Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases
Abstract
1. Introduction
2. Developmental Programming of Chronic Diseases by Pregnancy-Associated Disorders
2.1. (Patho)physiologic Role of Reactive Oxygen Species in Fetal Development
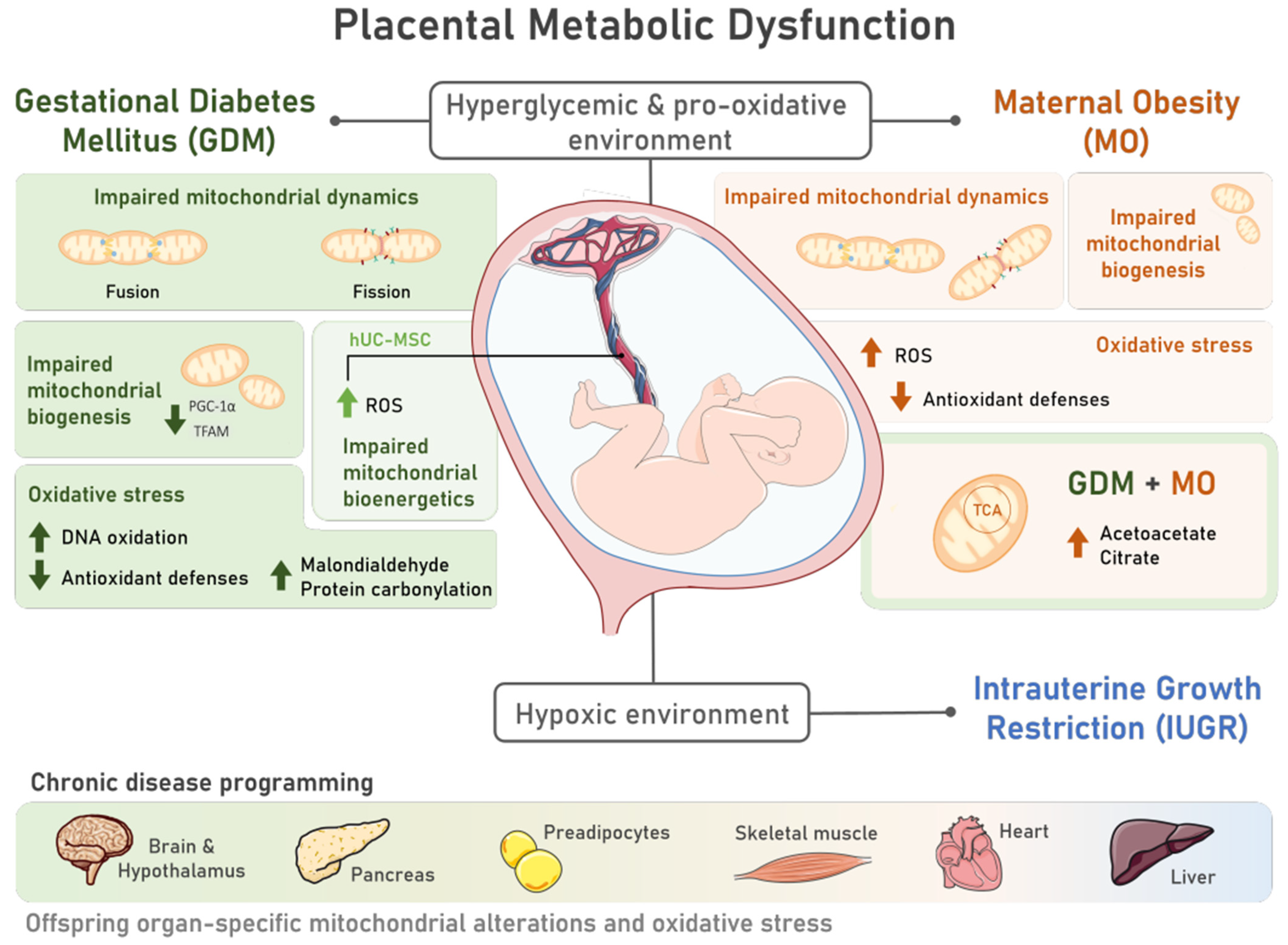
2.2. The Impact of Pregnancy-Associated Disorders on Offspring’s Organs Oxidative Stress and Mitochondrial Function
3. Antioxidants and Mitochondriotropic Activity of Naturally Occurring and Synthetic Antioxidants
3.1. Naturally Occurring Antioxidants
3.1.1. Endogenous Antioxidants
3.1.2. Dietary Antioxidants
3.2. Synthetic Antioxidants
Mitochondria-Targeted Antioxidants
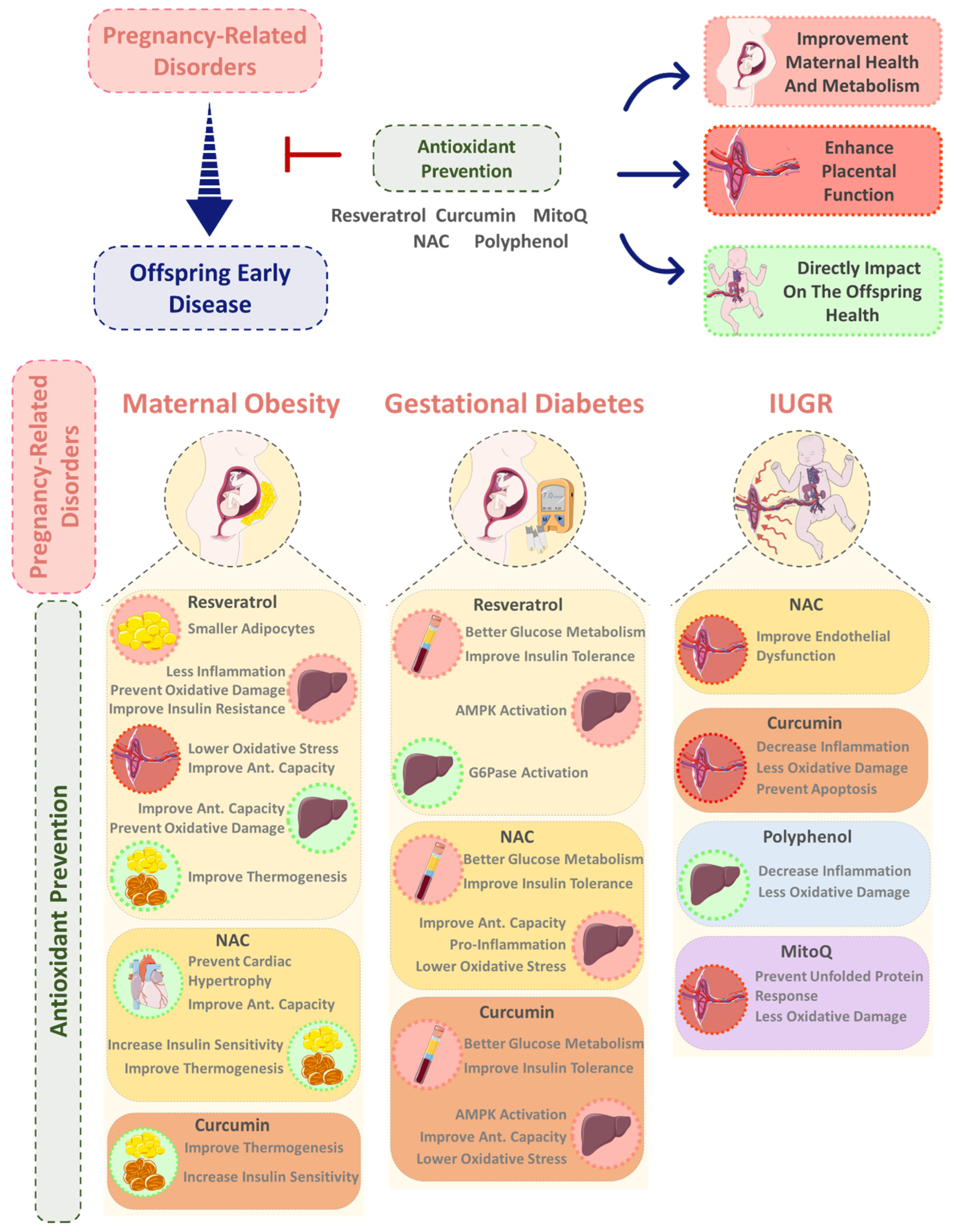
4. Exploring the Role of Dietary Antioxidant Supplementation during Pregnancy in Offspring Non-Communicable Disease Prevention
4.1. Vitamins
4.2. Resveratrol
4.3. Curcumin
4.4. NAC
4.5. MitoQ
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of Chronic Disease in the 21st Century: Elimination of the Leading Preventable Causes of Premature Death and Disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R. Preventing and Managing Chronic Diseases. BMJ 2019, 364, l459. [Google Scholar] [CrossRef]
- Adams, M.L.; Grandpre, J.; Katz, D.L.; Shenson, D. The Impact of Key Modifiable Risk Factors on Leading Chronic Conditions. Prev. Med. 2019, 120, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tocantins, C.; Diniz, M.S.; Grilo, L.F.; Pereira, S.P. The Birth of Cardiac Disease: Mechanisms Linking Gestational Diabetes Mellitus and Early Onset of Cardiovascular Disease in Offspring. WIREs Mech. Dis. 2022, 14, e1555. [Google Scholar] [CrossRef]
- Diniz, M.S.; Hiden, U.; Falcão-Pires, I.; Oliveira, P.J.; Sobrevia, L.; Pereira, S.P. Fetoplacental Endothelial Dysfunction in Gestational Diabetes Mellitus and Maternal Obesity: A Potential Threat for Programming Cardiovascular Disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166834. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; Grilo, L.F.; Tocantins, C.; Falcão-Pires, I.; Pereira, S.P. Made in the Womb: Maternal Programming of Offspring Cardiovascular Function by an Obesogenic Womb. Metabolites 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Grilo, L.F.; Tocantins, C.; Diniz, M.S.; Gomes, R.M.; Oliveira, P.J.; Matafome, P.; Pereira, S.P. Metabolic Disease Programming: From Mitochondria to Epigenetics, Glucocorticoid Signalling and Beyond. Eur. J. Clin. Investig. 2021, 51, e13625. [Google Scholar] [CrossRef]
- Pereira, S.P.; Grilo, L.F.; Tavares, R.S.; Gomes, R.M.; Ramalho-Santos, J.; Ozanne, S.E.; Matafome, P. Programming of Early Aging. In Aging; Elsevier: Amsterdam, The Netherlands, 2023; pp. 407–431. [Google Scholar]
- Holemans, K.; Aerts, L.; Van Assche, F.A. Fetal Growth Restriction and Consequences for the Offspring in Animal Models. J. Soc. Gynecol. Investig. 2003, 10, 392–399. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of Gestational Diabetes Mellitus Attributable to Overweight and Obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef]
- Li, H.P.; Chen, X.; Li, M.Q. Gestational Diabetes Induces Chronic Hypoxia Stress and Excessive Inflammatory Response in Murine Placenta. Int. J. Clin. Exp. Pathol. 2013, 6, 650. [Google Scholar]
- Fasoulakis, Z.; Koutras, A.; Antsaklis, P.; Theodora, M.; Valsamaki, A.; Daskalakis, G.; Kontomanolis, E.N. Intrauterine Growth Restriction Due to Gestational Diabetes: From Pathophysiology to Diagnosis and Management. Medicina 2023, 59, 1139. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks Associated with Obesity in Pregnancy, for the Mother and Baby: A Systematic Review of Reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zheng, Q.; Jin, L. Physiological Function of the Dynamic Oxygen Signaling Pathway at the Maternal-Fetal Interface. J. Reprod. Immunol. 2022, 151, 103626. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Carter, A.M. Fetoplacental Oxygen Homeostasis in Pregnancies with Maternal Diabetes Mellitus and Obesity. Nat. Rev. Endocrinol. 2022, 18, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and Oxidative Stress in the Perinatal Period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Mutinati, M.; Piccinno, M.; Roncetti, M.; Campanile, D.; Rizzo, A.; Sciorsci, R. Oxidative Stress during Pregnancy in the Sheep. Reprod. Domest. Anim. 2013, 48, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The Roles of Cellular Reactive Oxygen Species, Oxidative Stress and Antioxidants in Pregnancy Outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Sobrevia, L.; Valero, P.; Grismaldo, A.; Villalobos-Labra, R.; Pardo, F.; Subiabre, M.; Armstrong, G.; Toledo, F.; Vega, S.; Cornejo, M.; et al. Mitochondrial Dysfunction in the Fetoplacental Unit in Gestational Diabetes Mellitus. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165948. [Google Scholar] [CrossRef]
- Gyllenhammer, L.E.; Entringer, S.; Buss, C.; Wadhwa, P.D. Developmental Programming of Mitochondrial Biology: A Conceptual Framework and Review. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192713. [Google Scholar] [CrossRef]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental Mitochondrial Function and Structure in Gestational Disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shao, L.; Luo, X.; Mu, Y.; Xu, W.; Gao, C.; Gao, L.; Liu, J.; Cui, Y. Ultrastructure of Placenta of Gravidas with Gestational Diabetes Mellitus. Obstet. Gynecol. Int. 2015, 2015, 283124. [Google Scholar] [CrossRef]
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef]
- Fisher, J.J.; Vanderpeet, C.L.; Bartho, L.A.; McKeating, D.R.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V. Mitochondrial Dysfunction in Placental Trophoblast Cells Experiencing Gestational Diabetes Mellitus. J. Physiol. 2021, 599, 1291–1305. [Google Scholar] [CrossRef]
- Valent, A.M.; Choi, H.; Kolahi, K.S.; Thornburg, K.L. Hyperglycemia and Gestational Diabetes Suppress Placental Glycolysis and Mitochondrial Function and Alter Lipid Processing. FASEB J. 2021, 35, e21423. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Teague, A.M.; Tryggestad, J.B.; Aston, C.E.; Lyons, T.; Chernausek, S.D. Effects of Maternal Diabetes and Fetal Sex on Human Placenta Mitochondrial Biogenesis. Placenta 2017, 57, 26–32. [Google Scholar] [CrossRef]
- Abbade, J.; Klemetti, M.M.; Farrell, A.; Ermini, L.; Gillmore, T.; Sallais, J.; Tagliaferro, A.; Post, M.; Caniggia, I. Increased Placental Mitochondrial Fusion in Gestational Diabetes Mellitus: An Adaptive Mechanism to Optimize Feto-Placental Metabolic Homeostasis? BMJ Open Diabetes Res. Care 2020, 8, e000923. [Google Scholar] [CrossRef] [PubMed]
- Kolac, U.K.; Kurek Eken, M.; Ünübol, M.; Donmez Yalcin, G.; Yalcin, A. The Effect of Gestational Diabetes on the Expression of Mitochondrial Fusion Proteins in Placental Tissue. Placenta 2021, 115, 106–114. [Google Scholar] [CrossRef]
- Hori, A.; Yoshida, M.; Ling, F. Mitochondrial Fusion Increases the Mitochondrial DNA Copy Number in Budding Yeast. Genes Cells 2011, 16, 527–544. [Google Scholar] [CrossRef] [PubMed]
- McElwain, C.; McCarthy, C.M. Investigating Mitochondrial Dysfunction in Gestational Diabetes Mellitus and Elucidating If BMI Is a Causative Mediator. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 60–65. [Google Scholar] [CrossRef]
- Crovetto, F.; Lattuada, D.; Rossi, G.; Mangano, S.; Somigliana, E.; Bolis, G.; Fedele, L. A Role for Mitochondria in Gestational Diabetes Mellitus? Gynecol. Endocrinol. 2013, 29, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Hevner, K.; Abetew, D.; Sedensky, M.; Morgan, P.; Enquobahrie, D.; Williams, M. Mitochondrial DNA Copy Number and Oxidative DNA Damage in Placental Tissues from Gestational Diabetes and Control Pregnancies: A Pilot Study. Clin. Lab. 2013, 59, 655–660. [Google Scholar] [CrossRef]
- Chen, X.; Scholl, T.O. Oxidative Stress: Changes in Pregnancy and with Gestational Diabetes Mellitus. Curr. Diab. Rep. 2005, 5, 282–288. [Google Scholar] [CrossRef]
- Biri, A.; Onan, A.; Devrim, E.; Babacan, F.; Kavutcu, M.; Durak, İ. Oxidant Status in Maternal and Cord Plasma and Placental Tissue in Gestational Diabetes. Placenta 2006, 27, 327–332. [Google Scholar] [CrossRef]
- Diceglie, C.; Anelli, G.M.; Martelli, C.; Serati, A.; Lo Dico, A.; Lisso, F.; Parisi, F.; Novielli, C.; Paleari, R.; Cetin, I.; et al. Placental Antioxidant Defenses and Autophagy-Related Genes in Maternal Obesity and Gestational Diabetes Mellitus. Nutrients 2021, 13, 1303. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, Y.; Pak, Y.K.; Chung, D.; Han, Y.M.; Hong, J.S.; Jun, E.J.; Shim, J.-Y.; Choi, J.; Kim, C.J. Umbilical Cord Mesenchymal Stromal Cells Affected by Gestational Diabetes Mellitus Display Premature Aging and Mitochondrial Dysfunction. Stem Cells Dev. 2015, 24, 575–586. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key Transcription Factors in the Differentiation of Mesenchymal Stem Cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Hansen, N.S.; Strasko, K.S.; Hjort, L.; Kelstrup, L.; Houshmand-Øregaard, A.; Schrölkamp, M.; Schultz, H.S.; Scheele, C.; Pedersen, B.K.; Ling, C.; et al. Fetal Hyperglycemia Changes Human Preadipocyte Function in Adult Life. J. Clin. Endocrinol. Metab. 2017, 102, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Brar, N.; Morriseau, T.S.; Kereliuk, S.M.; Fonseca, M.A.; Cole, L.K.; Jha, A.; Xiang, B.; Hunt, K.L.; Seshadri, N.; et al. Gestational Diabetes Adversely Affects Pancreatic Islet Architecture and Function in the Male Rat Offspring. Endocrinology 2019, 160, 1907–1925. [Google Scholar] [CrossRef] [PubMed]
- Stevanović-Silva, J.; Beleza, J.; Coxito, P.; Pereira, S.; Rocha, H.; Gaspar, T.B.; Gärtner, F.; Correia, R.; Martins, M.J.; Guimarães, T.; et al. Maternal High-Fat High-Sucrose Diet and Gestational Exercise Modulate Hepatic Fat Accumulation and Liver Mitochondrial Respiratory Capacity in Mothers and Male Offspring. Metabolism 2021, 116, 154704. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Zhang, M.; Chen, L.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Supplemental Berberine in a High-Fat Diet Reduces Adiposity and Cardiac Dysfunction in Offspring of Mouse Dams with Gestational Diabetes Mellitus. J. Nutr. 2021, 151, 892–901. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine Elevates Cardiolipin in Heart of Offspring from Mouse Dams with High Fat Diet-Induced Gestational Diabetes Mellitus. Sci. Rep. 2021, 11, 15770. [Google Scholar] [CrossRef]
- Raji, S.R.; Nandini, R.J.; Ashok, S.; Anand, R.C.; Vivek, P.V.; Karunakaran, J.; Sreelatha, H.V.; Manjunatha, S.; Gopala, S. Diminished Substrate-mediated Cardiac Mitochondrial Respiration and Elevated Autophagy in Adult Male Offspring of Gestational Diabetic Rats. IUBMB Life 2021, 73, 676–689. [Google Scholar] [CrossRef]
- Huerta-Cervantes, M.; Peña-Montes, D.J.; Montoya-Pérez, R.; Trujillo, X.; Huerta, M.; López-Vázquez, M.Á.; Olvera-Cortés, M.E.; Saavedra-Molina, A. Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects. Nutrients 2020, 12, 376. [Google Scholar] [CrossRef]
- Cornejo, M.; Fuentes, G.; Valero, P.; Vega, S.; Grismaldo, A.; Toledo, F.; Pardo, F.; Moore-Carrasco, R.; Subiabre, M.; Casanello, P.; et al. Gestational Diabesity and Foetoplacental Vascular Dysfunction. Acta Physiol. 2021, 232, e13671. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity Increases Oxidative Stress in Placenta and It Is Associated With Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef]
- Mele, J.; Muralimanoharan, S.; Maloyan, A.; Myatt, L. Impaired Mitochondrial Function in Human Placenta with Increased Maternal Adiposity. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E419–E425. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Li, J.; Wang, H.; Cheng, C.; Yang, L.; Li, Q.; Deng, J.; Liang, Z.; Yin, Y.; et al. Maternal Diet-Induced Obesity Compromises Oxidative Stress Status and Angiogenesis in the Porcine Placenta by Upregulating Nox2 Expression. Oxid. Med. Cell. Longev. 2019, 2019, 2481592. [Google Scholar] [CrossRef]
- Ballesteros-Guzmán, A.K.; Carrasco-Legleu, C.E.; Levario-Carrillo, M.; Chávez-Corral, D.V.; Sánchez-Ramírez, B.; Mariñelarena-Carrillo, E.O.; Guerrero-Salgado, F.; Reza-López, S.A. Prepregnancy Obesity, Maternal Dietary Intake, and Oxidative Stress Biomarkers in the Fetomaternal Unit. Biomed. Res. Int. 2019, 2019, 5070453. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal High-Fat Feeding Primes Steatohepatitis in Adult Mice Offspring, Involving Mitochondrial Dysfunction and Altered Lipogenesis Gene Expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Borengasser, S.J.; Lau, F.; Kang, P.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Shankar, K. Maternal Obesity during Gestation Impairs Fatty Acid Oxidation and Mitochondrial SIRT3 Expression in Rat Offspring at Weaning. PLoS ONE 2011, 6, e24068. [Google Scholar] [CrossRef]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative Stress and Altered Lipid Homeostasis in the Programming of Offspring Fatty Liver by Maternal Obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 26–34. [Google Scholar] [CrossRef]
- Serafim, T.L.; Cunha-Oliveira, T.; Deus, C.M.; Sardão, V.A.; Cardoso, I.M.; Yang, S.; Odhiambo, J.F.; Ghnenis, A.B.; Smith, A.M.; Li, J.; et al. Maternal Obesity in Sheep Impairs Foetal Hepatic Mitochondrial Respiratory Chain Capacity. Eur. J. Clin. Investig. 2021, 51, e13375. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.F.P.; Woyames, J.; Miranda, R.A.; Oliveira, L.S.; Caetano, B.; Martins, I.L.; Souza, M.S.; Andrade, C.B.V.; Bento-Bernardes, T.; Bloise, F.F.; et al. Maternal Isocaloric High-Fat Diet Induces Liver Mitochondria Maladaptations and Homeostatic Disturbances Intensifying Mitochondria Damage in Response to Fructose Intake in Adult Male Rat Offspring. Mol. Nutr. Food Res. 2022, 66, 2100514. [Google Scholar] [CrossRef]
- Diniz, M.S.; Tocantins, C.; Grilo, L.F.; Pereira, S.P. The Bitter Side of Sugar Consumption: A Mitochondrial Perspective on Diabetes Development. Diabetology 2022, 3, 583–595. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rosario, F.J.; Chan, J.; Cox, L.A.; Ferchaud-Roucher, V.; Zemski-Berry, K.A.; Reusch, J.E.B.; Keller, A.C.; Powell, T.L.; Jansson, T. Maternal Obesity Causes Fetal Cardiac Hypertrophy and Alters Adult Offspring Myocardial Metabolism in Mice. J. Physiol. 2022, 600, 3169–3191. [Google Scholar] [CrossRef]
- Chiñas Merlin, A.; Gonzalez, K.; Mockler, S.; Perez, Y.; Jia, U.-T.A.; Chicco, A.J.; Ullevig, S.L.; Chung, E. Switching to a Standard Chow Diet at Weaning Improves the Effects of Maternal and Postnatal High-Fat and High-Sucrose Diet on Cardiometabolic Health in Adult Male Mouse Offspring. Metabolites 2022, 12, 563. [Google Scholar] [CrossRef]
- do Carmo, J.M.; Omoto, A.C.M.; Dai, X.; Moak, S.P.; Mega, G.S.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E.; da Silva, A.A. Sex Differences in the Impact of Parental Obesity on Offspring Cardiac SIRT3 Expression, Mitochondrial Efficiency, and Diastolic Function Early in Life. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H485–H495. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; MacFarlane, M.; Kim, K.; Patten, D.A.; Wei-LaPierre, L.; Fullerton, M.D.; Harper, M. Maternal Diet-induced Obesity Alters Muscle Mitochondrial Function in Offspring without Changing Insulin Sensitivity. FASEB J. 2019, 33, 13515–13526. [Google Scholar] [CrossRef] [PubMed]
- Ferey, J.L.A.; Boudoures, A.L.; Reid, M.; Drury, A.; Scheaffer, S.; Modi, Z.; Kovacs, A.; Pietka, T.; DeBosch, B.J.; Thompson, M.D.; et al. A Maternal High-Fat, High-Sucrose Diet Induces Transgenerational Cardiac Mitochondrial Dysfunction Independently of Maternal Mitochondrial Inheritance. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1202–H1210. [Google Scholar] [CrossRef]
- Cardenas-Perez, R.E.; Fuentes-Mera, L.; de la Garza, A.L.; Torre-Villalvazo, I.; Reyes-Castro, L.A.; Rodriguez-Rocha, H.; Garcia-Garcia, A.; Corona-Castillo, J.C.; Tovar, A.R.; Zambrano, E.; et al. Maternal Overnutrition by Hypercaloric Diets Programs Hypothalamic Mitochondrial Fusion and Metabolic Dysfunction in Rat Male Offspring. Nutr. Metab. 2018, 15, 38. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; D’Angelo, F.; Sciarretta, F.; Tatulli, G.; Tortolici, F.; Ciriolo, M.R.; Aquilano, K. Maternal High Calorie Diet Induces Mitochondrial Dysfunction and Senescence Phenotype in Subcutaneous Fat of Newborn Mice. Oncotarget 2017, 8, 83407–83418. [Google Scholar] [CrossRef]
- McCurdy, C.E.; Schenk, S.; Hetrick, B.; Houck, J.; Drew, B.G.; Kaye, S.; Lashbrook, M.; Bergman, B.C.; Takahashi, D.L.; Dean, T.A.; et al. Maternal Obesity Reduces Oxidative Capacity in Fetal Skeletal Muscle of Japanese Macaques. JCI Insight 2016, 1, e86612. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Blackmore, H.L.; Siggens, L.; Giussani, D.A.; Cross, C.M.; Foo, R.; Ozanne, S.E. The Programming of Cardiac Hypertrophy in the Offspring by Maternal Obesity Is Associated with Hyperinsulinemia, AKT, ERK, and MTOR Activation. Endocrinology 2012, 153, 5961–5971. [Google Scholar] [CrossRef]
- Saad, M.I.; Abdelkhalek, T.M.; Haiba, M.M.; Saleh, M.M.; Hanafi, M.Y.; Tawfik, S.H.; Kamel, M.A. Maternal Obesity and Malnourishment Exacerbate Perinatal Oxidative Stress Resulting in Diabetogenic Programming in F1 Offspring. J. Endocrinol. Investig. 2016, 39, 643–655. [Google Scholar] [CrossRef]
- Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Beltrán, A.A.; Ibáñez, C.A.; Vega, C.C.; Lomas-Soria, C.; Castro-Rodríguez, D.C.; Elías-López, A.L.; Nathanielsz, P.W.; et al. Maternal Obesity Accelerates Rat Offspring Metabolic Ageing in a Sex-dependent Manner. J. Physiol. 2019, 597, 5549–5563. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kwon, Y.H. Effects of Disturbed Liver Growth and Oxidative Stress of High-Fat Diet-Fed Dams on Cholesterol Metabolism in Offspring Mice. Nutr. Res. Pract. 2016, 10, 386. [Google Scholar] [CrossRef]
- Mandò, C.; De Palma, C.; Stampalija, T.; Anelli, G.M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental Mitochondrial Content and Function in Intrauterine Growth Restriction and Preeclampsia. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E404–E413. [Google Scholar] [CrossRef]
- Chiaratti, M.R.; Malik, S.; Diot, A.; Rapa, E.; Macleod, L.; Morten, K.; Vatish, M.; Boyd, R.; Poulton, J. Is Placental Mitochondrial Function a Regulator That Matches Fetal and Placental Growth to Maternal Nutrient Intake in the Mouse? PLoS ONE 2015, 10, e0130631. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Kirschenman, R.; Phillips, T.; Case, C.P.; Cooke, C.-L.M.; Davidge, S.T. Maternal Treatment with a Placental-Targeted Antioxidant (MitoQ) Impacts Offspring Cardiovascular Function in a Rat Model of Prenatal Hypoxia. Pharmacol. Res. 2018, 134, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.G.; Camm, E.J.; Modi, B.N.; Naeem, F.; Cross, C.M.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Dunster, C.; Mudway, I.S.; Kelly, F.J.; et al. Ascorbate Prevents Placental Oxidative Stress and Enhances Birth Weight in Hypoxic Pregnancy in Rats. J. Physiol. 2012, 590, 1377–1387. [Google Scholar] [CrossRef]
- Keenaghan, M.; Sun, L.; Wang, A.; Hyodo, E.; Homma, S.; Ten, V.S. Intrauterine Growth Restriction Impairs Right Ventricular Response to Hypoxia in Adult Male Rats. Pediatr. Res. 2016, 80, 547–553. [Google Scholar] [CrossRef][Green Version]
- Pereira, S.P.; Tavares, L.C.; Duarte, A.I.; Baldeiras, I.; Cunha-Oliveira, T.; Martins, J.D.; Santos, M.S.; Maloyan, A.; Moreno, A.J.; Cox, L.A.; et al. Sex-Dependent Vulnerability of Fetal Nonhuman Primate Cardiac Mitochondria to Moderate Maternal Nutrient Reduction. Clin. Sci. 2021, 135, 1103–1126. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Mampel, M.; Gonzalez-Tendero, A.; Niñerola, S.; Morén, C.; Catalán-Garcia, M.; González-Casacuberta, I.; Juárez-Flores, D.L.; Ugarteburu, O.; Matalonga, L.; Cascajo, M.V.; et al. Cardiac and Placental Mitochondrial Characterization in a Rabbit Model of Intrauterine Growth Restriction. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1157–1167. [Google Scholar] [CrossRef]
- Peterside, I.E.; Selak, M.A.; Simmons, R.A. Impaired Oxidative Phosphorylation in Hepatic Mitochondria in Growth-Retarded Rats. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E1258–E1266. [Google Scholar] [CrossRef]
- Rains, M.E.; Muncie, C.B.; Pang, Y.; Fan, L.-W.; Tien, L.-T.; Ojeda, N.B. Oxidative Stress and Neurodevelopmental Outcomes in Rat Offspring with Intrauterine Growth Restriction Induced by Reduced Uterine Perfusion. Brain Sci. 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, A.; Rajasekaran, N.S.; Valburg, C.; Ganapathy, E.; Bindra, S.; Freije, W.A. Maternal Perinatal Calorie Restriction Temporally Regulates the Hepatic Autophagy and Redox Status in Male Rat. Free Radic. Biol. Med. 2019, 130, 592–600. [Google Scholar] [CrossRef]
- Grilo, L.F.; Diniz, M.S.; Tocantins, C.; Areia, A.L.; Pereira, S.P. The Endocrine–Metabolic Axis Regulation in Offspring Exposed to Maternal Obesity—Cause or Consequence in Metabolic Disease Programming? Obesities 2022, 2, 236–255. [Google Scholar] [CrossRef]
- Grilo, L.F.; Martins, J.D.; Diniz, M.S.; Tocantins, C.; Cavallaro, C.H.; Baldeiras, I.; Cunha-Oliveira, T.; Ford, S.; Nathanielsz, P.W.; Oliveira, P.J.; et al. Maternal Hepatic Adaptations during Obese Pregnancy Encompass Lobe-Specific Mitochondrial Alterations and Oxidative Stress. Clin. Sci. 2023, 137, 1347–1372. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The Antioxidant Role of Coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Teixeira, J.; Chavarria, D.; Borges, F.; Wojtczak, L.; Wieckowski, M.R.; Karkucinska-Wieckowska, A.; Oliveira, P.J. Dietary Polyphenols and Mitochondrial Function: Role in Health and Disease. Curr. Med. Chem. 2019, 26, 3376–3406. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Croft, K.D. Dietary Polyphenols: Antioxidants or Not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary Polyphenols Suppress Chronic Inflammation by Modulation of Multiple Inflammation-Associated Cell Signaling Pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting CAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The Effects of Resveratrol on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Cao, M.; Lu, X.; Liu, G.; Su, Y.; Li, Y.; Zhou, J. Resveratrol Attenuates Type 2 Diabetes Mellitus by Mediating Mitochondrial Biogenesis and Lipid Metabolism via Sirtuin Type 1. Exp. Ther. Med. 2018, 15, 576–584. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, Y.; Liu, S.; Cheng, X.; Yang, X.; Cui, X.; Zhao, X.; Zhao, H.; Hao, M.; Li, M.; et al. Curcumin Alleviates Oxidative Stress and Inhibits Apoptosis in Diabetic Cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt Signalling Pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef] [PubMed]
- Hamidie, R.D.R.; Shibaguchi, T.; Yamada, T.; Koma, R.; Ishizawa, R.; Saito, Y.; Hosoi, T.; Masuda, K. Curcumin Induces Mitochondrial Biogenesis by Increasing Cyclic AMP Levels via Phosphodiesterase 4A Inhibition in Skeletal Muscle. Br. J. Nutr. 2021, 126, 1642–1650. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Cheng, X.; Xiang, H.; He, B.; Zhang, Q.; Peng, W. Curcumin Attenuates Isoniazid-induced Hepatotoxicity by Upregulating the SIRT1/PGC-1α/NRF1 Pathway. J. Appl. Toxicol. 2022, 42, 1192–1204. [Google Scholar] [CrossRef]
- Ren, T.; Huang, C.; Cheng, M. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxid. Med. Cell. Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef]
- Jiménez-Flores, L.; López-Briones, S.; Macías-Cervantes, M.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. A PPARγ, NF-ΚB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic Db/Db Mice Liver. Molecules 2014, 19, 8289–8302. [Google Scholar] [CrossRef]
- Zendedel, E.; Butler, A.E.; Atkin, S.L.; Sahebkar, A. Impact of Curcumin on Sirtuins: A Review. J. Cell Biochem. 2018, 119, 10291–10300. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry Juice Protects Osteocytes and Bone Precursor Cells against Oxidative Stress Partly through SIRT1. FEBS Open Bio 2019, 9, 1082–1096. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol Confers Endothelial Protection via Activation of the Antioxidant Transcription Factor Nrf2. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Wei, Z.; Shaohuan, Q.; Pinfang, K.; Chao, S. Curcumin Attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 Pathway. Cardiovasc. Ther. 2022, 2022, 3159717. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef]
- Li, C.; Tan, Y.; Wu, J.; Ma, Q.; Bai, S.; Xia, Z.; Wan, X.; Liang, J. Resveratrol Improves Bnip3-Related Mitophagy and Attenuates High-Fat-Induced Endothelial Dysfunction. Front. Cell. Dev. Biol. 2020, 8, 00796. [Google Scholar] [CrossRef]
- Brenjian, S.; Moini, A.; Yamini, N.; Kashani, L.; Faridmojtahedi, M.; Bahramrezaie, M.; Khodarahmian, M.; Amidi, F. Resveratrol Treatment in Patients with Polycystic Ovary Syndrome Decreased Pro-inflammatory and Endoplasmic Reticulum Stress Markers. Am. J. Reprod. Immunol. 2020, 83, e13186. [Google Scholar] [CrossRef]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol Attenuates TNF-α-Induced Activation of Coronary Arterial Endothelial Cells: Role of NF-ΚB Inhibition. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1694–H1699. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q.; et al. Curcumin Attenuates Collagen-Induced Rat Arthritis via Anti-Inflammatory and Apoptotic Effects. Int. Immunopharmacol. 2019, 72, 292–300. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-Targeted Antioxidants for Treatment of Parkinson’s Disease: Preclinical and Clinical Outcomes. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1282–1294. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and Potential Therapeutic Uses for N-Acetylcysteine: The Need for Conversion to Intracellular Glutathione for Antioxidant Benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, L.G.; Paulino, L.R.F.M.; Silva, B.R.; Barbalho, E.C.; Nascimento, D.R.; Neto, M.F.L.; Silva, J.R.V. N-Acetyl-Cysteine and the Control of Oxidative Stress during in Vitro Ovarian Follicle Growth, Oocyte Maturation, Embryo Development and Cryopreservation. Anim. Reprod. Sci. 2021, 231, 106801. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, G.; Jia, T.; Wang, C.; Lu, X.; Tian, L.; Yang, Q.; Zhu, C. Protection Against Post-Resuscitation Acute Kidney Injury by N-Acetylcysteine via Activation of the Nrf2/HO-1 Pathway. Front. Med. 2022, 9, 848491. [Google Scholar] [CrossRef]
- Teixeira, J.; Deus, C.M.; Borges, F.; Oliveira, P.J. Mitochondria: Targeting Mitochondrial Reactive Oxygen Species with Mitochondriotropic Polyphenolic-Based Antioxidants. Int. J. Biochem. Cell Biol. 2018, 97, 98–103. [Google Scholar] [CrossRef]
- Amorim, R.; Cagide, F.; Tavares, L.C.; Simões, R.F.; Soares, P.; Benfeito, S.; Baldeiras, I.; Jones, J.G.; Borges, F.; Oliveira, P.J.; et al. Mitochondriotropic Antioxidant Based on Caffeic Acid AntiOxCIN4 Activates Nrf2-Dependent Antioxidant Defenses and Quality Control Mechanisms to Antagonize Oxidative Stress-Induced Cell Damage. Free Radic. Biol. Med. 2022, 179, 119–132. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A.J. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Ji, Y.; Leng, Y.; Lei, S.; Qiu, Z.; Ming, H.; Zhang, Y.; Zhang, A.; Wu, Y.; Xia, Z. The Mitochondria-Targeted Antioxidant MitoQ Ameliorates Myocardial Ischemia–Reperfusion Injury by Enhancing PINK1/Parkin-Mediated Mitophagy in Type 2 Diabetic Rats. Cell Stress Chaperones 2022, 27, 353–367. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, P.; Budbazar, E.; Zhu, Q.; Sun, C.; Mo, J.; Peng, J.; Gospodarev, V.; Tang, J.; Shi, H.; et al. Mitophagy Reduces Oxidative Stress Via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) Pathway After Subarachnoid Hemorrhage in Rats. Stroke 2019, 50, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The Mitochondria-Targeted Antioxidant MitoQ Ameliorated Tubular Injury Mediated by Mitophagy in Diabetic Kidney Disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, X.; Zhang, M.; Zhu, Z.; Zhou, L.; Chen, Q.; Zhang, Q.; Ma, B. MitoQ Ameliorates Testis Injury from Oxidative Attack by Repairing Mitochondria and Promoting the Keap1-Nrf2 Pathway. Toxicol. Appl. Pharmacol. 2019, 370, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Cagide, F.; Benfeito, S.; Soares, P.; Garrido, J.; Baldeiras, I.; Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Andrade, P.B.; et al. Development of a Mitochondriotropic Antioxidant Based on Caffeic Acid: Proof of Concept on Cellular and Mitochondrial Oxidative Stress Models. J. Med. Chem. 2017, 60, 7084–7098. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Benfeito, S.; Amorim, R.; Teixeira, J.; Oliveira, P.J.; Remião, F.; Borges, F. Desrisking the Cytotoxicity of a Mitochondriotropic Antioxidant Based on Caffeic Acid by a PEGylated Strategy. Bioconjug. Chem. 2018, 29, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Basit, F.; Willems, P.H.G.M.; Wagenaars, J.A.; van de Westerlo, E.; Amorim, R.; Cagide, F.; Benfeito, S.; Oliveira, C.; Borges, F.; et al. Mitochondria-Targeted Phenolic Antioxidants Induce ROS-Protective Pathways in Primary Human Skin Fibroblasts. Free Radic. Biol. Med. 2021, 163, 314–324. [Google Scholar] [CrossRef]
- Amorim, R.; Simões, I.C.M.; Teixeira, J.; Cagide, F.; Potes, Y.; Soares, P.; Carvalho, A.; Tavares, L.C.; Benfeito, S.; Pereira, S.P.; et al. Mitochondria-Targeted Anti-Oxidant AntiOxCIN4 Improved Liver Steatosis in Western Diet-Fed Mice by Preventing Lipid Accumulation Due to Upregulation of Fatty Acid Oxidation, Quality Control Mechanism and Antioxidant Defense Systems. Redox Biol. 2022, 55, 102400. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The Importance of Nutrition in Pregnancy and Lactation: Lifelong Consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Ota, E.; Tobe-Gai, R.; Mori, R.; Farrar, D. Antenatal Dietary Advice and Supplementation to Increase Energy and Protein Intake. In Cochrane Database of Systematic Reviews; Ota, E., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Weckman, A.M.; McDonald, C.R.; Baxter, J.-A.B.; Fawzi, W.W.; Conroy, A.L.; Kain, K.C. Perspective: L-Arginine and L-Citrulline Supplementation in Pregnancy: A Potential Strategy to Improve Birth Outcomes in Low-Resource Settings. Adv. Nutr. 2019, 10, 765–777. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. L-Arginine Induces Antioxidant Response to Prevent Oxidative Stress via Stimulation of Glutathione Synthesis and Activation of Nrf2 Pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Imdad, A.; Bhutta, Z.A. Routine Iron/Folate Supplementation during Pregnancy: Effect on Maternal Anaemia and Birth Outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, E.; Ugur, A.; Baltaci, A.K.; Undar, L. Effect of Iron Supplementation on Oxidative Stress and Antioxidant Status in Iron-Deficiency Anemia. Biol. Trace Elem. Res. 2003, 96, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu. Rev. Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Hovdenak, N.; Haram, K. Influence of Mineral and Vitamin Supplements on Pregnancy Outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Swaney, P.; Thorp, J.; Allen, I. Vitamin C Supplementation in Pregnancy—Does It Decrease Rates of Preterm Birth? A Systematic Review. Am. J. Perinatol. 2013, 31, 091–098. [Google Scholar] [CrossRef]
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E Supplementation in Pregnancy. Cochrane Database Syst. Rev. 2015, 2016, CD004069. [Google Scholar] [CrossRef]
- McCauley, M.E.; van den Broek, N.; Dou, L.; Othman, M. Vitamin A Supplementation during Pregnancy for Maternal and Newborn Outcomes. Cochrane Database Syst. Rev. 2015, 2016, CD008666. [Google Scholar] [CrossRef]
- Hanrahan, V.; Gillies, K.; Biesty, L. Recruiters’ Perspectives of Recruiting Women during Pregnancy and Childbirth to Clinical Trials: A Qualitative Evidence Synthesis. PLoS ONE 2020, 15, e0234783. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.-J.; Nathanielsz, P.W.; Du, M. Resveratrol Supplementation of High-Fat Diet-Fed Pregnant Mice Promotes Brown and Beige Adipocyte Development and Prevents Obesity in Male Offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Induces Brown-like Adipocyte Formation in White Fat through Activation of AMP-Activated Protein Kinase (AMPK) A1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef]
- Rodríguez-González, G.L.; Vargas-Hernández, L.; Reyes-Castro, L.A.; Ibáñez, C.A.; Bautista, C.J.; Lomas-Soria, C.; Itani, N.; Estrada-Gutierrez, G.; Espejel-Nuñez, A.; Flores-Pliego, A.; et al. Resveratrol Supplementation in Obese Pregnant Rats Improves Maternal Metabolism and Prevents Increased Placental Oxidative Stress. Antioxidants 2022, 11, 1871. [Google Scholar] [CrossRef]
- Ding, K.-N.; Lu, M.-H.; Guo, Y.-N.; Liang, S.-S.; Mou, R.-W.; He, Y.-M.; Tang, L.-P. Resveratrol Relieves Chronic Heat Stress-Induced Liver Oxidative Damage in Broilers by Activating the Nrf2-Keap1 Signaling Pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114411. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of Resveratrol Supplementation in Regulation of Glucose Hemostasis, Inflammation and Oxidative Stress in Patients with Diabetes Mellitus Type 2: A Randomized, Placebo-Controlled Trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Yao, L.; Wan, J.; Li, H.; Ding, J.; Wang, Y.; Wang, X.; Li, M. Resveratrol Relieves Gestational Diabetes Mellitus in Mice through Activating AMPK. Reprod. Biol. Endocrinol. 2015, 13, 118. [Google Scholar] [CrossRef]
- Lu, X.; Wu, F.; Jiang, M.; Sun, X.; Tian, G. Curcumin Ameliorates Gestational Diabetes in Mice Partly through Activating AMPK. Pharm. Biol. 2019, 57, 250–254. [Google Scholar] [CrossRef]
- Xing, S.; Guo, Z.; Lang, J.; Zhou, M.; Cao, J.; He, H.; Yu, L.; Zhou, Y. N-Acetyl-L-Cysteine Ameliorates Gestational Diabetes Mellitus by Inhibiting Oxidative Stress. Gynecol. Endocrinol. 2023, 39, 2189969. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Amaro, L.B.R.; Batista Jorge, A.H.; Lelis, S.d.F.; Lelis, D.d.F.; Guimarães, A.L.S.; Santos, S.H.S.; Andrade, J.M.O. Curcumin Improves Metabolic Response and Increases Expression of Thermogenesis-Associated Markers in Adipose Tissue of Male Offspring from Obese Dams. Mol. Cell. Endocrinol. 2023, 563, 111840. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Maternal Curcumin Supplementation Ameliorates Placental Function and Fetal Growth in Mice with Intrauterine Growth Retardation†. Biol. Reprod. 2020, 102, 1090–1101. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Yu, G.; Zhang, X.; Qi, X.; Zhang, J.; Zhang, L.; Wang, T. Dietary Curcumin Supplementation Ameliorates Placental Inflammation in Rats with Intra-Uterine Growth Retardation by Inhibiting the NF-ΚB Signaling Pathway. J. Nutr. Biochem. 2022, 104, 108973. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Dietary Curcumin Supplementation Increases Antioxidant Capacity, Upregulates Nrf2 and Hmox1 Levels in the Liver of Piglet Model with Intrauterine Growth Retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Ji, S.; Jia, P.; Zhang, H.; Wang, T.; Song, Z.; Zhang, L.; Wang, T. Resveratrol Improves Hepatic Redox Status and Lipid Balance of Neonates with Intrauterine Growth Retardation in a Piglet Model. Biomed. Res. Int. 2020, 2020, 7402645. [Google Scholar] [CrossRef]
- He, J.; Niu, Y.; Wang, F.; Wang, C.; Cui, T.; Bai, K.; Zhang, J.; Zhong, X.; Zhang, L.; Wang, T. Dietary Curcumin Supplementation Attenuates Inflammation, Hepatic Injury and Oxidative Damage in a Rat Model of Intra-Uterine Growth Retardation. Br. J. Nutr. 2018, 120, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Charron, M.J.; Williams, L.; Seki, Y.; Du, X.Q.; Chaurasia, B.; Saghatelian, A.; Summers, S.A.; Katz, E.B.; Vuguin, P.M.; Reznik, S.E. Antioxidant Effects of N-Acetylcysteine Prevent Programmed Metabolic Disease in Mice. Diabetes 2020, 69, 1650–1661. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, L.; Tan, Y.; Zheng, Y.; Gui, Y. N-Acetylcysteine Protects Neonatal Mice from Ventricular Hypertrophy Induced by Maternal Obesity in a Sex-Specific Manner. Biomed. Pharmacother. 2021, 133, 110989. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Turdi, S.; Ford, S.P.; Hua, Y.; Nijland, M.J.; Zhu, M.; Nathanielsz, P.W.; Ren, J. Influence of Gestational Overfeeding on Cardiac Morphometry and Hypertrophic Protein Markers in Fetal Sheep. J. Nutr. Biochem. 2011, 22, 30–37. [Google Scholar] [CrossRef]
- Herrera, E.A.; Cifuentes-Zúñiga, F.; Figueroa, E.; Villanueva, C.; Hernández, C.; Alegría, R.; Arroyo-Jousse, V.; Peñaloza, E.; Farías, M.; Uauy, R.; et al. N-Acetylcysteine, a Glutathione Precursor, Reverts Vascular Dysfunction and Endothelial Epigenetic Programming in Intrauterine Growth Restricted Guinea Pigs. J. Physiol. 2017, 595, 1077–1092. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.; Cindrova-Davies, T.; Spiroski, A.-M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal. 2021, 34, 118–136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, M.S.; Magalhães, C.C.; Tocantins, C.; Grilo, L.F.; Teixeira, J.; Pereira, S.P. Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients 2023, 15, 4623. https://doi.org/10.3390/nu15214623
Diniz MS, Magalhães CC, Tocantins C, Grilo LF, Teixeira J, Pereira SP. Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients. 2023; 15(21):4623. https://doi.org/10.3390/nu15214623
Chicago/Turabian StyleDiniz, Mariana S., Carina C. Magalhães, Carolina Tocantins, Luís F. Grilo, José Teixeira, and Susana P. Pereira. 2023. "Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases" Nutrients 15, no. 21: 4623. https://doi.org/10.3390/nu15214623
APA StyleDiniz, M. S., Magalhães, C. C., Tocantins, C., Grilo, L. F., Teixeira, J., & Pereira, S. P. (2023). Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients, 15(21), 4623. https://doi.org/10.3390/nu15214623






