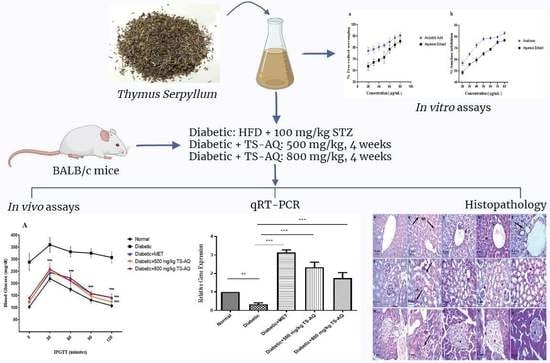
Thymus serpyllum Exhibits Anti-Diabetic Potential in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice: A Combined Biochemical and In Vivo Study
Abstract
:1. Introduction
2. Material and Methods
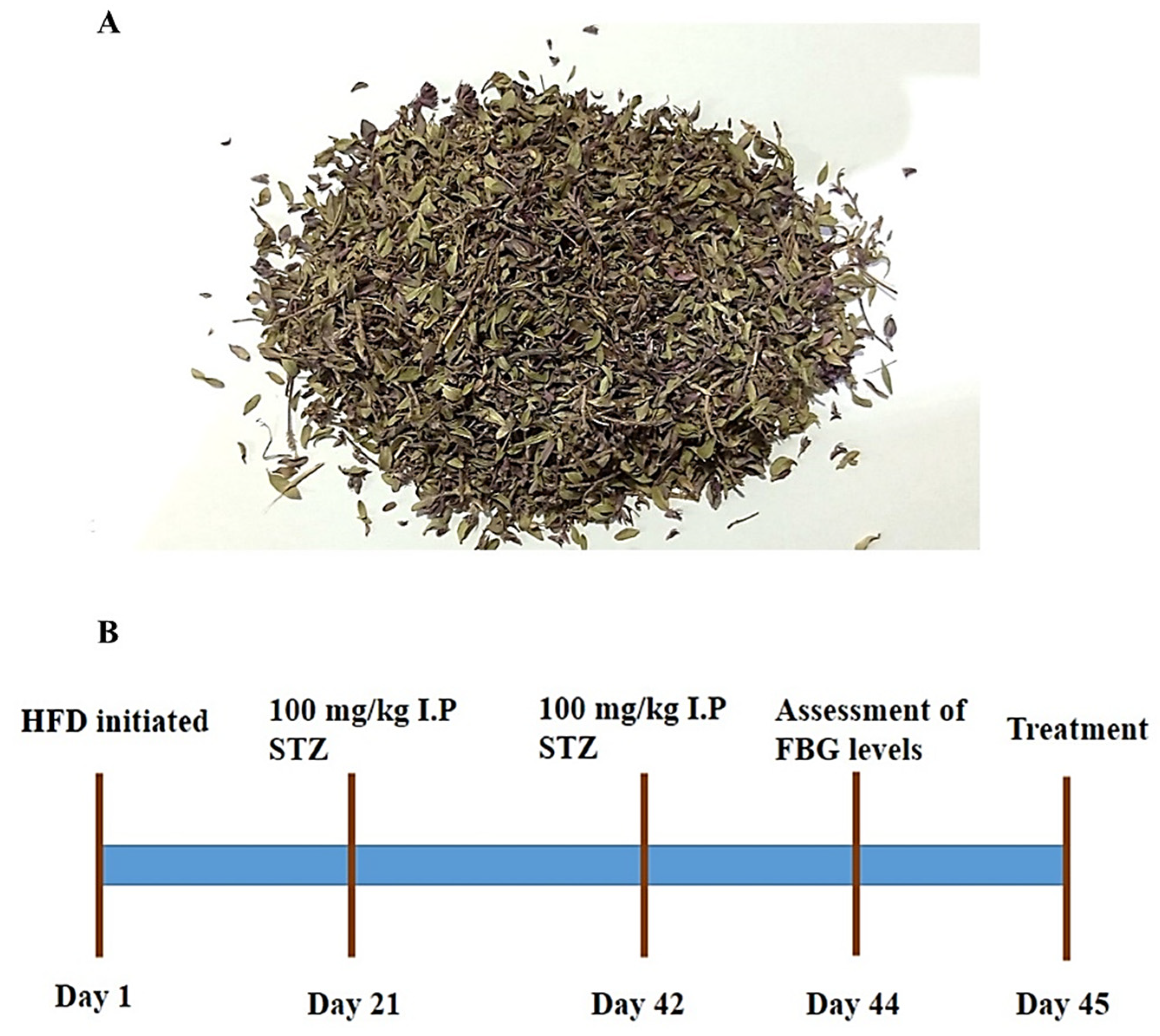
2.1. Plant Collection and Extract Preparation
2.2. Phytochemicals Screening
2.3. Antioxidant and Alpha Amylase Inhibition Assay
2.4. In Vivo Assessment of Antidiabetic Potential
2.4.1. Animal Procurement and Model Establishment
- Group 1: Normal control mice (n = 10).
- Group 2: Diabetic or untreated mice group (n = 10).
- Group 3: Mice group treated with standard drug metformin at 100 mg/kg dose (n = 10).
- Group 4: Mice group treated with 500 mg/kg dose of Thymus serpyllum extract (n = 10).
- Group 5: Mice group treated with 800 mg/kg dose of Thymus serpyllum extract (n = 10).
2.4.2. Administration of Thymus serpyllum for Treatment
2.4.3. Glucose and Insulin Tolerance Test
2.4.4. Expression Analysis of AMPK, IRS1 and GLUT2 gene by Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.4.5. Histopathological Examination
2.5. Statistical Analysis
3. Results
3.1. Phytochemicals Screening, Antioxidant and Alpha Amylase Inhibition Activity
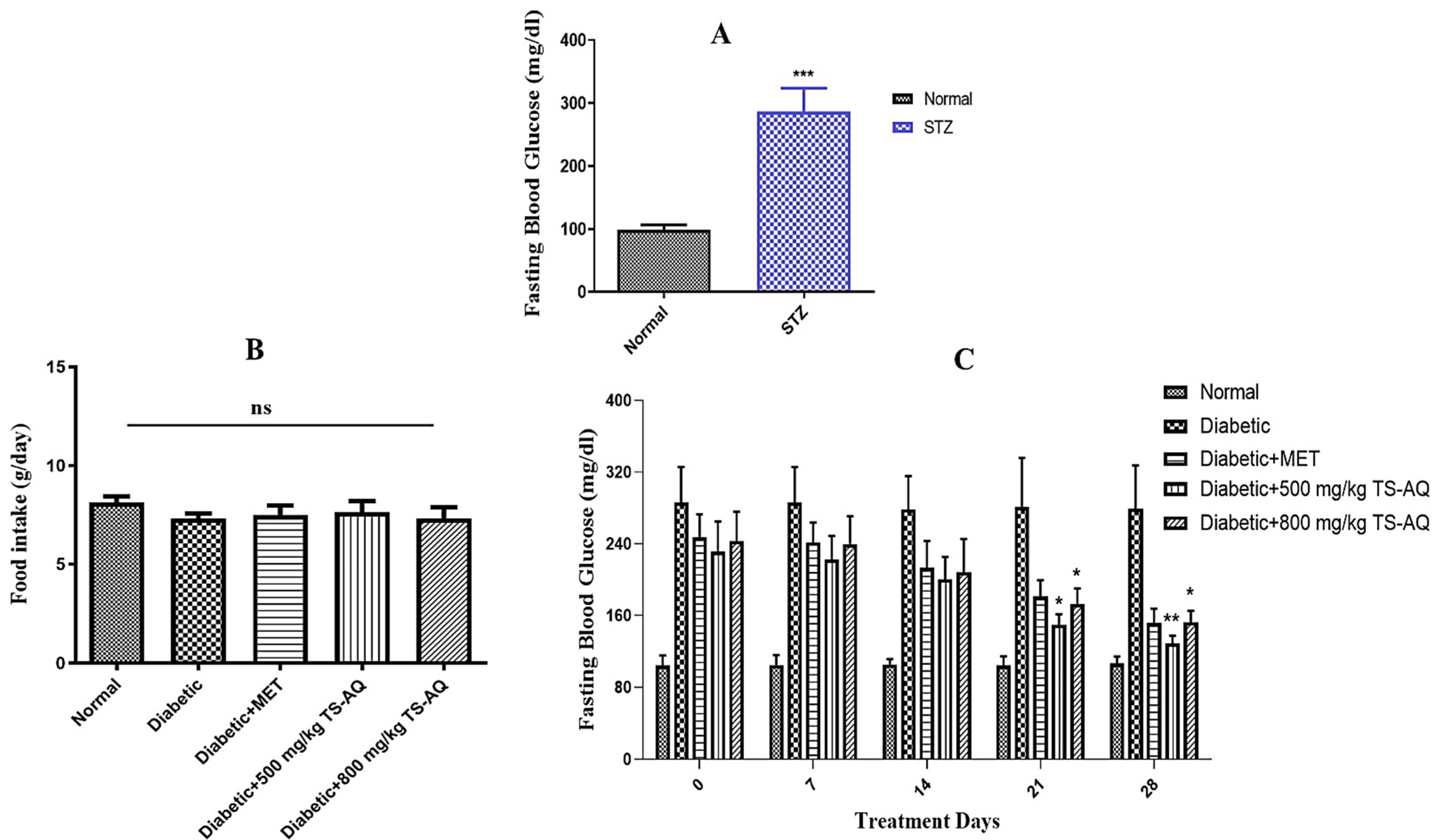
3.2. Thyme Extract Reduced Fasting Blood Glucose Levels in BALB/c Mice
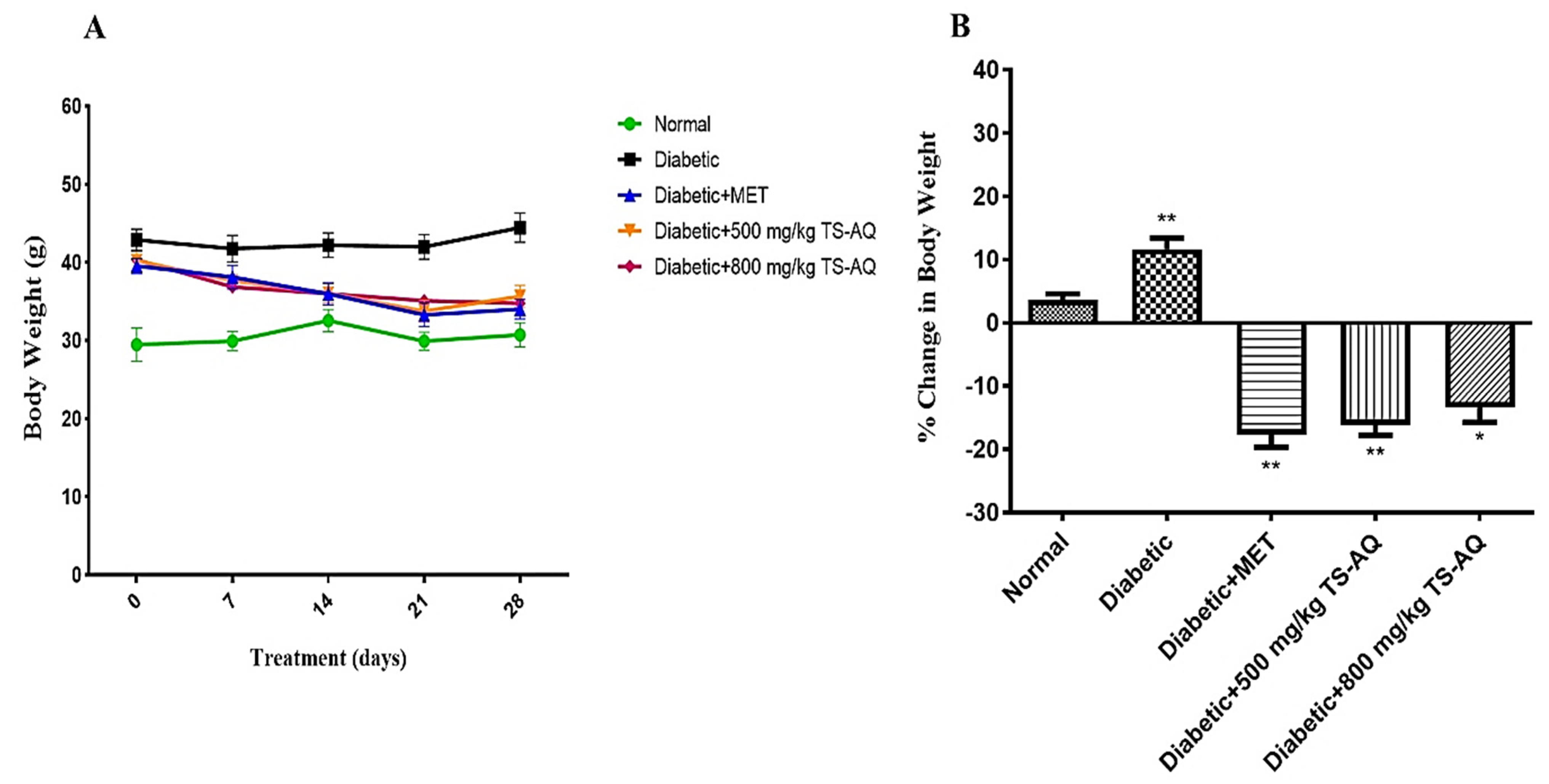
3.3. Effect of Thyme Extract on Body Weight
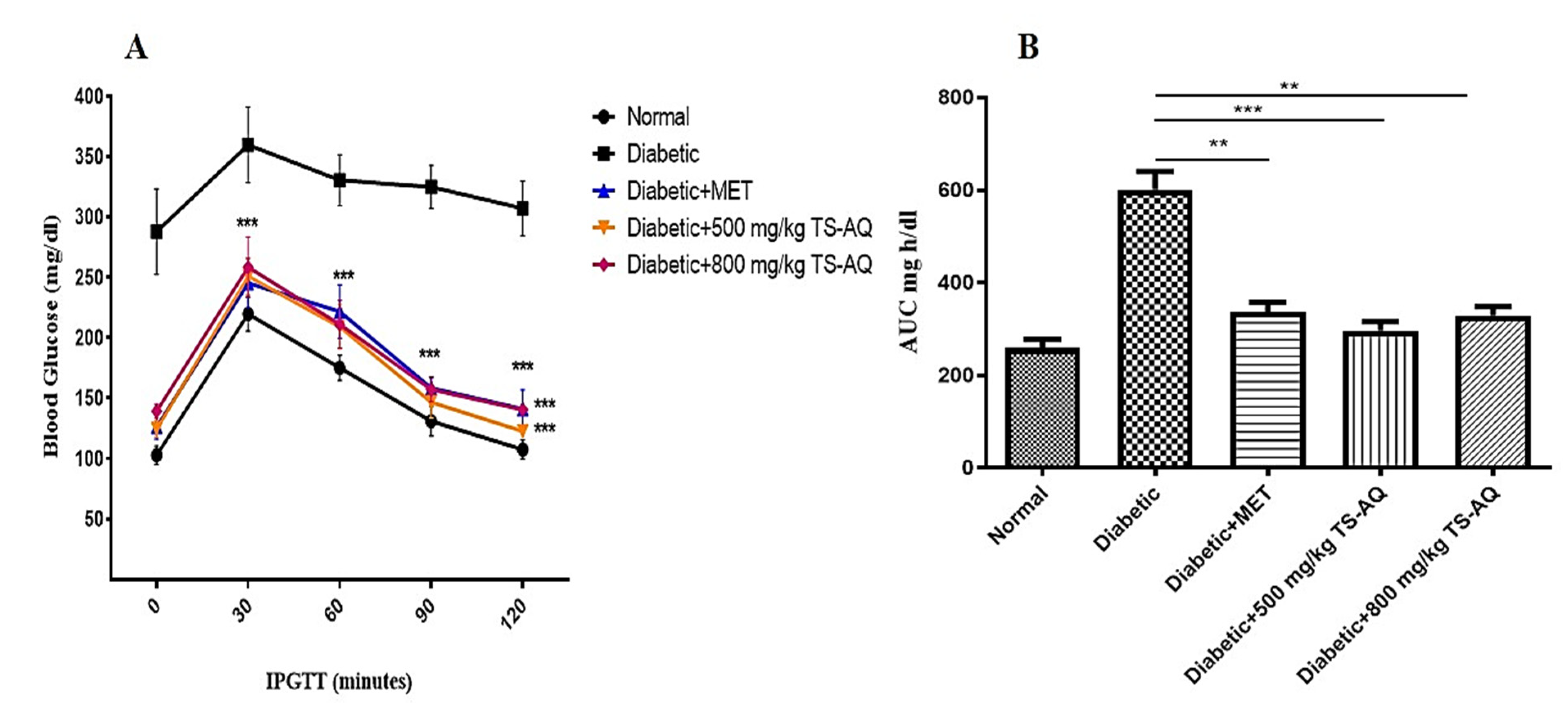
3.4. Thyme Extract Improved Glucose Tolerance In Vivo
3.5. Effect of Thyme Extract on Insulin Action
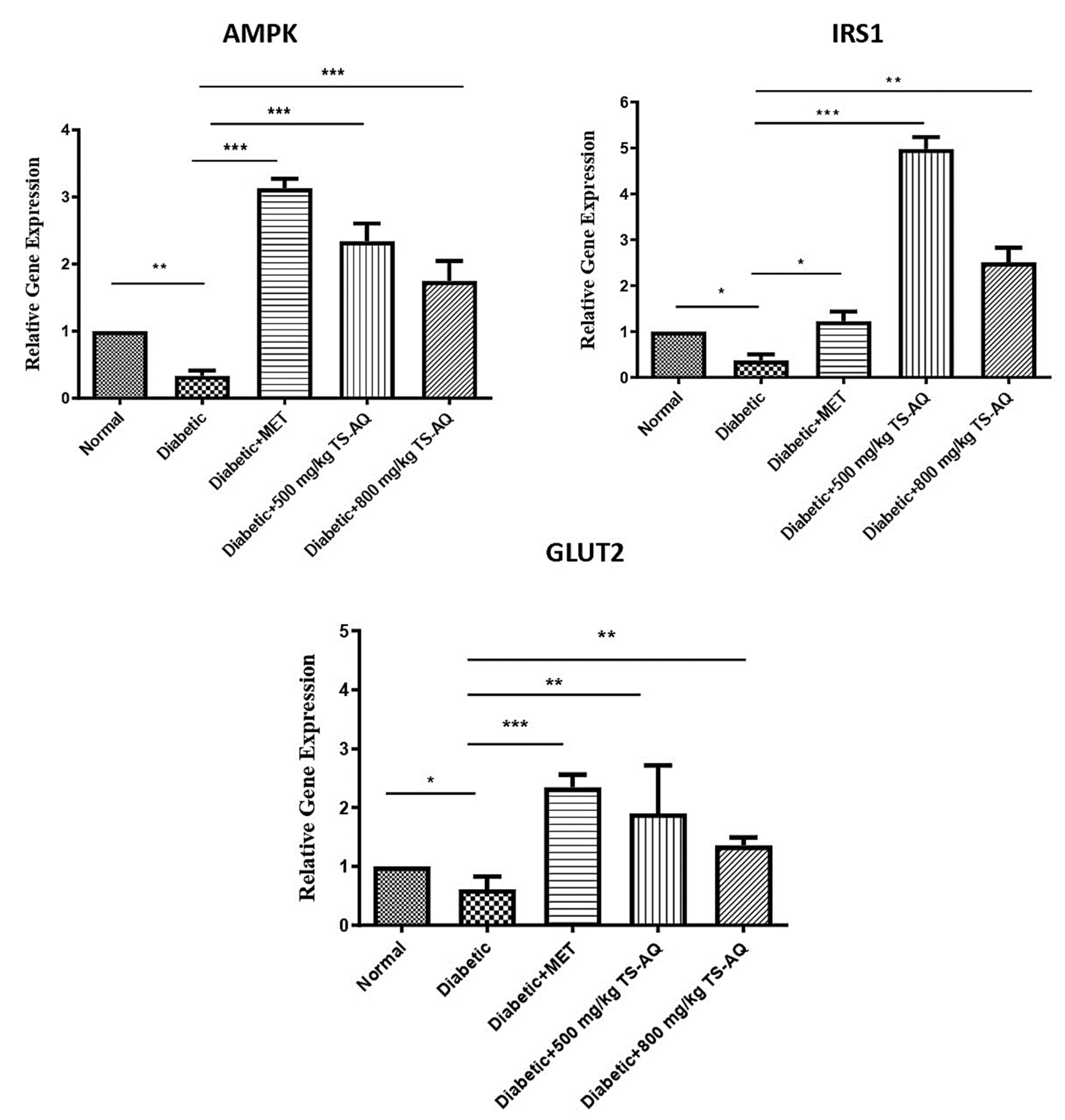
3.6. Thyme Extract Improved the AMPK, IRS1 and GLUT2 Expression at RNA Level
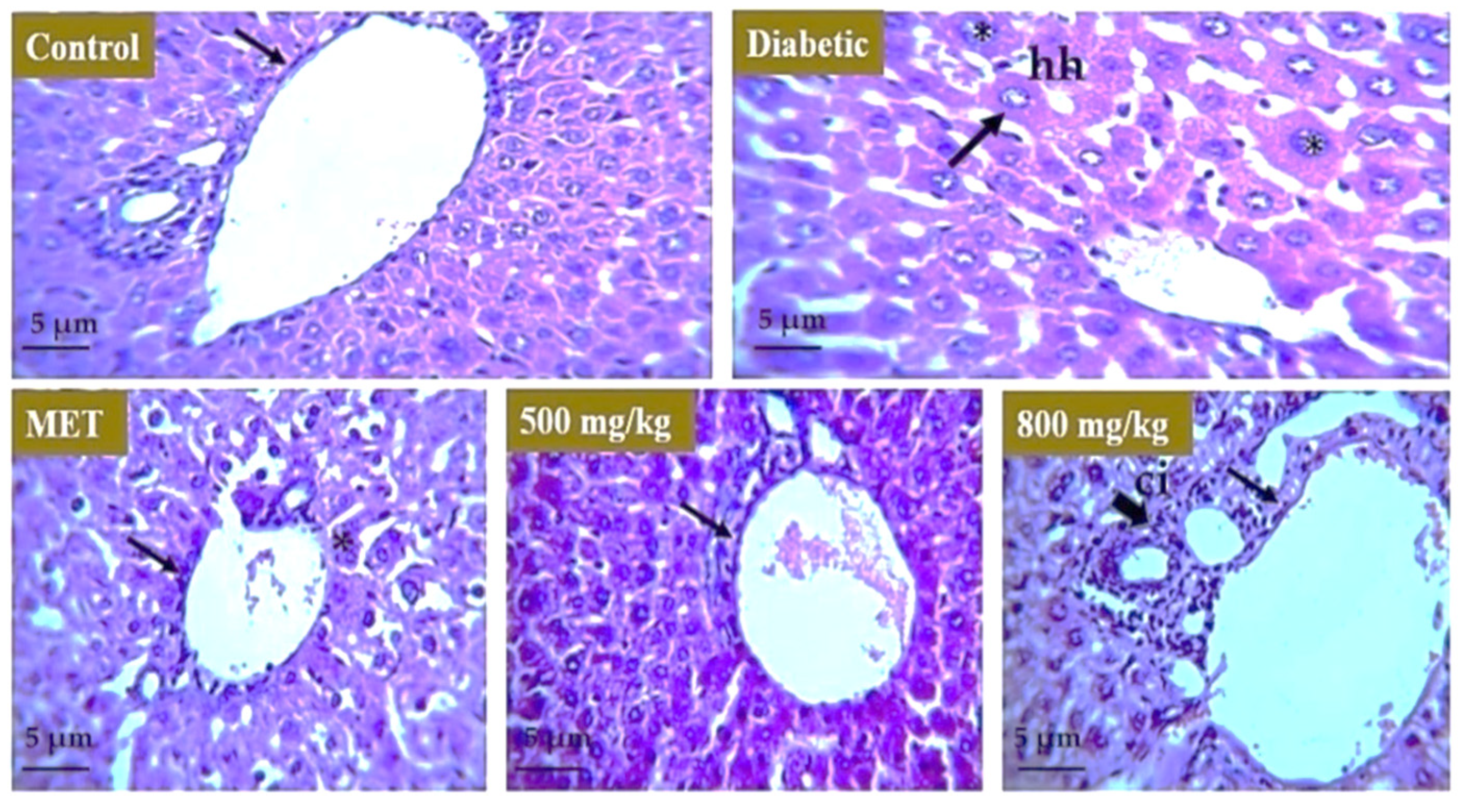
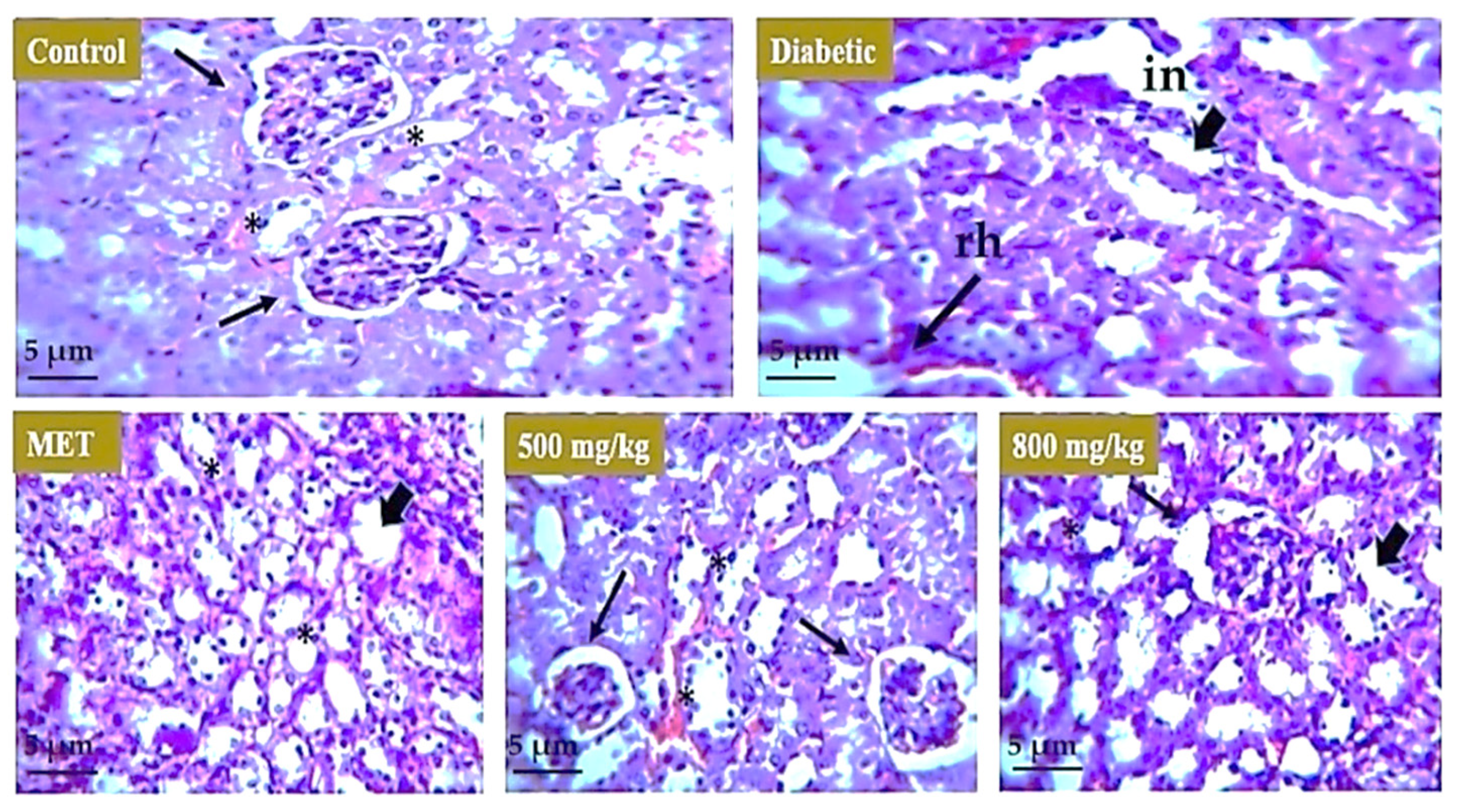
3.7. Effect of Extract on Liver, Kidney, and Pancreas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FBG | Fasting blood glucose |
| HFD | High-fat diet |
| MET | Metformin |
| TS-AQ | Aqueous extract of Thymus serpyllum |
| T2DM | Type 2 diabetes mellitus |
| AUC | Area under the curve |
References
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar]
- Lochhead, P.A.; Salt, I.P.; Walker, K.S.; Hardie, D.G.; Sutherland, C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 2000, 49, 896–903. [Google Scholar]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar]
- Chopra, I.; Li, H.; Wang, H.; Webster, K. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia 2012, 55, 783–794. [Google Scholar]
- Carvalho, E.; Jansson, P.-A.; Axelsen, M.; Eriksson, J.W.; Huang, X.; Groop, L.; Rondinone, C.; Sjostrom, L.; Smith, U.P. Low Cellular IRS 1 Gene and Protein Expression Predict Insulin Resistance and Type 2 Diabetes. Diabetes 1999, 48, SA23. [Google Scholar]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Hoffmann, I.S.; Roa, M.; Torrico, F.; Cubeddu, L.X. Ondansetron and metformin-induced gastrointestinal side effects. Am. J. Ther. 2003, 10, 447–451. [Google Scholar]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354. [Google Scholar]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar]
- Bouchoucha, M.; Uzzan, B.; Cohen, R. Metformin and digestive disorders. Diabetes Metab. 2011, 37, 90–96. [Google Scholar]
- Jabeen, N.; Ajaib, M.; Siddiqui, M.F. A survey of ethnobotanically important plants of district Ghizer, Gilgit-Baltistan. FUUAST J. Biol. 2015, 5, 153–160. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.d.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar]
- Jannat, A.; John, P.; Bhatti, A.; Hayat, M.Q. Tomorou attenuates progression of rheumatoid arthritis through alteration in ULK-1 independent autophagy pathway in collagen induced arthritis mice model. Cell Death Discov. 2019, 5, 1–13. [Google Scholar]
- Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, N.; Zorrilla, P.; Burkard, N.; Pischel, I.; Sievers, H.; Benedek, B.; Feistel, B.; Walbroel, B. Intestinal anti-inflammatory activity of the Serpylli herba extract in experimental models of rodent colitis. J. Crohn’s Colitis 2014, 8, 775–788. [Google Scholar]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevao-Silva, C.F.; Carvalho, M.D.d.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar]
- Tsuji, T.; Mori, T.; Taniguchi, M.; Shimizu, K.; Kobayashi, T. Solvent extraction of plant pigments from leaf protein concentrate. J. Chem. Eng. Jpn. 1985, 18, 539–544. [Google Scholar]
- Kaur, G.J.; Arora, D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 1–10. [Google Scholar]
- Sanganna, B.; Chitme, H.R.; Vrunda, K.; Jamadar, M.J. Antiproliferative and antioxidant activity of leaves extracts of Moringa oleifera. Int. J. Curr. Pharm. Res. 2016, 8, 54–56. [Google Scholar]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar]
- Noor, A.; Zahid, S. Alterations in adult hippocampal neurogenesis, aberrant protein s-nitrosylation, and associated spatial memory loss in streptozotocin-induced diabetes mellitus type 2 mice. Iran. J. Basic Med. Sci. 2017, 20, 1159. [Google Scholar]
- Shin, J.-W.; Seol, I.-C.; Son, C.-G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Med. 2010, 31, 1–7. [Google Scholar]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Singh, A.; Yadav, A.; Chauhan, A.; Yadav, A.K.; Singh, A.K. Essential oil composition of Thymus serpyllum cultivated in the Kumaon region of western Himalaya, India. Nat. Prod. Commun. 2009, 4, 1934578X0900400723. [Google Scholar]
- Verma, R.; Verma, R.; Chauhan, A.; Yadav, A. Seasonal variation in essential oil content and composition of Thyme, Thymus serpyllum L. cultivated in Uttarakhand Hills. Indian J. Pharm. Sci. 2011, 73, 233. [Google Scholar]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar]
- Goyal, S.; Pathak, R.; Pandey, H.K.; Kumari, A.; Tewari, G.; Bhandari, N.S.; Bala, M. Comparative study of the volatile constituents of Thymus serpyllum L. grown at different altitudes of Western Himalayas. SN Appl. Sci. 2020, 2, 1208. [Google Scholar] [CrossRef]
- Abramovic, H.; Abram, V.; Cuk, A.; Ceh, B.; SMOLE-MOZINA, S.; Vidmar, M.; Pavlovic, M.; ULRIH, N.P. Antioxidative and antibacterial properties of organically grown thyme (Thymus sp.) and basil (Ocimum basilicum L.). Turk. J. Agric. For. 2018, 42, 185–194. [Google Scholar]
- Aralbaeva, A.N.; Mamataeva, A.T.; Zhaparkulova, N.I.; Utegalieva, R.S.; Khanin, M.; Danilenko, M.; Murzakhmetova, M.K. A composition of medicinal plants with an enhanced ability to suppress microsomal lipid peroxidation and a protective activity against carbon tetrachloride-induced hepatotoxicity. Biomed. Pharm. 2017, 96, 1283–1291. [Google Scholar]
- Mushtaq, M.N.; Bashir, S.; Ullah, I.; Karim, S.; Rashid, M.; Hayat Malik, M.N. Comparative hypoglycemic activity of different fractions of Thymus serpyllum L. in alloxan induced diabetic rabbits. Pak. J. Pharm. Sci. 2016, 29, 1483–1488. [Google Scholar]
- Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Hidalgo-García, L.; Molina-Tijeras, J.A.; García, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.; Rodríguez-Cabezas, M.E. The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants 2022, 11, 1073. [Google Scholar]
- Lucidi, P.; Rossetti, P.; Porcellati, F.; Pampanelli, S.; Candeloro, P.; Andreoli, A.M.; Perriello, G.; Bolli, G.B.; Fanelli, C.G. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: The role of lipolysis. Diabetes 2010, 59, 1349–1357. [Google Scholar]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary extract as a potential anti-hyperglycemic agent: Current evidence and future perspectives. Nutrients 2017, 9, 968. [Google Scholar]
- Elbadawi, M.; Ammar, R.M.; Aziz-Kalbhenn, H.; Rabini, S.; Klauck, S.M.; Dawood, M.; Saeed, M.E.; Kampf, C.J.; Efferth, T. Anti-inflammatory and tight junction protective activity of the herbal preparation STW 5-II on mouse intestinal organoids. Phytomedicine 2021, 88, 153589. [Google Scholar]
- Xie, Z.; Zhong, L.; Wu, Y.; Wan, X.; Yang, H.; Xu, X.; Li, P. Carnosic acid improves diabetic nephropathy by activating Nrf2/ARE and inhibition of NF-κB pathway. Phytomedicine 2018, 47, 161–173. [Google Scholar]
- He, T.; Li, X.; Wang, X.; Xu, X.; Yan, X.; Li, X.; Sun, S.; Dong, Y.; Ren, X.; Liu, X. Chemical composition and anti-oxidant potential on essential oils of Thymus quinquecostatus Celak. from Loess Plateau in China, regulating Nrf2/Keap1 signaling pathway in zebrafish. Sci. Rep. 2020, 10, 1–18. [Google Scholar]
- Saravanan, S.; Pari, L. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. Eur. J. Pharm. 2015, 761, 279–287. [Google Scholar]
- Kabbaoui, M.; Chda, A.; Mejrhit, N.; Azdad, O.; Farah, A.; Aarab, L.; Bencheikh, R.; Tazi, A. Antidiabetic effect of Thymus satureioides aqueous extract in streptozotocin-induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 140–145. [Google Scholar]
- Aljarah, A.K.; Hameed, I.H. In Vitro anti-diabetic properties of Methanolic extract of Thymus vulgaris using α-glucosidase and α-amylase inhibition assay and determination of its bioactive chemical compounds. Indian J. Public Health Res. Dev. 2018, 9, 388–392. [Google Scholar]



| Phytochemical | Aqueous Extract |
|---|---|
| Alkaloids | ++ |
| Phenols | +++ |
| Flavonoids | ++ |
| Anthraquinones | ++ |
| Anthocyanins | - |
| Phlobatannins | + |
| Coumarins | ++ |
| Terpenoids | + |
| Sterols | ++ |
| Steroids | +++ |
| Saponins | - |
| Glycosides | + |
| Tannins | +++ |
| Amino acids | +++ |
| Carbohydrates | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, J.; John, P.; Bhatti, A. Thymus serpyllum Exhibits Anti-Diabetic Potential in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice: A Combined Biochemical and In Vivo Study. Nutrients 2022, 14, 3561. https://doi.org/10.3390/nu14173561
Azhar J, John P, Bhatti A. Thymus serpyllum Exhibits Anti-Diabetic Potential in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice: A Combined Biochemical and In Vivo Study. Nutrients. 2022; 14(17):3561. https://doi.org/10.3390/nu14173561
Chicago/Turabian StyleAzhar, Jahanzaib, Peter John, and Attya Bhatti. 2022. "Thymus serpyllum Exhibits Anti-Diabetic Potential in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice: A Combined Biochemical and In Vivo Study" Nutrients 14, no. 17: 3561. https://doi.org/10.3390/nu14173561
APA StyleAzhar, J., John, P., & Bhatti, A. (2022). Thymus serpyllum Exhibits Anti-Diabetic Potential in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice: A Combined Biochemical and In Vivo Study. Nutrients, 14(17), 3561. https://doi.org/10.3390/nu14173561