Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review
Abstract
:1. Introduction
2. Malnutrition and Aging: A Close but Unclear Link in the Elderly
3. Dysphagia in Older Subjects
4. Oral Frailty: A Detrimental Issue
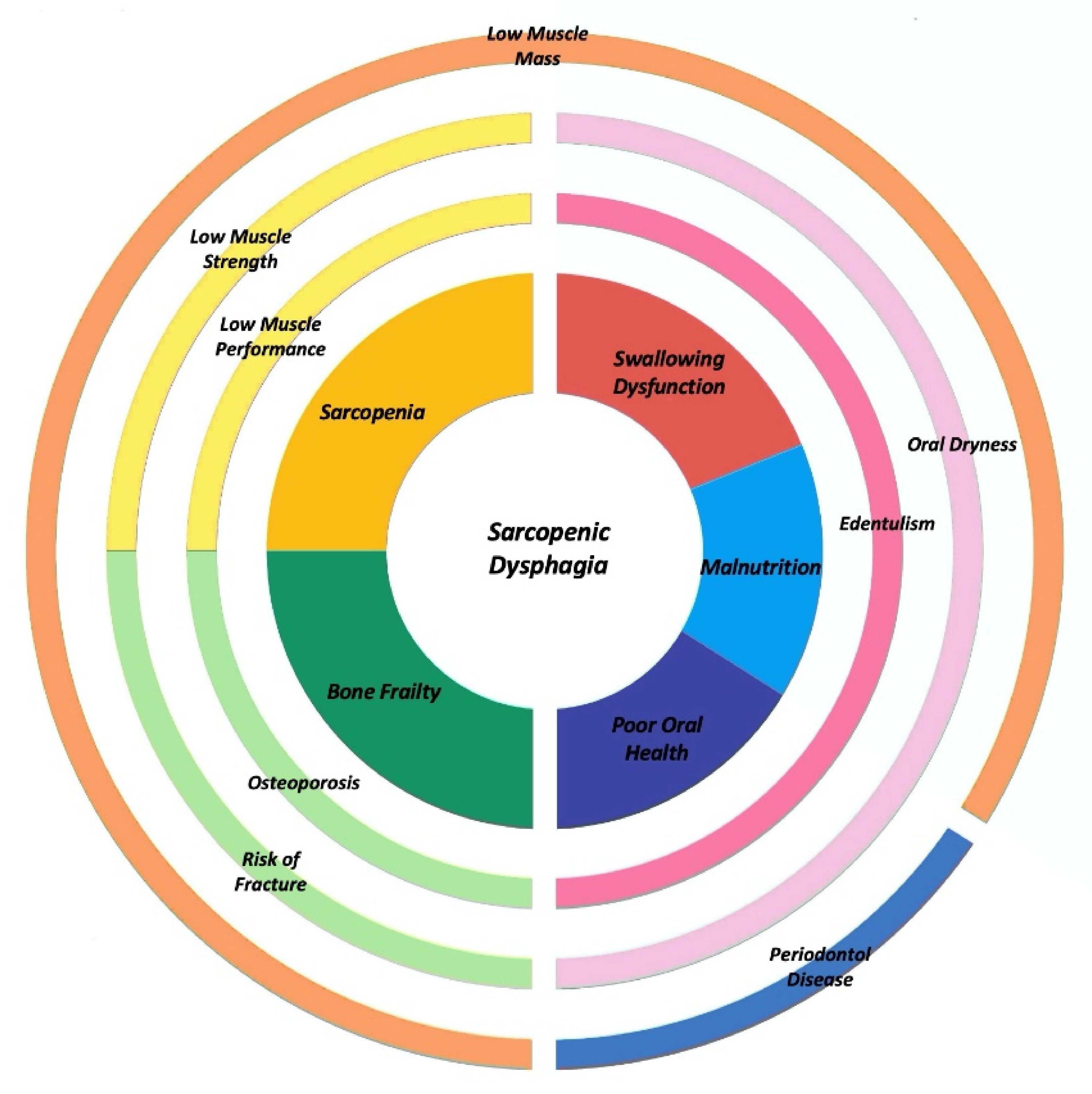
5. Sarcopenic Dysphagia: An Old and New Concept
6. Nutritional Supplementation to Counteract Sarcopenic Dysphagia
7. Oropharyngeal Rehabilitation
8. Oral Health Management for Older Subjects
9. Study Limitations
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullah, B.; Wolbring, G. Analysis of newspaper coverage of active aging through the lens of the 2002 World Health Organization Active Ageing Report: A Policy Framework and the 2010 Toronto Charter for Physical Activity: A Global Call for Action. Int. J. Environ. Res. Public Health 2013, 10, 6799–6819. [Google Scholar] [CrossRef] [Green Version]
- Branca, S.; Bennati, E.; Ferlito, L.; Spallina, G.; Cardillo, E.; Malaguarnera, M.; Motta, M. The health-care in the extreme longevity. Arch. Gerontol. Geriatr. 2009, 49, 32–34. [Google Scholar] [CrossRef]
- Hoeksema, A.R.; Spoorenberg, S.; Peters, L.L.; Meijer, H.; Raghoebar, G.M.; Vissink, A.; Visser, A. Elderly with remaining teeth report less frailty and better quality of life than edentulous elderly: A cross-sectional study. Oral. Dis. 2017, 23, 526–536. [Google Scholar] [CrossRef]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty syndrome: Implications and challenges for health care policy. Risk Manag. Healthc. Policy 2019, 12, 23–30. [Google Scholar] [CrossRef] [Green Version]
- De Sire, A.; Giachero, A.; De Santi, S.; Inglese, K.; Solaro, C. Screening dysphagia risk in 534 older patients undergoing rehabilitation after total joint replacement: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 131–136. [Google Scholar] [CrossRef]
- Fan, Y.; Ni, S.; Zhang, H. Association between Healthy Eating Index-2015 total and component food scores with osteoporosis in middle-aged and older Americans: A cross-sectional study with U.S. National Health and Nutrition Examination Survey. Osteoporos. Int. 2021, 1–9. [Google Scholar] [CrossRef]
- Leigheb, M.; de Sire, A.; Colangelo, M.; Zagaria, D.; Grassi, F.A.; Rena, O.; Ferraro, E. Sarcopenia Diagnosis: Reliability of the Ultrasound Assessment of the Tibialis Anterior Muscle as an Alternative Evaluation Tool. Diagnostics 2021, 11, 2158. [Google Scholar] [CrossRef]
- Palmer, K.; Onder, G.; Cesari, M. The geriatric condition of frailty. Eur. J. Intern. Med. 2018, 56, 1–2. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Chatterji, S. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Di Monaco, M.; Castiglioni, C.; Bardesono, F.; Milano, E.; Massazza, G. Sarcopenic obesity and function in women with subacute hip fracture: A short-term prospective study. Eur. J. Phys. Rehabil. Med. 2021, 57, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Zamboni, M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Agostini, F.; Bernetti, A.; Di Giacomo, G.; Viva, M.G.; Paoloni, M.; Mangone, M.; Masiero, S. Rehabilitative Good Practices in the Treatment of Sarcopenia: A Narrative Review. Am. J. Phys. Med. Rehabil. 2021, 100, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.; Kim, W.S.; Kim, K.W.; Han, J.W.; Jang, H.C.; Lim, S.; Paik, N.J. Sarcopenia is an Independent Risk Factor for Dysphagia in Community-Dwelling Older Adults. Dysphagia 2019, 34, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takaki, M.; Akagi, J. Decreased Skeletal Muscle Mass and Risk Factors of Sarcopenic Dysphagia: A Prospective Observational Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Baijens, L.W.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Walshe, M. European Society for Swallowing Disorders—European Union Geriatric Medicine Society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef] [Green Version]
- Dziewas, R.; Beck, A.M.; Clave, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.E.; Wirth, R. Recognizing the Importance of Dysphagia: Stumbling Blocks and Stepping Stones in the Twenty-First Century. Dysphagia 2017, 32, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clavé, P.; Hamdy, S.; Heppner, H.J.; Volkert, D. Oropharyngeal dysphagia in older persons—from pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Kuroda, R. Relationship between thinness and swallowing function in Japanese older adults: Implications for sarcopenic dysphagia. J. Am. Geriatr. Soc. 2012, 60, 1785–1786. [Google Scholar] [CrossRef]
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Baricich, A.; Riso, S.; Lippi, L.; Cisari, C.; de Sire, A. Sarcopenic dysphagia: A narrative review. Clin. Cases Miner. Bone Metab. 2019, 16, 147–149. [Google Scholar]
- Mann, T.; Heuberger, R.; Wong, H. The association between chewing and swallowing difficulties and nutritional status in older adults. Aust. Dent. J. 2013, 58, 200–206. [Google Scholar] [CrossRef]
- N’Gom, P.I.; Woda, A. Influence of impaired mastication on nutrition. J. Prosthet. Dent. 2002, 87, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Veldee, M.S.; Peth, L.D. Can protein-calorie malnutrition cause dysphagia? Dysphagia 1992, 7, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, Y.; Vijayageetha, M.; Kumar, S.G.; Rajaa, S.; Rehman, T. Prevalence of malnutrition and its associated factors among elderly population in rural Puducherry using mini-nutritional assessment questionnaire. J. Fam. Med. Prim. Care 2018, 7, 1429–1433. [Google Scholar] [CrossRef]
- Lieu, P.K.; Chong, M.S.; Seshadri, R. The impact of swallowing disorders in the elderly. Ann. Acad. Med. Singap. 2001, 30, 148–154. [Google Scholar]
- De Sire, A.; Baricich, A.; Ferrillo, M.; Migliario, M.; Cisari, C.; Invernizzi, M. Buccal hemineglect: Is it useful to evaluate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors? A cross-sectional study. Top. Stroke Rehabil. 2020, 27, 208–214. [Google Scholar] [CrossRef]
- De Sire, A.; Invernizzi, M.; Ferrillo, M.; Gimigliano, F.; Baricich, A.; Cisari, C.; Migliario, M. Functional status and oral health in patients with amyotrophic lateral sclerosis: A cross-sectional study. NeuroRehabilitation 2021, 48, 49–57. [Google Scholar] [CrossRef]
- Kloukos, D.; Roccuzzo, A.; Stähli, A.; Sculean, A.; Katsaros, C.; Salvi, G.E. Effect of combined periodontal and orthodontic treatment of tilted molars and of teeth with intra-bony and furcation defects in stage-IV periodontitis patients: A systematic review. J. Clin. Periodontol. 2021, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Toniazzo, M.P.; Amorim, P.S.; Muniz, F.; Weidlich, P. Relationship of nutritional status and oral health in elderly: Systematic review with meta-analysis. Clin. Nutr. 2018, 37, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Plassman, B.L.; Pan, W.; Wu, B. Mediation Effect of Oral Hygiene on the Relationship Between Cognitive Function and Oral Health in Older Adults. J. Gerontol. Nurs. 2016, 42, 30–37. [Google Scholar] [CrossRef] [PubMed]
- El Osta, N.; Hennequin, M.; Tubert-Jeannin, S.; Abboud Naaman, N.B.; El Osta, L.; Geahchan, N. The pertinence of oral health indicators in nutritional studies in the elderly. Clin. Nutr. 2014, 33, 316–321. [Google Scholar] [CrossRef]
- Petersen, P.E.; Yamamoto, T. Improving the oral health of older people: The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2005, 33, 81–92. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Saura-Perez, M.; Llevat-Espinosa, N. Effect of oral health dental state and risk of malnutrition in elderly people. Geriatr. Gerontol. Int. 2013, 13, 43–49. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Sieber, C.C. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Garry, P. Overview of the MNA--Its history and challenges. J. Nutr. Health Aging 2006, 10, 456–463; discussion 455–463. [Google Scholar]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Singer, P. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Fleurke, M.; Voskuil, D.; Kolmer, D. The role of the dietitian in the management of malnutrition in the elderly: A systematic review of current practices. Nutr. Diet. 2019, 77, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA(®). Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Wojzischke, J.; van Wijngaarden, J.; van den Berg, C.; Cetinyurek-Yavuz, A.; Diekmann, R.; Luiking, Y.; Bauer, J. Nutritional status and functionality in geriatric rehabilitation patients: A systematic review and meta-analysis. Eur. Geriatr. Med. 2020, 11, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Naruishi, K.; Nishikawa, Y. Swallowing impairment is a significant factor for predicting life prognosis of elderly at the end of life. Aging Clin. Exp. Res. 2018, 30, 77–80. [Google Scholar] [CrossRef]
- Hägglund, P.; Fält, A.; Hägg, M.; Wester, P.; Levring Jäghagen, E. Swallowing dysfunction as risk factor for undernutrition in older people admitted to Swedish short-term care: A cross-sectional study. Aging Clin. Exp. Res. 2019, 31, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, S.; Okamoto, T.; Takayama, M.; Urushihara, M.; Watanabe, M.; Kiriya, Y.; Kageyama, N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study). Clin. Nutr. 2017, 36, 1089–1096. [Google Scholar] [CrossRef]
- Saarela, R.K.; Soini, H.; Hiltunen, K.; Muurinen, S.; Suominen, M.; Pitkälä, K. Dentition status, malnutrition and mortality among older service housing residents. J. Nutr. Health Aging 2014, 18, 34–38. [Google Scholar] [CrossRef]
- Anastassiadou, V.; Heath, M.R. Food choices and eating difficulty among elderly edentate patients in Greece. Gerodontology 2002, 19, 17–24. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Willett, W.C.; Douglass, C.W. The impact of edentulousness on food and nutrient intake. J. Am. Dent. Assoc. 1996, 127, 459–467. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Milledge, K.L.; O’Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2020, 78, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Chrepa, V.; Shivappa, N.; Wirth, M.; Hébert, J.; Koyanagi, A.; Tyrovolas, S. Diet-borne systemic inflammation is associated with prevalent tooth loss. Clin. Nutr. 2018, 37, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, G.J.; Vanobbergen, J.; De Visschere, L.; Schols, J.; de Baat, C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: A systematic literature review. Nutrition 2009, 25, 717–722. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Yoshie, H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Keller, H.H.; Goy, R.; Kane, S.L. Validity and reliability of SCREEN II (Seniors in the community: Risk evaluation for eating and nutrition, Version II). Eur. J. Clin. Nutr. 2005, 59, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Stratton, R.J.; King, C.L.; Stroud, M.A.; Jackson, A.A.; Elia, M. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br. J. Nutr. 2006, 95, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Bai, A.V.; Agostini, F.; Bernetti, A.; Mangone, M.; Fidenzi, G.; D’Urzo, R.; Masiero, S. State of the evidence about rehabilitation interventions in patients with dysphagia. Eur. J. Phys. Rehabil. Med. 2021, 57, 900–911. [Google Scholar] [CrossRef]
- Eroğlu, A.G. Malnutrition and the heart. Turk. Pediatri. Ars. 2019, 54, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras Roubín, S.; Abu Assi, E.; Cespón Fernandez, M.; Barreiro Pardal, C.; Lizancos Castro, A.; Parada, J.A.; Íñiguez Romo, A. Prevalence and Prognostic Significance of Malnutrition in Patients with Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 76, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef]
- Cantorna, M.T.; McDaniel, K.; Bora, S.; Chen, J.; James, J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med. 2014, 239, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Blicklé, J.F. Vitamin B12 (cobalamin) deficiency in elderly patients. Cmaj 2004, 171, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Cunha, D.F.; Cunha, S.F.; Unamuno, M.R.; Vannucchi, H. Serum levels assessment of vitamin A, E, C, B2 and carotenoids in malnourished and non-malnourished hospitalized elderly patients. Clin. Nutr. 2001, 20, 167–170. [Google Scholar] [CrossRef]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; González-Gross, M. Vitamin B6 status, deficiency and its consequences—An overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar]
- Vaquero, M.P. Magnesium and trace elements in the elderly: Intake, status and recommendations. J. Nutr. Health Aging 2002, 6, 147–153. [Google Scholar] [PubMed]
- Carmel, R. Nutritional anemias and the elderly. Semin. Hematol. 2008, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutritional aspects of bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Postmenopausal osteoporosis: Assessment and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 739–757. [Google Scholar] [CrossRef]
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I signaling in muscle bone interactions. Bone 2015, 80, 79–88. [Google Scholar] [CrossRef] [Green Version]
- De Sire, A.; Invernizzi, M.; Baricich, A.; Lippi, L.; Ammendolia, A.; Grassi, F.A.; Leigheb, M. Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: A patient-tailored plan from orthopaedics to rehabilitation. World J. Orthop. 2021, 12, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Namasivayam, A.M.; Steele, C.M. Malnutrition and Dysphagia in long-term care: A systematic review. J. Nutr. Gerontol. Geriatr. 2015, 34, 1–21. [Google Scholar] [CrossRef]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damanti, S.; Azzolino, D.; Roncaglione, C.; Arosio, B.; Rossi, P.; Cesari, M. Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients 2019, 11, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logrippo, S.; Ricci, G.; Sestili, M.; Cespi, M.; Ferrara, L.; Palmieri, G.F.; Blasi, P. Oral drug therapy in elderly with dysphagia: Between a rock and a hard place! Clin. Interv. Aging 2017, 12, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebens, H.; Trupe, E.; Siebens, A.; Cook, F.; Anshen, S.; Hanauer, R.; Oster, G. Correlates and consequences of eating dependency in institutionalized elderly. J. Am. Geriatr. Soc. 1986, 34, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Dellis, S.; Papadopoulou, S.; Krikonis, K.; Zigras, F. Sarcopenic Dysphagia. A Narrative Review. J. Frailty Sarcopenia Falls 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Feng, X.; Todd, T.; Lintzenich, C.R.; Ding, J.; Carr, J.J.; Ge, Y.; Butler, S.G. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 853–860. [Google Scholar] [CrossRef]
- Nakao, Y.; Uchiyama, Y.; Honda, K.; Yamashita, T.; Saito, S.; Domen, K. Age-related composition changes in swallowing-related muscles: A Dixon MRI study. Aging Clin. Exp. Res. 2021, 33, 3205–3213. [Google Scholar] [CrossRef]
- Miyashita, T.; Kikutani, T.; Nagashima, K.; Igarashi, K.; Tamura, F. The effects of sarcopenic dysphagia on the dynamics of swallowing organs observed on videofluoroscopic swallowing studies. J. Oral Rehabil. 2020, 47, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Kunieda, K.; Fujishima, I.; Wakabayashi, H.; Ohno, T.; Shigematsu, T.; Itoda, M.; Ogawa, S. Relationship Between Tongue Pressure and Pharyngeal Function Assessed Using High-Resolution Manometry in Older Dysphagia Patients with Sarcopenia: A Pilot Study. Dysphagia 2021, 36, 33–40. [Google Scholar] [CrossRef]
- Nawaz, S.; Tulunay-Ugur, O.E. Dysphagia in the Older Patient. Otolaryngol. Clin. N. Am. 2018, 51, 769–777. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Cheney, D.M.; Siddiqui, M.T.; Litts, J.K.; Kuhn, M.A.; Belafsky, P.C. The Ability of the 10-Item Eating Assessment Tool (EAT-10) to Predict Aspiration Risk in Persons with Dysphagia. Ann. Otol. Rhinol. Laryngol. 2015, 124, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Tohara, H.; Saitoh, E.; Mays, K.A.; Kuhlemeier, K.; Palmer, J.B. Three tests for predicting aspiration without videofluorography. Dysphagia 2003, 18, 126–134. [Google Scholar] [CrossRef]
- Yagi, N.; Oku, Y.; Nagami, S.; Yamagata, Y.; Kayashita, J.; Ishikawa, A.; Takahashi, R. Inappropriate Timing of Swallow in the Respiratory Cycle Causes Breathing-Swallowing Discoordination. Front. Physiol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audag, N.; Goubau, C.; Toussaint, M.; Reychler, G. Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: A systematic review. Ther. Adv. Chronic. Dis. 2019, 10, 2040622318821622. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Giraldo-Cadavid, L.F.; Pantoja, J.A.; Forero, Y.; Gutierrez, H.M.; Bastidas, A. Aspiration in the Fiberoptic Endoscopic Evaluation of Swallowing Associated with an Increased Risk of Mortality in a Cohort of Patients Suspected of Oropharyngeal Dysphagia. Dysphagia 2019, 35, 369–377. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, E.; Chung, J.P.; Mojon, P. Successful aging—The case for prosthetic therapy. J. Public Health Dent. 2000, 60, 308–312. [Google Scholar] [CrossRef]
- Avlund, K.; Schultz-Larsen, K.; Christiansen, N.; Holm-Pedersen, P. Number of Teeth and Fatigue in Older Adults. J. Am. Geriatr. Soc. 2011, 59, 1459–1464. [Google Scholar] [CrossRef]
- Coleman, P. Improving oral health care for the frail elderly: A review of widespread problems and best practices. Geriatr. Nurs. 2002, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Taylor, G.W.; Manz, M.C.; Yoshihara, A.; Sato, M.; Muramatsu, K.; Miyazaki, H. Oral health status: Relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent. Oral Epidemiol. 2014, 42, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Punzi, L.; Sieber, C.; Bauer, J.; Reginster, J.Y.; Maggi, S.; the Task Finish Group on “Arthritis” of the European Geriatric Medicine Society. Sarcopenic osteoarthritis: A new entity in geriatric medicine? Eur. Geriatr. Med. 2018, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Kantawalla, R.F.; Dickie, S.; Suarez-Durall, P.; Enciso, R.; Mulligan, R. Association of Oral Health and Mini Nutritional Assessment in Older Adults: A Systematic Review with Meta-analyses. J. Prosthodont. Res. 2021. [Google Scholar] [CrossRef]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Edahiro, A.; Awata, S. Association between Oral Frailty and Nutritional Status among Community-Dwelling Older Adults: The Takashimadaira Study. J. Nutr. Health Aging 2020, 24, 1003–1010. [Google Scholar] [CrossRef]
- Lamy, M.; Mojon, P.; Kalykakis, G.; Legrand, R.; Butz-Jorgensen, E. Oral status and nutrition in the institutionalized elderly. J. Dent. 1999, 27, 443–448. [Google Scholar] [CrossRef]
- Anil, S.; Vellappally, S.; Hashem, M.; Preethanath, R.S.; Patil, S.; Samaranayake, L.P. Xerostomia in geriatric patients: A burgeoning global concern. J. Investig. Clin. Dent. 2016, 7, 5–12. [Google Scholar] [CrossRef]
- Liu, B.; Dion, M.R.; Jurasic, M.M.; Gibson, G.; Jones, J.A. Xerostomia and salivary hypofunction in vulnerable elders: Prevalence and etiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 52–60. [Google Scholar] [CrossRef]
- Ouanounou, A. Xerostomia in the Geriatric Patient: Causes, Oral Manifestations, and Treatment. Compend. Contin. Educ. Dent. 2016, 37, 306–311, quiz312. [Google Scholar]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health 1999, 16, 12–17. [Google Scholar]
- Nederfors, T.; Isaksson, R.; Mörnstad, H.; Dahlöf, C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population--relation to age, sex and pharmacotherapy. Community Dent. Oral. Epidemiol. 1997, 25, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Aliko, A.; Wolff, A.; Dawes, C.; Aframian, D.; Proctor, G.; Ekström, J.; Vissink, A. World Workshop on Oral Medicine VI: Clinical implications of medication-induced salivary gland dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Von Bültzingslöwen, I.; Sollecito, T.P.; Fox, P.C.; Daniels, T.; Jonsson, R.; Lockhart, P.B.; Schiødt, M. Salivary dysfunction associated with systemic diseases: Systematic review and clinical management recommendations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, S57-e1. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C.; Hegarty, A.M. An update of the etiology and management of xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 28–46. [Google Scholar] [CrossRef]
- Napeñas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef]
- Carrozzo, M. Oral diseases associated with hepatitis C virus infection. Part 1. sialadenitis and salivary glands lymphoma. Oral Dis. 2008, 14, 123–130. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Salles, C.; Chagnon, M.-C.; Feron, G.; Guichard, E.; Laboure, H.; Morzel, M.; Yven, C. In-mouth mechanisms leading to flavor release and perception. Crit. Rev. Food Sci. Nutr. 2011, 51, 67–90. [Google Scholar] [CrossRef]
- Han, P.; Suarez-Durall, P.; Mulligan, R. Dry mouth: A critical topic for older adult patients. J. Prosthodont. Res. 2015, 59, 6–19. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Vandenberghe-Descamps, M.; Feron, G.; Canon, F.; Labouré, H.; Sulmont-Rossé, C. Association between Salivary Hypofunction and Food Consumption in the Elderlies. A Systematic Literature Review. J. Nutr. Health Aging 2017, 22, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Lauritano, D.; Minervini, G.; Paparella, R.S.; Petruzzi, M.; Romano, A.; Lucchese, A. Management of denture stomatitis: A narrative review. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 113–116. [Google Scholar] [PubMed]
- Kaplan, I.; Zuk-Paz, L.; Wolff, A. Association between salivary flow rates, oral symptoms, and oral mucosal status. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 235–241. [Google Scholar] [CrossRef]
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing xerostomia and salivary gland hypofunction: Executive summary of a report from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2014, 145, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; van Winkelhoff, A.J. Periodontal infections: Understanding the complexity--consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; de Sire, A. Periodontal Disease and Vitamin D Deficiency in Pregnant Women: Which Correlation with Preterm and Low-Weight Birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef]
- Aimetti, M.; Romano, F.; Nessi, F. Microbiologic Analysis of Periodontal Pockets and Carotid Atheromatous Plaques in Advanced Chronic Periodontitis Patients. J. Periodontol. 2007, 78, 1718–1723. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Zhang, R.; Li, Y.; Wang, J.; Zhang, X.; Lin, L. Is periodontal disease a risk indicator for colorectal cancer? A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 336–347. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460s–463s. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, S. Analytical Methods for Evaluation of Immune Response in Nutrient Intervention. Nutr. Rev. 1998, 56, S27–S37. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Kuzmanova, D.; Jönsson, D.; Grant, M.; Chapple, I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol. 2000 2018, 78, 129–153. [Google Scholar] [CrossRef]
- Džopalić, T.; Božić-Nedeljković, B.; Jurišić, V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent. Eur. J. Immunol. 2021, 46, 264–269. [Google Scholar] [CrossRef]
- Meydani, S.N.; Meydani, M.; Blumberg, J.B.; Leka, L.S.; Siber, G.; Loszewski, R.; Stollar, B.D. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA 1997, 277, 1380–1386. [Google Scholar] [CrossRef]
- Boyd, L.D.; Madden, T.E. Nutrition, infection, and periodontal disease. Dent. Clin. N. Am. 2003, 47, 337–354. [Google Scholar] [CrossRef]
- Hatta, K.; Ikebe, K. Association between oral health and sarcopenia: A literature review. J. Prosthodont. Res. 2021, 65, 131–136. [Google Scholar] [CrossRef]
- Chang, H.R.; Bistrian, B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J. Parenter. Enteral Nutr. 1998, 22, 156–166. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-α With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A 2002, 57, M326–M332. [Google Scholar] [CrossRef] [Green Version]
- Payette, H.; Roubenoff, R.; Jacques, P.F.; Dinarello, C.A.; Wilson, P.W.F.; Abad, L.W.; Harris, T. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: The Framingham Heart Study. J. Am. Geriatr. Soc. 2003, 51, 1237–1243. [Google Scholar] [CrossRef]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin. Nutr. 2018, 37, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Ortega, O.; Parra, C.; Zarcero, S.; Nart, J.; Sakwinska, O.; Clavé, P. Oral health in older patients with oropharyngeal dysphagia. Age Ageing 2014, 43, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poisson, P.; Laffond, T.; Campos, S.; Dupuis, V.; Bourdel-Marchasson, I. Relationships between oral health, dysphagia and undernutrition in hospitalised elderly patients. Gerodontology 2016, 33, 161–168. [Google Scholar] [CrossRef]
- Osterberg, T.; Tsuga, K.; Rothenberg, E.; Carlsson, G.E.; Steen, B. Masticatory ability in 80-year-old subjects and its relation to intake of energy, nutrients and food items. Gerodontology 2002, 19, 95–101. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Rantanen, T.; Keskinen, M.; Meurman, J.H. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology 2004, 21, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nakayama, E.; Tohara, H.; Maeda, T.; Sugimoto, M.; Takehisa, T.; Ueda, K. Tongue Strength is Associated with Grip Strength and Nutritional Status in Older Adult Inpatients of a Rehabilitation Hospital. Dysphagia 2017, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Manes-Gravina, E.; Spaziani, L.; Landi, F.; Bernabei, R.; Marzetti, E. Bone-Muscle Crosstalk: Unraveling New Therapeutic Targets for Osteoporosis. Curr. Pharm. Des. 2017, 23, 6256–6263. [Google Scholar] [CrossRef]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Yoshimura, Y. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Pizzoferrato, M.; de Sire, R.; Ingravalle, F.; Mentella, M.C.; Petito, V.; Martone, A.M.; Gasbarrini, A. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients 2019, 11, 2281. [Google Scholar] [CrossRef] [Green Version]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.i.; Thompson, W.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.G.; Justice, J.N.; Freitas, E.C.; Kershaw, E.E.; Sparks, L.M. Adipose Tissue Quality in Aging: How Structural and Functional Aspects of Adipose Tissue Impact Skeletal Muscle Quality. Nutrients 2019, 11, 2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Invernizzi, M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Azzolino, D.; Damanti, S.; Bertagnoli, L.; Lucchi, T.; Cesari, M. Sarcopenia and swallowing disorders in older people. Aging Clin. Exp. Res. 2019, 31, 799–805. [Google Scholar] [CrossRef]
- Molfenter, S.M.; Amin, M.R.; Branski, R.C.; Brumm, J.D.; Hagiwara, M.; Roof, S.A.; Lazarus, C.L. Age-Related Changes in Pharyngeal Lumen Size: A Retrospective MRI Analysis. Dysphagia 2015, 30, 321–327. [Google Scholar] [CrossRef]
- Suzuki, M.; Koyama, S.; Kimura, Y.; Ishiyama, D.; Ohji, S.; Otobe, Y.; Yamada, M. Relationship between tongue muscle quality and swallowing speed in community-dwelling older women. Aging Clin. Exp. Res. 2019, 32, 2073–2079. [Google Scholar] [CrossRef]
- Mizuno, S.; Wakabayashi, H.; Wada, F. Rehabilitation nutrition for individuals with frailty, disability, sarcopenic dysphagia, or sarcopenic respiratory disability. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 29–36. [Google Scholar] [CrossRef]
- Ogawa, N.; Mori, T.; Fujishima, I.; Wakabayashi, H.; Itoda, M.; Kunieda, K.; Ogawa, S. Ultrasonography to Measure Swallowing Muscle Mass and Quality in Older Patients with Sarcopenic Dysphagia. J. Am. Med. Dir. Assoc. 2017, 19, 516–522. [Google Scholar] [CrossRef]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Landi, F. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed. Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef]
- Matsuo, H.; Yoshimura, Y.; Fujita, S.; Maeno, Y. Dysphagia is associated with poor physical function in patients with acute heart failure: A prospective cohort study. Aging Clin. Exp. Res. 2020, 32, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.T.; Yang, M.; Wu, H.M.; Yang, L.; Zhang, X.M.; Huang, Y. Systematic Review and Meta-Analysis of the Association between Sarcopenia and Dysphagia. J. Nutr. Health Aging 2018, 22, 1003–1009. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Ogawa, S. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Sporns, P.B.; Muhle, P.; Hanning, U.; Suntrup-Krueger, S.; Schwindt, W.; Eversmann, J.; Dziewas, R. Atrophy of Swallowing Muscles Is Associated With Severity of Dysphagia and Age in Patients With Acute Stroke. J. Am. Med. Dir. Assoc. 2017, 18, 635.e1–635.e7. [Google Scholar] [CrossRef] [PubMed]
- Can, B.; İsmagulova, N.; Enver, N.; Tufan, A.; Cinel, İ. Sarcopenic dysphagia following COVID-19 infection: A new danger. Nutr. Clin. Pract. 2021, 36, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.B.; Gilbert, R.J. The Long-Term Effects of COVID-19 on Dysphagia Evaluation and Treatment. Arch. Phys. Med. Rehabil. 2020, 101, 1662–1664. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Ruiu, P.; Paolucci, T.; Santilli, V.; Bernetti, A. Rehabilitation setting during and after Covid-19: An overview on recommendations. J. Rehabil. Med. 2021, 53, jrm00141. [Google Scholar] [CrossRef]
- Roberts, S.; Collins, P.; Rattray, M. Identifying and Managing Malnutrition, Frailty and Sarcopenia in the Community: A Narrative Review. Nutrients 2021, 13, 2316. [Google Scholar] [CrossRef]
- Kiss, N.; Bauer, J.; Boltong, A.; Brown, T.; Isenring, L.; Loeliger, J.; Findlay, M. Awareness, perceptions and practices regarding cancer-related malnutrition and sarcopenia: A survey of cancer clinicians. Support. Care Cancer 2020, 28, 5263–5270. [Google Scholar] [CrossRef]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cesari, M. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-C.; Jeng, Y.; Wu, W.-T.; Wang, T.-G.; Han, D.-S.; Özçakar, L.; Chang, K.-V. Sarcopenic Dysphagia: A Narrative Review from Diagnosis to Intervention. Nutrients 2021, 13, 4043. [Google Scholar] [CrossRef]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T. The Japanese Working Group on Sarcopenic, D. Nutritional Management Enhances the Recovery of Swallowing Ability in Older Patients with Sarcopenic Dysphagia. Nutrients 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.; Maeda, K.; Shimizu, A.; Nagami, S.; Takigawa, N.; Ueshima, J.; Suenaga, M. Association of Sarcopenic Dysphagia with Underlying Sarcopenia Following Hip Fracture Surgery in Older Women. Nutrients 2020, 12, 1365. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Bischoff, S.C. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Boirie, Y. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Nagano, A.; Maeda, K.; Koike, M.; Murotani, K.; Ueshima, J.; Shimizu, A.; Mori, N. Effects of Physical Rehabilitation and Nutritional Intake Management on Improvement in Tongue Strength in Sarcopenic Patients. Nutrients 2020, 12, 3104. [Google Scholar] [CrossRef]
- Hudson, H.M.; Daubert, C.R.; Mills, R.H. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia 2000, 15, 31–38. [Google Scholar] [CrossRef]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Rowan, K.M. Trial of the Route of Early Nutritional Support in Critically Ill Adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef]
- Lewis, S.R.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Database Syst. Rev. 2018, 6, Cd012276. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.Y. Age-Related Changes to Eating and Swallowing Impact Frailty: Aspiration, Choking Risk, Modified Food Texture and Autonomy of Choice. Geriatrics 2018, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.A.; Kimlin, M.G. Vitamin D, Aging, and the 2005 Dietary Guidelines for Americans. Nutr. Rev. 2006, 64, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; de Sire, A.; D’Andrea, F.; Carrera, D.; Renò, F.; Migliaccio, S.; Cisari, C. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: A pilot randomized controlled trial. Aging Clin. Exp. Res. 2019, 31, 1517–1524. [Google Scholar] [CrossRef]
- Iolascon, G.; Mauro, G.L.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicenter retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef]
- Gimigliano, F.; Moretti, A.; de Sire, A.; Calafiore, D.; Iolascon, G. The combination of vitamin D deficiency and overweight affects muscle mass and function in older post-menopausal women. Aging Clin. Exp. Res. 2018, 30, 625–631. [Google Scholar] [CrossRef]
- Hashida, N.; Shamoto, H.; Maeda, K.; Wakabayashi, H.; Suzuki, M.; Fujii, T. Rehabilitation and nutritional support for sarcopenic dysphagia and tongue atrophy after glossectomy: A case report. Nutrition 2017, 35, 128–131. [Google Scholar] [CrossRef]
- Yamada, Y.; Shamoto, H.; Maeda, K.; Wakabayashi, H. Home-based Combined Therapy with Rehabilitation and Aggressive Nutrition Management for a Parkinson’s Disease Patient with Sarcopenic Dysphagia: A Case Report. Prog. Rehabil. Med. 2018, 3, 20180019. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, H.; Uwano, R. Rehabilitation Nutrition for Possible Sarcopenic Dysphagia After Lung Cancer Surgery: A Case Report. Am. J. Phys. Med. Rehabil. 2016, 95, e84–e89. [Google Scholar] [CrossRef]
- Borda, M.G.; Venegas-Sanabria, L.C.; Puentes-Leal, G.A.; Garcia-Cifuentes, E.; Chavarro-Carvajal, D.A.; Cano, C.A. Oropharyngeal dysphagia in older adults: The well-known tale. Geriatr. Gerontol. Int. 2017, 17, 1031–1033. [Google Scholar] [CrossRef]
- Paolucci, T.; Cardarola, A.; Colonnelli, P.; Ferracuti, G.; Gonnella, R.; Murgia, M.; Mangone, M. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur. J. Phys. Rehabil. Med. 2020, 56, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Treatment of Sarcopenic Dysphagia with Rehabilitation and Nutritional Support: A Comprehensive Approach. J. Acad. Nutr. Diet. 2016, 116, 573–577. [Google Scholar] [CrossRef]
- Nakayama, E.; Tohara, H.; Sato, M.; Hino, H.; Sakai, M.; Nagashima, Y.; Ooshima, M. Time Course and Recovery of the Movements of Hyoid Bone and Thyroid Cartilage During Swallowing in a Patient with Sarcopenic Dysphagia. Am. J. Phys. Med. Rehabil. 2019, 99, e64–e67. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Takahashi, R.; Murakami, T. The Prevalence and Prognosis of Sarcopenic Dysphagia in Patients Who Require Dysphagia Rehabilitation. J. Nutr. Health Aging 2019, 23, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Arai, H. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr. Gerontol. Int. 2019, 19, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Uchida, K.; Jeong, S.; Yamaga, M. Effects of Nutritional Supplements on Muscle Mass and Activities of Daily Living in Elderly Rehabilitation Patients with Decreased Muscle Mass: A Randomized Controlled Trial. J. Nutr. Health Aging 2016, 20, 185–191. [Google Scholar] [CrossRef]
- Almeida, T.M.d.; Gomes, L.M.S.; Afonso, D.; Magnoni, D.; Mota, I.C.P.; França, J.Í.D.; Silva, R.G.d. Risk factors for oropharyngeal dysphagia in cardiovascular diseases. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190489. [Google Scholar] [CrossRef]
- Neloska, L.; Damevska, K.; Nikolchev, A.; Pavleska, L.; Petreska-Zovic, B.; Kostov, M. The Association between Malnutrition and Pressure Ulcers in Elderly in Long-Term Care Facility. Open Access Maced. J. Med. Sci. 2016, 4, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Byeon, H. Predicting the Swallow-Related Quality of Life of the Elderly Living in a Local Community Using Support Vector Machine. Int. J. Environ. Res. Public Health 2019, 16, 4269. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, H.; Kishima, M.; Itoda, M.; Fujishima, I.; Kunieda, K.; Ohno, T.; Ogawa, S. Diagnosis and Treatment of Sarcopenic Dysphagia: A Scoping Review. Dysphagia 2021, 36, 523–531. [Google Scholar] [CrossRef]
- Di Pede, C.; Mantovani, M.E.; Del Felice, A.; Masiero, S. Dysphagia in the elderly: Focus on rehabilitation strategies. Aging Clin. Exp. Res. 2016, 28, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Zafar, H.; Al-Eisa, E.S.; Iqbal, Z.A. Effect of posture on swallowing. Afr. Health Sci. 2017, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Namiki, C.; Hara, K.; Tohara, H.; Kobayashi, K.; Chantaramanee, A.; Nakagawa, K.; Minakuchi, S. Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin. Interv. Aging 2019, 14, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichero, J.A.Y.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Stanschus, S. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [Green Version]
- Steele, C.M.; Namasivayam-MacDonald, A.M.; Guida, B.T.; Cichero, J.A.; Duivestein, J.; Hanson, B.; Riquelme, L.F. Creation and Initial Validation of the International Dysphagia Diet Standardisation Initiative Functional Diet Scale. Arch. Phys. Med. Rehabil. 2018, 99, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-C.; Lee, T.-M.; Wu, W.-T.; Wang, T.-G.; Han, D.-S.; Chang, K.-V. Assessment of Tongue Strength in Sarcopenia and Sarcopenic Dysphagia: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 684840. [Google Scholar] [CrossRef]
- Vose, A.; Nonnenmacher, J.; Singer, M.L.; González-Fernández, M. Dysphagia Management in Acute and Sub-acute Stroke. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Carnaby, G.; Crary, M. Examining the Evidence on Neuromuscular Electrical Stimulation for Swallowing. Arch. Otolaryngol.—Head Neck Surg. 2007, 133, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Carnaby-Mann, G.; Crary, M.A.; Schmalfuss, I.; Amdur, R. ‘’Pharyngocise’’: Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 210–219. [Google Scholar] [CrossRef]
- Carnaby-Mann, G.D.; Crary, M.A. McNeill dysphagia therapy program: A case-control study. Arch. Phys. Med. Rehabil. 2010, 91, 743–749. [Google Scholar] [CrossRef]
- Crary, M.A.; Carnaby, G.D.; LaGorio, L.A.; Carvajal, P.J. Functional and physiological outcomes from an exercise-based dysphagia therapy: A pilot investigation of the McNeill Dysphagia Therapy Program. Arch. Phys. Med. Rehabil. 2012, 93, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging; National Institutes of Health; U.S. Department of Health and Human Services. Why Population. 2008. Available online: https://2001-2009.state.gov/documents/organization/81775.pdf (accessed on 25 January 2022).
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Petersen, P.E. Global policy for improvement of oral health in the 21st century--implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent. Oral Epidemiol. 2009, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Henne, K.; Taylor, J.J.; Valentine, R.A.; Conrads, G. Age-related changes in immune function (immune senescence) in caries and periodontal diseases: A systematic review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S153–S177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, R.; Smith, P.C.; Göstemeyer, G.; Schwendicke, F. Ageing, dental caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S145–S152. [Google Scholar] [CrossRef] [Green Version]
- Azarpazhooh, A.; Leake, J.L. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef] [PubMed]
- Dizdar, O.; Hayran, M.; Guven, D.C.; Yılmaz, T.B.; Taheri, S.; Akman, A.C.; Berker, E. Increased cancer risk in patients with periodontitis. Curr. Med. Res. Opin. 2017, 33, 2195–2200. [Google Scholar] [CrossRef]
- Wu, B.; Fillenbaum, G.G.; Plassman, B.L.; Guo, L. Association Between Oral Health and Cognitive Status: A Systematic Review. J. Am. Geriatr. Soc. 2016, 64, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Tonsekar, P.P.; Jiang, S.S.; Yue, G. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology 2017, 34, 151–163. [Google Scholar] [CrossRef]
- Zarb, G.A. The edentulous milieu. J. Prosthet. Dent. 1983, 49, 825–831. [Google Scholar] [CrossRef]
- Niesten, D.; van Mourik, K.; van der Sanden, W. The impact of frailty on oral care behavior of older people: A qualitative study. BMC Oral Health 2013, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Eberhard, J.; Jepsen, S.; Jervøe-Storm, P.M.; Needleman, I.; Worthington, H.V. Full-mouth treatment modalities (within 24 hours) for chronic periodontitis in adults. Cochrane Database Syst. Rev. 2015, 4, Cd004622. [Google Scholar] [CrossRef] [Green Version]
- Manresa, C.; Sanz-Miralles, E.C.; Twigg, J.; Bravo, M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018, 1, Cd009376. [Google Scholar] [CrossRef]
- Castro-Calderón, A.; Roccuzzo, A.; Ferrillo, M.; Gada, S.; González-Serrano, J.; Fonseca, M.; Molinero-Mourelle, P. Hyaluronic acid injection to restore the lost interproximal papilla: A systematic review. Acta Odontol. Scand. 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Molinero-Mourelle, P.; Ferrillo, M.; Cobo-Vázquez, C.; Sanchez-Labrador, L.; Ammendolia, A.; de Sire, A. Type I Collagen-Based Devices to Treat Nerve Injuries after Oral Surgery Procedures. A Systematic Review. Appl. Sci. 2021, 11, 3927. [Google Scholar] [CrossRef]
- Togni, L.; Mascitti, M.; Santarelli, A.; Contaldo, M.; Romano, A.; Serpico, R.; Rubini, C. Unusual Conditions Impairing Saliva Secretion: Developmental Anomalies of Salivary Glands. Front. Physiol. 2019, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.N.; Shorthose, K. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database Syst. Rev. 2007, 3, CD003782. [Google Scholar]
- Fedele, S.; Wolff, A.; Strietzel, F.; López, R.M.; Porter, S.R.; Konttinen, Y.T. Neuroelectrostimulation in treatment of hyposalivation and xerostomia in Sjögren's syndrome: A salivary pacemaker. J. Rheumatol. 2008, 35, 1489–1494. [Google Scholar]
- Sugiura, Y.; Soga, Y.; Yamabe, K.; Tsutani, S.; Tanimoto, I.; Maeda, H.; Takashiba, S. Total bacterial counts on oral mucosa after using a commercial saliva substitute in patients undergoing hematopoietic cell transplantation. Support. Care Cancer 2010, 18, 395–398. [Google Scholar] [CrossRef]
- Assery, M.K.A. Efficacy of Artificial Salivary Substitutes in Treatment of Xerostomia: A Systematic Review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S1), S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Vadcharavivad, S.; Boonroung, T. Original article. Effects of two carboxymethylcellulose-containing saliva substitutes on post-radiation xerostomia in head and neck cancer patients related to quality of life. Asian Biomed. 2013, 7, 193–202. [Google Scholar]
- Ameri, A.; Heydarirad, G.; Rezaeizadeh, H.; Choopani, R.; Ghobadi, A.; Gachkar, L. Evaluation of Efficacy of an Herbal Compound on Dry Mouth in Patients with Head and Neck Cancers: A Randomized Clinical Trial. J. Evid. Based Complement. Altern. Med. 2016, 21, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaldo, L.; Montaldo, P.; Papa, A.; Caramico, N.; Toro, G. Effects of saliva substitutes on oral status in patients with Type 2 diabetes. Diabet. Med. 2010, 27, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Carr, A.B.; Worthington, H.V. Interventions for replacing missing teeth: Partially absent dentition. Cochrane Database Syst. Rev. 2012, 2, Cd003814. [Google Scholar] [CrossRef]
- Marra, R.; Acocella, A.; Alessandra, R.; Ganz, S.D.; Blasi, A. Rehabilitation of Full-Mouth Edentulism: Immediate Loading of Implants Inserted with Computer-Guided Flapless Surgery Versus Conventional Dentures: A 5-Year Multicenter Retrospective Analysis and OHIP Questionnaire. Implant. Dent. 2017, 26, 54–58. [Google Scholar] [CrossRef]
- Allen, P.F.; McMillan, A.S.; Walshaw, D. A patient-based assessment of implant-stabilized and conventional complete dentures. J. Prosthet. Dent. 2001, 85, 141–147. [Google Scholar] [CrossRef]
- Ornstein, K.A.; DeCherrie, L.; Gluzman, R.; Scott, E.S.; Kansal, J.; Shah, T.; Soriano, T.A. Significant unmet oral health needs of homebound elderly adults. J. Am. Geriatr. Soc. 2015, 63, 151–157. [Google Scholar] [CrossRef]
- Chen, X.; Kistler, C.E. Oral Health Care for Older Adults with Serious Illness: When and How? J. Am. Geriatr. Soc. 2015, 63, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Paquette, D.W. The periodontal infection-systemic disease link: A review of the truth or myth. J. Int. Acad. Periodontol. 2003, 4, 101–109. [Google Scholar]
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84 (Suppl. S4), S135–S152. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, L.M.B.; Muniz, F.W.M.G.; Colussi, P.R.G.; Rösing, C.K.; Colussi, E.L. Nutritional assessment and associated factors in the elderly: Apopulation-based cross-sectional study. Nutrition 2018, 55–56, 104–110. [Google Scholar] [CrossRef]
- Kossioni, A.E.; Hajto-Bryk, J.; Maggi, S.; McKenna, G.; Petrovic, M.; Roller-Wirnsberger, R.E.; Müller, F. An Expert Opinion from the European College of Gerodontology and the European Geriatric Medicine Society: European Policy Recommendations on Oral Health in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. https://doi.org/10.3390/nu14050982
de Sire A, Ferrillo M, Lippi L, Agostini F, de Sire R, Ferrara PE, Raguso G, Riso S, Roccuzzo A, Ronconi G, et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients. 2022; 14(5):982. https://doi.org/10.3390/nu14050982
Chicago/Turabian Stylede Sire, Alessandro, Martina Ferrillo, Lorenzo Lippi, Francesco Agostini, Roberto de Sire, Paola Emilia Ferrara, Giuseppe Raguso, Sergio Riso, Andrea Roccuzzo, Gianpaolo Ronconi, and et al. 2022. "Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review" Nutrients 14, no. 5: 982. https://doi.org/10.3390/nu14050982
APA Stylede Sire, A., Ferrillo, M., Lippi, L., Agostini, F., de Sire, R., Ferrara, P. E., Raguso, G., Riso, S., Roccuzzo, A., Ronconi, G., Invernizzi, M., & Migliario, M. (2022). Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients, 14(5), 982. https://doi.org/10.3390/nu14050982










