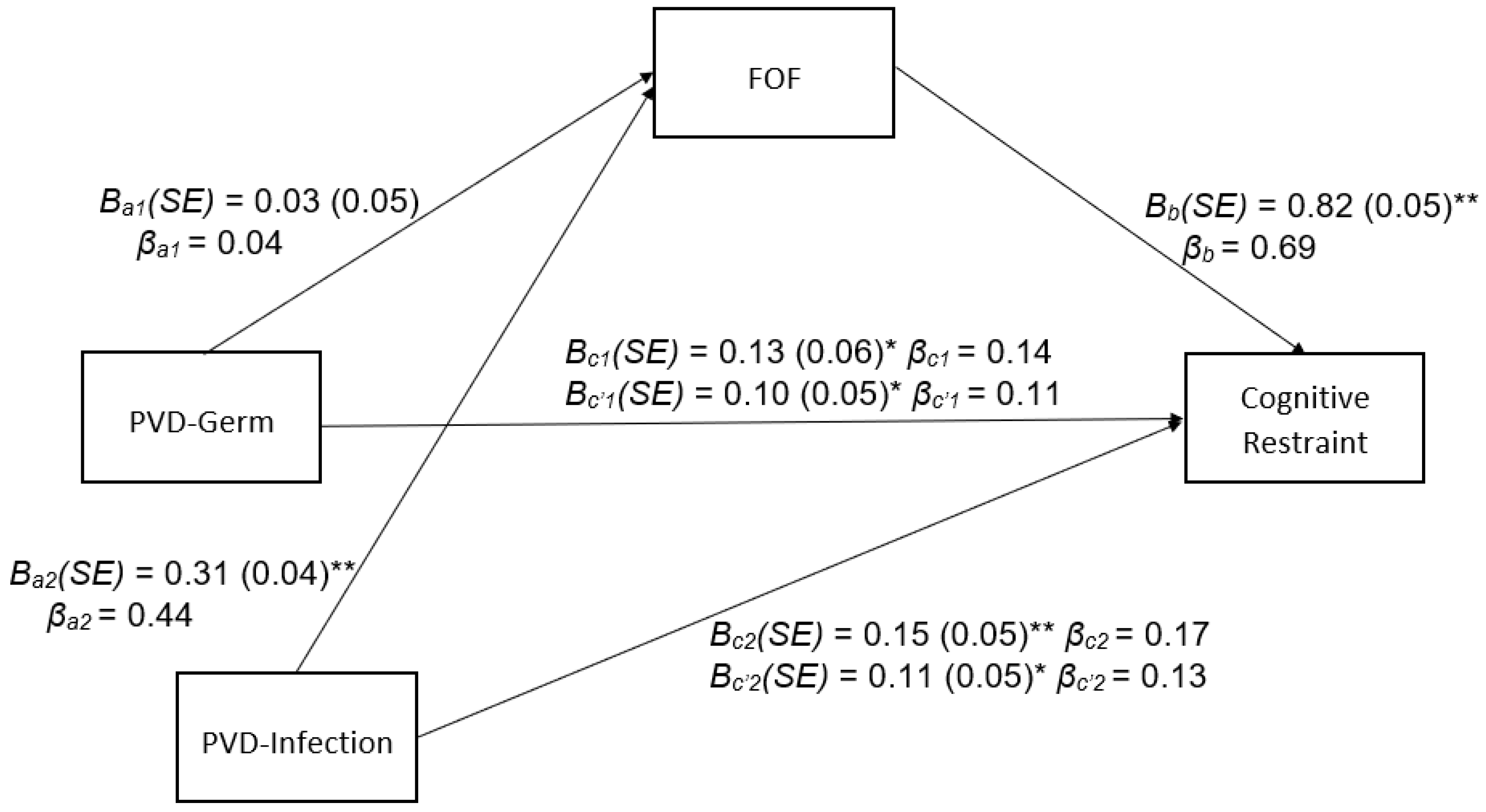
3.2. Indirect Effects of PVD Subscales on Cognitive Restraint through FOF
3.3. Indirect Effects of PVD Subscales on Compensatory Behaviors through FOF
3.4. Sex-Stratified Analyses
4. Discussion
4.1. PVD, FOF, Cognitive Restraint, and Compensatory Behaviors
4.2. Sex-Stratified Analyses
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PVD-Germ | PVD—Inf | FOF | Cog Restraint | Comp Behaviors | BMI | Age | Sex | Education | Race/Ethnicity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PVD—Germ | r | 0.25 *** | 0.15 * | 0.18 * | 0.03 | −0.13 | 0.02 | −0.22 ** | −0.06 | 0.11 | |
| n | 245 | 245 | 245 | 245 | 226 | 245 | 244 | 245 | 245 | ||
| PVD—Inf | r | 0.45 *** | 0.21 ** | 0.35 *** | 0.06 | 0.09 | −0.10 | 0.14 * | 0.13 * | ||
| n | 245 | 245 | 245 | 226 | 245 | 244 | 245 | 245 | |||
| FOF | r | 0.65 *** | 0.60 *** | 0.07 | <0.01 | −0.03 | 0.19 ** | ≤0.01 | |||
| n | 246 | 246 | 227 | 246 | 245 | 246 | 246 | ||||
| Cog Restraint | r | 0.48 *** | 0.01 | 0.02 | −0.02 | 0.21 ** | −0.04 | ||||
| n | 247 | 228 | 247 | 246 | 247 | 247 | |||||
| Comp Behaviors | r | −0.12 | ≤0.01 | −0.07 | 0.29 *** | ≤0.01 | |||||
| n | 228 | 247 | 246 | 247 | 247 | ||||||
| BMI | r | −0.03 | −0.05 | −0.16 * | −0.03 | ||||||
| n | 228 | 227 | 228 | 228 | |||||||
| Age | r | 0.18 ** | 0.09 | 0.19 ** | |||||||
| n | 246 | 247 | 247 | ||||||||
| Sex | r | −0.10 | 0.16 * | ||||||||
| n | 246 | 246 | |||||||||
| Education | r | −0.16 * | |||||||||
| n | 247 | ||||||||||
| Race/Ethnicity | r | ||||||||||
| n |
Appendix B
| t | p | b (SE) | β | f | p | Adj. R2 | |
|---|---|---|---|---|---|---|---|
| Cog Restraint | |||||||
| Overall model | 9.51 | <0.001 | 0.07 | ||||
| Constant | 7.40 | <0.001 | 2.03 (0.28) | ||||
| Education | 3.29 | 0.001 | 0.42 (0.13) | 0.20 | |||
| PVD—Germ | 3.04 | 0.003 | 0.18 (0.06) | 0.19 | |||
| Cog Restraint | |||||||
| Overall model | 8.86 | <0.001 | 0.06 | ||||
| Constant | 11.67 | <0.001 | 2.31 (0.20) | ||||
| Education | 2.68 | 0.01 | 0.35 (0.13) | 0.17 | |||
| PVD—Infection | 2.93 | 0.004 | 0.16 (0.05) | 0.18 | |||
| Comp Behaviors | |||||||
| Overall model | 11.16 | <0.001 | 0.08 | ||||
| Constant | 0.62 | 0.54 | 2.58 (4.19) | ||||
| Education | 4.70 | <0.001 | 9.22 (1.96) | 0.29 | |||
| PVD-Germ | 0.71 | 0.48 | 0.63 (0.88) | 0.04 | |||
| Comp Behaviors | |||||||
| Overall model | 26.72 | <0.001 | 0.17 | ||||
| Constant | −2.77 | 0.01 | −7.91 (2.85) | ||||
| Education | 4.13 | <0.001 | 7.72 (1.87) | 0.24 | |||
| PVD-Infection | 5.39 | <0.001 | 4.15 (0.77) | 0.32 |
Appendix C
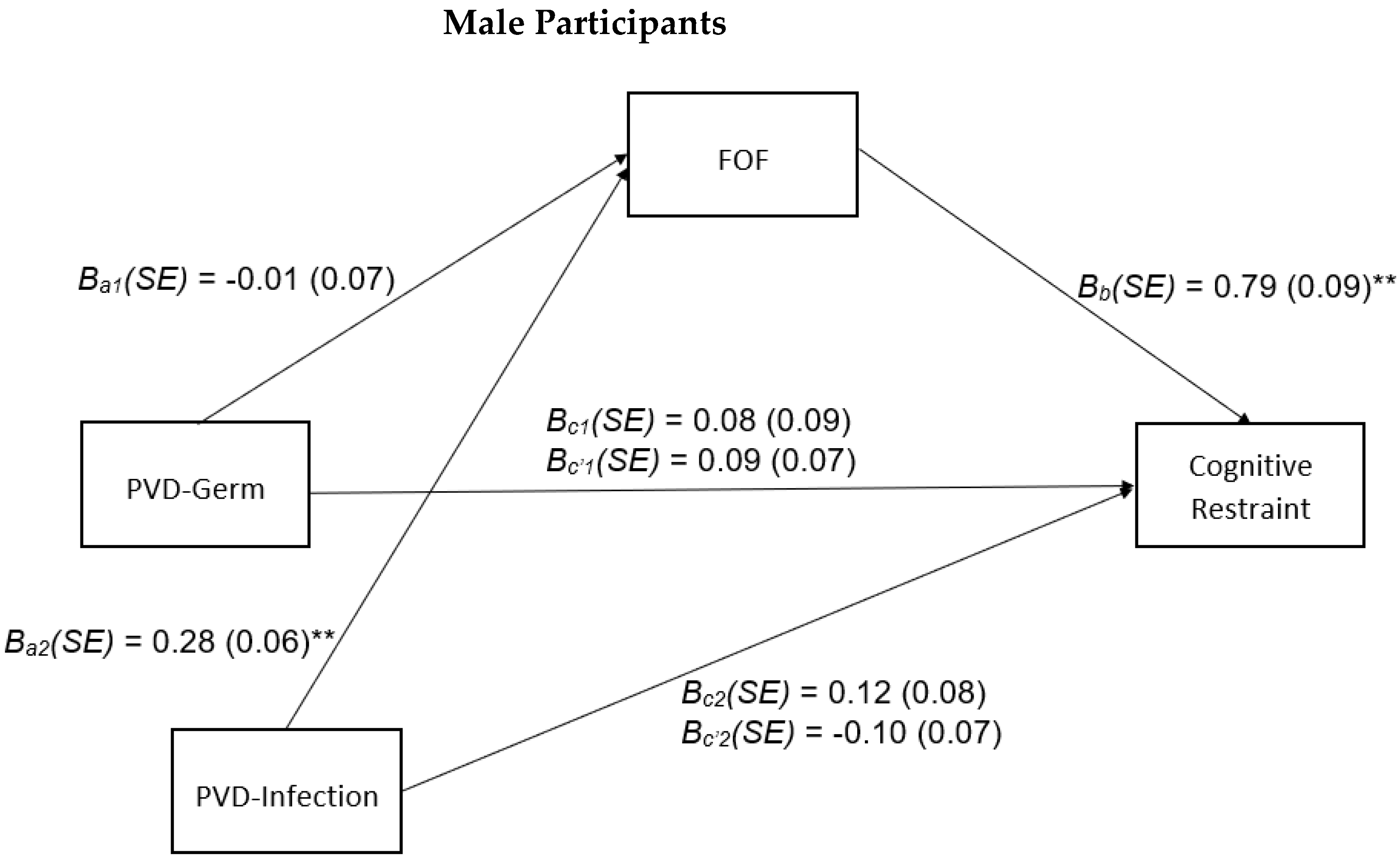
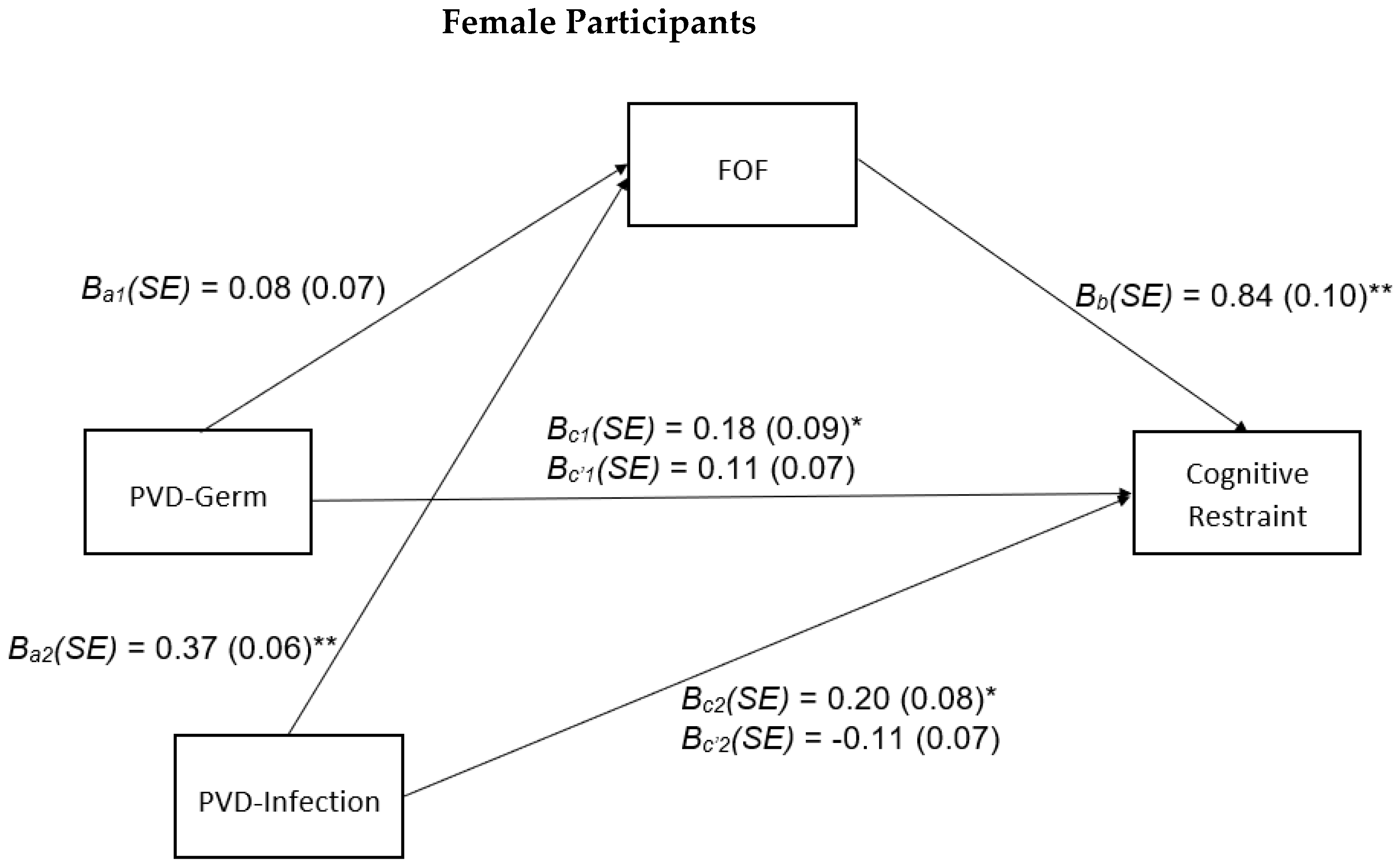
Appendix D

References
- Ackerman, J.M.; Hill, S.E.; Murray, D.R. The behavioral immune system: Current concerns and future directions. Soc. Personal. Psychol. Compass 2018, 12, e12371. [Google Scholar] [CrossRef]
- Park, J.H.; Van Leeuwen, F.; Chochorelou, Y. Disease-Avoidance Processes and Stigmatization: Cues of Substandard Health Arouse Heightened Discomfort with Physical Contact. J. Soc. Psychol. 2013, 153, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Park, J.H. The behavioral immune system (and why it matters). Curr. Psychol. 2011, 20, 99–103. [Google Scholar] [CrossRef]
- Duncan, L.A.; Schaller, M.; Park, J.H. Perceived vulnerability to disease: Development and validation of a 15-item self-report instrument. Personal. Individ. Differ. 2009, 47, 541–546. [Google Scholar] [CrossRef]
- Park, J.H.; Faulkner, J.; Schaller, M. Evolved Disease-Avoidance Processes and Contemporary Anti-Social Behavior: Prejudicial Attitudes and Avoidance of People with Physical Disabilities. J. Nonverbal Behav. 2003, 27, 65–87. [Google Scholar] [CrossRef]
- Park, J.H.; Schaller, M.; Crandall, C.S. Pathogen-avoidance mechanisms and the stigmatization of obese people. Evol. Hum. Behav. 2007, 28, 410–414. [Google Scholar] [CrossRef]
- Tapp, C.; Oaten, M.; Stevenson, R.; Occhipinti, S.; Thandi, R. Is obesity treated like a contagious disease? J. Appl. Soc. Psychol. 2019, 50, 205–212. [Google Scholar] [CrossRef]
- Miller, S.L.; Maner, J.K. Overperceiving disease cues: The basic cognition of the behavioral immune system. J. Personal. Soc. Psychol. 2012, 102, 1198–1213. [Google Scholar] [CrossRef]
- Lieberman, D.L.; Tybur, J.M.; Latner, J.D. Disgust Sensitivity, Obesity Stigma, and Gender: Contamination Psychology Predicts Weight Bias for Women, Not Men. Obesity 2012, 20, 1803–1814. [Google Scholar] [CrossRef]
- Lund, E.M.; Miller, S.L. Is obesity un-American? Disease concerns bias implicit perceptions of national identity. Evol. Hum. Behav. 2014, 35, 336–340. [Google Scholar] [CrossRef]
- Magallares, A.; Jáuregui-Lobera, I.; Carbonero-Carreño, R.; Ruiz-Prieto, I.; Bolaños-Ríos, P.; Cano-Escoriaza, A. Perceived vulnerability to disease and antifat attitudes in a sample of children and teenagers. Eat. Weight Disord. 2015, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.M.; Tybur, J.M.; Mortensen, C.R. Infectious Disease and Imperfections of Self-Image. Psychol. Sci. 2018, 29, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.M.; Hinman, N.; Koball, A.; Hoffmann, D.A.; Carels, R.A. Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite 2013, 60, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Durso, L.E.; Latner, J.D.; White, M.A.; Masheb, R.M.; Blomquist, K.K.; Morgan, P.T.; Grilo, C.M. Internalized weight bias in obese patients with binge eating disorder: Associations with eating disturbances and psychological functioning. Int. J. Eat. Disord. 2012, 45, 423–427. [Google Scholar] [CrossRef]
- American Psychiatric Association. Feeding and Eating Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Dalley, S.E.; Buunk, A.P. “Thinspiration” vs. “fear of fat”. Using prototypes to predict frequent weight-loss dieting in females. Appetite 2009, 52, 217–221. [Google Scholar] [CrossRef]
- Levitt, D.H. Drive for Thinness and Fear of Fat: Separate Yet Related Constructs? Eat. Disord. 2003, 11, 221–234. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Shafran, R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behav. Res. Ther. 2003, 41, 509–528. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.; Bergers, G.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Calugi, S.; Grave, R.D. Body image concern and treatment outcomes in adolescents with anorexia nervosa. Int. J. Eat. Disord. 2019, 52, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Calugi, S.; El Ghoch, M.; Conti, M.; Grave, R.D. Preoccupation with shape or weight, fear of weight gain, feeling fat and treatment outcomes in patients with anorexia nervosa: A longitudinal study. Behav. Res. Ther. 2018, 105, 63–68. [Google Scholar] [CrossRef]
- Chow, C.M.; Ruhl, H.; Tan, C.C.; Ellis, L. Fear of fat and restrained eating: Negative body talk between female friends as a moderator. Eat. Weight Disord. 2019, 24, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Dalley, S.E.; Toffanin, P.; Pollet, T.V. Dietary restraint in college women: Fear of an imperfect fat self is stronger than hope of a perfect thin self. Body Image 2012, 9, 441–447. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.S.; MacLeod, C.; Dondzilo, L.; Bell, J. The Role of Fear of Fatness and Avoidance of Fatness in Predicting Eating Restraint. Cogn. Ther. Res. 2020, 44, 196–207. [Google Scholar] [CrossRef]
- Wellman, J.D.; Araiza, A.M.; Newell, E.E.; McCoy, S.K. Weight stigma facilitates unhealthy eating and weight gain via fear of fat. Stigma Health 2018, 3, 186–194. [Google Scholar] [CrossRef]
- Goldfarb, L.A.; Dykens, E.M.; Gerrard, M. The Goldfarb Fear of Fat Scale. J. Personal. Assess. 1985, 49, 329–332. [Google Scholar] [CrossRef]
- Goldschmidt, A.B.; Crosby, R.D.; Cao, L.; Moessner, M.; Forbush, K.T.; Accurso, E.C.; Le Grange, D. Network analysis of pediatric eating disorder symptoms in a treatment-seeking, transdiagnostic sample. J. Abnorm. Psychol. 2018, 127, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Levinson, C.A.; Zerwas, S.; Calebs, B.; Forbush, K.; Kordy, H.; Watson, H.; Hofmeier, S.; Levine, M.; Crosby, R.D.; Peat, C.; et al. The core symptoms of bulimia nervosa, anxiety, and depression: A network analysis. J. Abnorm. Psychol. 2017, 126, 340–354. [Google Scholar] [CrossRef]
- De Coninck, D.; D’Haenens, L.; Matthijs, K. Perceived vulnerability to disease and attitudes towards public health measures: COVID-19 in Flanders, Belgium. Personal. Individ. Differ. 2020, 166, 110220. [Google Scholar] [CrossRef]
- Cummings, J.R.; Joyner, M.A.; Gearhardt, A.N. Development and preliminary validation of the Anticipated Effects of Food Scale. Psychol. Addict. Behav. 2020, 34, 403–413. [Google Scholar] [CrossRef]
- Stice, E.; Telch, C.F.; Rizvi, S.L. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol. Assess. 2000, 12, 123–131. [Google Scholar] [CrossRef]
- IBM Corporation. Released 2020. IBM SPSS Statistics for Windows; Version 27; IBM Corp.: Armonk, NY, USA.
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Pearl, R.L.; Hopkins, C.H.; Berkowitz, R.I.; Wadden, T.A. Group cognitive-behavioral treatment for internalized weight stigma: A pilot study. Eat. Weight Disord. 2018, 23, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Makhanova, A.; Shepherd, M.A.; Plant, E.A.; Gerend, M.A.; Maner, J.K. Childhood illness as an antecedent of perceived vulnerability to disease. Evol. Behav. Sci. 2020, 16, 53–66. [Google Scholar] [CrossRef]
- Anderson, L.M.; Berg, H.; Brown, T.A.; Menzel, J.; Reilly, E.E. The Role of Disgust in Eating Disorders. Curr. Psychiatry Rep. 2021, 23, 4. [Google Scholar] [CrossRef]
- Mitchison, D.; Mond, J.M. Epidemiology of eating disorders, eating disordered behaviour, and body image disturbance in males: A narrative review. J. Eat. Disord. 2015, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.; Peterson, C.B.; Frazier, P.; Crow, S.J. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. Int. J. Eat. Disord. 2011, 45, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, C.G.; Beglin, S.J. Assessment of eating disorders: Interview or self-report questionnaire? Int. J. Eat. Disord. 1994, 16, 363–370. [Google Scholar] [CrossRef]
- Keel, P.K.; Crow, S.; Davis, T.L.; Mitchell, J.E. Assessment of eating disorders comparison of interview and questionnaire data from a long-term follow-up study of bulimia nervosa. J. Psychosom. Res. 2002, 53, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Buhrmester, M.D.; Talaifar, S.; Gosling, S.D. An Evaluation of Amazon’s Mechanical Turk, Its Rapid Rise, and Its Effective Use. Perspect. Psychol. Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- De Pasquale, C.; Pistorio, M.L.; Sciacca, F.; Hichy, Z. Relationships Between Anxiety, Perceived Vulnerability to Disease, and Smartphone Use during Coronavirus Disease 2019 Pandemic in a Sample of Italian College Students. Front. Psychol. 2021, 12, 692503. [Google Scholar] [CrossRef]
- Swinbourne, J.M.; Touyz, S. The co-morbidity of eating disorders and anxiety disorders: A review. Eur. Eat. Disord. Rev. 2007, 15, 253–274. [Google Scholar] [CrossRef]
- Brady, R.E.; Badour, C.L.; Arega, E.A.; Levy, J.J.; Adams, T.G. Evaluating the mediating effects of perceived vulnerability to disease in the relation between disgust and contamination-based OCD. J. Anxiety Disord. 2021, 79, 102384. [Google Scholar] [CrossRef] [PubMed]
- Bektas, S.; Keeler, J.L.; Anderson, L.M.; Mutwalli, H.; Himmerich, H.; Treasure, J. Disgust and Self-Disgust in Eating Disorders: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1728. [Google Scholar] [CrossRef] [PubMed]
- Eyal, T.; Dar, R.; Liberman, N. Is disgust in obsessive-compulsive disorder mediated by fear of pathogens? J. Anxiety Disord. 2021, 77, 102340. [Google Scholar] [CrossRef] [PubMed]
- Altman, S.E.; Shankman, S.A. What is the association between obsessive–compulsive disorder and eating disorders? Clin. Psychol. Rev. 2009, 29, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ackerman, J.M.; Preston, S.D. Dissociating compulsive washing and hoarding tendencies through differences in comorbidities and the content of concerns. J. Behav. Cogn. Ther. 2021, 31, 291–308. [Google Scholar] [CrossRef]
- Da Luz, F.Q.; Hay, P.; Touyz, S.; Sainsbury, A. Obesity with Comorbid Eating Disorders: Associated Health Risks and Treatment Approaches. Nutrients 2018, 10, 829. [Google Scholar] [CrossRef]
- Burton, A.L.; Abbott, M.J. Processes and pathways to binge eating: Development of an integrated cognitive and behavioural model of binge eating. J. Eat. Disord. 2019, 7, 18. [Google Scholar] [CrossRef]
- Mathes, W.F.; Brownley, K.A.; Mo, X.; Bulik, C.M. The biology of binge eating. Appetite 2009, 52, 545–553. [Google Scholar] [CrossRef]
- Mortensen, C.R.; Becker, D.V.; Ackerman, J.M.; Neuberg, S.L.; Kenrick, D.T. Infection Breeds Reticencer: The Effects of Disease Salience on Self-Perceptions of Personality and Behavioral Avoidance Tendencies. Psychol. Sci. 2010, 21, 440–447. [Google Scholar] [CrossRef]
- Wang, I.M.; Ackerman, J.M. The Infectiousness of Crowds: Crowding Experiences Are Amplified by Pathogen Threats. Personal. Soc. Psychol. Bull. 2019, 45, 120–132. [Google Scholar] [CrossRef]
- Allen, K.L.; Crosby, R.D.; Oddy, W.H.; Byrne, S.M. Eating disorder symptom trajectories in adolescence: Effects of time, participant sex, and early adolescent depressive symptoms. J. Eat. Disord. 2013, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Termorshuizen J., D.; Watson, H.J.; Thornton, L.M.; Borg, S.; Flatt, R.E.; MacDermod, C.M.; Harper, L.E.; van Furth, E.F.; Peat, C.M.; Bulik, C.M. Early impact of COVID-19 on individuals with self-reported eating disorders: A survey of ~1000 individuals in the United States and the Netherlands. Int. J. Eat. Disord. 2020, 53, 1780–1790. [Google Scholar] [CrossRef] [PubMed]

| n (%) | |
|---|---|
| Age (M = 36.8, SD = 11.3, min–max = 21–70) | |
| 21–29 | 73 (29.6%) |
| 30–39 | 101 (40.8%) |
| 40–49 | 33 (13.4%) |
| 50–59 | 23 (9.3%) |
| 60–69 | 16 (6.5%) |
| 70 | 1 (0.4%) |
| Sex at Birth | |
| Male | 131 (53.3%) |
| Female | 115 (46.7%) |
| Racial Identity ± | |
| American Indian/Alaskan Native | 6 (2.4%) |
| Hispanic/Latino | 17 (6.9%) |
| Asian | 11 (4.5%) |
| Black/African American | 38 (15.4%) |
| White | 184 (74.5%) |
| Other | 2 (0.8%) |
| Education | |
| High school graduate | 30 (12.1%) |
| Some college | 45 (18.2%) |
| Associates degree | 24 (9.7%) |
| Bachelor’s degree | 117 (47.4%) |
| Advanced degree | 31 (12.6%) |
| Income | |
| Less than USD 10,000 | 15 (6.1%) |
| USD 10,000–USD 19,999 | 18 (7.3%) |
| USD 20,000–USD 29,999 | 31 (12.7%) |
| USD 30,000–USD 39,999 | 45 (18.4%) |
| USD 40,000–USD 49,999 | 31 (12.7%) |
| USD 50,000–USD 59,999 | 25 (10.2%) |
| USD 60,000–USD 69,999 | 19 (7.8%) |
| USD 70,000–USD 79,999 | 20 (8.2%) |
| USD 80,000–USD 89,999 | 8 (3.3%) |
| USD 90,000–USD 99,999 | 10 (4.1%) |
| USD 100,000–USD 149,999 | 16 (6.5%) |
| More than USD 150,000 | 7 (2.9%) |
| Subjective Socioeconomic Status ¥ | |
| 1 | 3 (1.2%) |
| 2 | 19 (7.7%) |
| 3 | 32 (13.0%) |
| 4 | 32 (13.0%) |
| 5 | 60 (24.3%) |
| 6 | 32 (13.0%) |
| 7 | 35 (14.2%) |
| 8 | 23 (9.3%) |
| 9 | 9 (3.6%) |
| 10 | 2 (0.8%) |
| BMI (M = 26.3, SD = 5.9, min–max = 17.7–55.8) | |
| Underweight (BMI < 18.5) | 7 (3.1%) |
| Normal Weight (BMI 18.5–24.9) | 96 (42.3%) |
| Overweight (BMI 25.0–29.9) | 84 (36.6%) |
| Obese (BMI > 30) | 41 (18.1%) |
| PVD-Germ | PVD-Infection | FOF | Cognitive Restraint | Comp Behaviors | ||
|---|---|---|---|---|---|---|
| PVD–Germ | r | 0.25 *** | 0.15 * | 0.18 ** | 0.03 | |
| n | 245 | 245 | 245 | 245 | ||
| PVD–Infection | r | 0.45 *** | 0.21 ** | 0.35 *** | ||
| n | 245 | 245 | 245 | |||
| FOF | r | 0.65 *** | 0.60 *** | |||
| n | 246 | 246 | ||||
| Cognitive Restraint | r | 0.48 *** | ||||
| n | 247 | |||||
| Comp Behaviors | r | |||||
| n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoover, L.V.; Ackerman, J.M.; Cummings, J.R.; Gearhardt, A.N. The Association of Perceived Vulnerability to Disease with Cognitive Restraint and Compensatory Behaviors. Nutrients 2023, 15, 8. https://doi.org/10.3390/nu15010008
Hoover LV, Ackerman JM, Cummings JR, Gearhardt AN. The Association of Perceived Vulnerability to Disease with Cognitive Restraint and Compensatory Behaviors. Nutrients. 2023; 15(1):8. https://doi.org/10.3390/nu15010008
Chicago/Turabian StyleHoover, Lindzey V., Joshua M. Ackerman, Jenna R. Cummings, and Ashley N. Gearhardt. 2023. "The Association of Perceived Vulnerability to Disease with Cognitive Restraint and Compensatory Behaviors" Nutrients 15, no. 1: 8. https://doi.org/10.3390/nu15010008
APA StyleHoover, L. V., Ackerman, J. M., Cummings, J. R., & Gearhardt, A. N. (2023). The Association of Perceived Vulnerability to Disease with Cognitive Restraint and Compensatory Behaviors. Nutrients, 15(1), 8. https://doi.org/10.3390/nu15010008





