The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review
Abstract
1. Introduction
1.1. Obesity and Inflammation
1.2. Influence of Dietary Habits during Childhood on Obesity and Inflammation
2. Arthritis and Inflammation
2.1. Osteoarthritis and Inflammation
2.2. Brief Pathophysiology of Rheumatoid Arthritis (RA)
2.2.1. RA and Inflammation
2.2.2. RA, Gut Dysbiosis, and Inflammation
3. Relationships between Obesity and Arthritis
4. Current Drugs for the Management of Obesity and Arthritis
5. Research Methodology
6. Obesity and Arthritis Management
6.1. Ayurvedic Medicines against Arthritis and Obesity
6.2. Essential Oils for Use against Arthritis and Obesity
6.3. Medicinal Plants Used to Treat Obesity and Arthritis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 August 2021).
- Kortt, M.; Baldry, J. The association between musculoskeletal disorders and obesity. Aust. Health Rev. 2002, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Després, J.-P. Obésité et maladies cardiovasculaires. M/S Méd. Sci. 2003, 19, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.Y. Obesity and type 2 diabetes mellitus. Integr. Obes. Diabetes 2018, 4, 1–2. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Chang, M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2010, 13, 71–76. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef]
- Redinger, R.N. The pathophysiology of obesity and its clinical manifestations. Gastroenterol. Hepatol. 2007, 3, 856–863. [Google Scholar]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Knebel, B.; Fahlbusch, P.; Poschmann, G.; Dille, M.; Wahlers, N.; Stühler, K.; Hartwig, S.; Lehr, S.; Schiller, M.; Jacob, S.; et al. Adipokinome signatures in obese mouse models reflect adipose tissue health and are associated with serum lipid composition. Int. J. Mol. Sci. 2019, 20, 2559. [Google Scholar] [CrossRef]
- Eissing, L.; Scherer, T.; Tödter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef]
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and non-alcoholic fatty liver disease: Multiple interactions. Int. J. Mol. Sci. 2017, 18, 1649. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gomez-Ambrosi, J.; Muruzabal, F.J.; Burrell, M. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Metab. 2001, 280, E827–E847. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Das, U. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Hill, J.O. A new way of looking at obesity. Nutrition 2001, 17, 975–976. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Ciotola, M.; Di Palo, C.; Grella, E.; Nicoletti, G.; Giugliano, D. Weight loss reduces interleukin-18 levels in obese women. J. Clin. Endocrinol. Metab. 2002, 87, 3864–3866. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Méd. Bras. 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Burris, A.D.; Pizzarello, C.; Järvinen, K.M. Immunologic components in human milk and allergic diseases with focus on food allergy. Semin. Perinatol. 2020, 45, 151386. [Google Scholar] [CrossRef]
- Dain, A.; Repossi, G.; Diaz-Gerevini, G.T.; Vanamala, J.; Das, U.N.; Eynard, A.R. Long chain polyunsaturated fatty acids (LCPUFAs) and nordihydroguaiaretic acid (NDGA) modulate metabolic and inflammatory markers in a spontaneous type 2 diabetes mellitus model (Stillman Salgado rats). Lipids Health Dis. 2016, 15, 205. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids and diabetes mellitus. Am. J. Clin. Nutr. 2002, 75, 780–781. [Google Scholar] [CrossRef][Green Version]
- Hanson, L.; Korotkova, M. The role of breastfeeding in prevention of neonatal infection. Semin. Neonatol. 2002, 7, 275–281. [Google Scholar] [CrossRef]
- Quinello, C.; Quintilio, W.; Carneiro-Sampaio, M.; Palmeira, P. Passive Acquisition of protective antibodies reactive with Bordetella pertussis in newborns via placental transfer and breast-feeding. Scand. J. Immunol. 2010, 72, 66–73. [Google Scholar] [CrossRef]
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2012, 109, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Q. Adipokines: New therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Senthelal, S.; Li, J.; Goyal, A.; Bansal, P.; Thomas, M.A. Arthritis. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wallace, J.L. Polypharmacy of osteoarthritis: The perfect intestinal storm. Am. J. Dig. Dis. 2013, 58, 3088–3093. [Google Scholar] [CrossRef]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Res. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- So, A. Developments in the scientific and clinical understanding of gout. Arthritis Res. Ther. 2008, 10, 221. [Google Scholar] [CrossRef]
- Goo, B.; Lee, J.; Park, C.; Yune, T.; Park, Y. Bee venom alleviated edema and pain in monosodium urate crystals-induced gouty arthritis in rat by inhibiting inflammation. Toxins 2021, 13, 661. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 48–56. [Google Scholar]
- Zhao, L.; Xing, R.; Wang, P.; Zhang, N.; Yin, S.; Li, X.; Zhang, L. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol. Med. Rep. 2018, 17, 5463–5469. [Google Scholar] [CrossRef]
- Denoble, A.E.; Huffman, K.M.; Stabler, T.V.; Kelly, S.J.; Hershfield, M.S.; McDaniel, G.E.; Coleman, R.E.; Kraus, V.B. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA 2011, 108, 2088–2093. [Google Scholar] [CrossRef]
- Fiddis, R.W.; Vlachos, N.; Calvert, P.D. Studies of urate crystallisation in relation to gout. Ann. Rheum. Dis. 1983, 42 (Suppl. 1), 12–15. [Google Scholar] [CrossRef]
- Wilson, L.; Saseen, J.J. Gouty Arthritis: A review of acute management and prevention. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 906–922. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European league against rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2019, 79, 31–38. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Reginato, A.M.; Olsen, B.R. The role of structural genes in the pathogenesis of osteoarthritic disorders. Arthritis Res. Ther. 2002, 4, 337–345. [Google Scholar] [CrossRef]
- Held, F.P.; Blyth, F.; Gnjidic, D.; Hirani, V.; Naganathan, V.; Waite, L.M.; Seibel, M.J.; Rollo, J.; Handelsman, D.J.; Cumming, R.G.; et al. Association rules analysis of comorbidity and multimorbidity: The concord health and aging in men project. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 625–631. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- Wang, Z.; Singh, A.; Jones, G.; Winzenberg, T.; Ding, C.; Chopra, A.; Das, S.; Danda, D.; Laslett, L.; Antony, B. Efficacy and safety of turmeric extracts for the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomised controlled trials. Curr. Rheumatol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Hunt, R.H. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. N. Am. 2010, 39, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C. Piroxicam-β-cyclodextrin: A GI safer piroxicam. Curr. Med. Chem. 2013, 20, 2415–2437. [Google Scholar] [CrossRef] [PubMed]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef]
- Van Laar, M.; Pergolizzi, J.V., Jr.; Mellinghoff, H.-U.; Merchante, I.M.; Nalamachu, S.; O’Brien, J.; Perrot, S.; Raffa, R.B. Pain treatment in arthritis-related pain: Beyond NSAIDs. Open Rheumatol. J. 2012, 6, 320–330. [Google Scholar] [CrossRef]
- Courtney, P. Key questions concerning paracetamol and NSAIDs for osteoarthritis. Ann. Rheum. Dis. 2002, 61, 767–773. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2015, 45, S22–S27. [Google Scholar] [CrossRef]
- Da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef]
- Solomon, D.H.; Husni, M.E.; Mph, K.E.W.; Rn, L.M.W.; Borer, J.S.; Graham, D.Y.; Libby, P.; Lincoff, A.M.; Lüscher, T.F.; Menon, V.; et al. Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis. Arthritis Rheumatol. 2017, 70, 537–546. [Google Scholar] [CrossRef]
- Paul, A.K.; Gueven, N.; Dietis, N. Morphine dosing strategy plays a key role in the generation and duration of the produced antinociceptive tolerance. Neuropharmacology 2017, 121, 158–166. [Google Scholar] [CrossRef]
- Malfait, A.-M.; Schnitzer, T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 654–664. [Google Scholar] [CrossRef]
- Ragni, E.; Mangiavini, L.; Viganò, M.; Brini, A.T.; Peretti, G.M.; Banfi, G.; De Girolamo, L. Management of osteoarthritis during the COVID-19 pandemic. Clin. Pharmacol. Ther. 2020, 108, 719–729. [Google Scholar] [CrossRef]
- The Royal Australian College of General Practitioners. Guideline for the Management of Knee and Hip Osteoarthritis, 2nd ed.; The Royal Australian College of General Practitioners: East Melbourne, Australia, 2018. [Google Scholar]
- Alamanda, V.K.; Wally, M.K.; Seymour, R.B.; Springer, B.D.; Hsu, J.R.; Beuhler, M.; Bosse, M.J.; Gibbs, M.; Griggs, C.; Jarrett, S.; et al. Prevalence of opioid and benzodiazepine prescriptions for osteoarthritis. Arthritis Care Res. 2019, 72, 1081–1086. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Paul, A.K.; Lewis, R.J. Pain management in older adults: Facts to consider. Pain 2022, 163, e497–e498. [Google Scholar] [CrossRef]
- Mushtaq, S.; Choudhary, R.; Scanzello, C.R. Non-surgical treatment of osteoarthritis-related pain in the elderly. Curr. Rev. Musculoskelet. Med. 2011, 4, 113–122. [Google Scholar] [CrossRef][Green Version]
- Serhal, L.; Lwin, M.N.; Holroyd, C.; Edwards, C.J. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 2020, 19, 102528. [Google Scholar] [CrossRef]
- Xu, L.; Feng, X.; Tan, W.; Gu, W.; Guo, D.; Zhang, M.; Wang, F. IL-29 enhances Toll-like receptor-mediated IL-6 and IL-8 production by the synovial fibroblasts from rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R170. [Google Scholar] [CrossRef]
- Thompson, C.; Davies, R.; Choy, E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine 2016, 86, 92–99. [Google Scholar] [CrossRef]
- Kishimoto, T. Discovery of IL-6 and Development of anti-IL-6R antibody. Keio J. Med. 2019, 68, 96. [Google Scholar] [CrossRef]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81. [Google Scholar] [CrossRef]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Szodoray, P.; Zeher, M. Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 2015, 50, 1–17. [Google Scholar] [CrossRef]
- McGarry, T.; Veale, D.J.; Gao, W.; Orr, C.; Fearon, U.; Connolly, M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 153. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Williams, L.; McCann, F.E.; Midwood, K.S. Investigating the role of toll-like receptors in models of arthritis. In Toll-Like Receptors; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1390, pp. 351–381. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Matrix metalloproteinases and synovial joint pathology. Prog. Mol. Biol. Transl. Sci. 2017, 148, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): A complex story about association and causality. Front. Immunol. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Favalli, E.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Raimondo, M.G.; Meroni, P.L. Sex and management of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2018, 56, 333–345. [Google Scholar] [CrossRef]
- Islander, U.; Jochems, C.; Lagerquist, M.K.; Forsblad-D’Elia, H.; Carlsten, H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol. Cell. Endocrinol. 2011, 335, 14–29. [Google Scholar] [CrossRef]
- Fert-Bober, J.; Darrah, E.; Andrade, F. Insights into the study and origin of the citrullinome in rheumatoid arthritis. Immunol. Rev. 2019, 294, 133–147. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- He, X.; Zhao, S.; Li, Y. Faecalibacterium prausnitzii: A next-generation probiotic in gut disease improvement. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6666114. [Google Scholar] [CrossRef]
- Chu, X.-J.; Cao, N.-W.; Zhou, H.-Y.; Meng, X.; Guo, B.; Zhang, H.-Y.; Li, B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology 2020, 60, 1054–1066. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshida, M.; Murakami, Y.; Ogiwara, T.; Shoji, M.; Kobayashi, S.; Watanabe, S.; Machino, M.; Fujisawa, S. The Relationship of Prevotella intermedia, Prevotella nigrescens and Prevotella melaninogenica in the supragingival plaque of children, caries and oral malodor. J. Clin. Pediatr. Dent. 2008, 32, 195–200. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and rheumatoid arthritis: The same inflammatory mediators? Mediat. Inflamm. 2019, 2019, 6034546. [Google Scholar] [CrossRef]
- Paul, A.K.; Paul, A.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Nissapatorn, V.; Pereira, M.L.; Wilairatana, P.; Rahmatullah, M. Probiotics and amelioration of rheumatoid arthritis: Significant roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms 2021, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.-M.; Fu, S.-M.; He, J.-J.; Zhang, M. Prevotella intermedia induces prostaglandin E2 via multiple signaling pathways. J. Dent. Res. 2010, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Drago, L.; Zuccotti, G.V.; Romanò, C.L.; Goswami, K.; Villafañe, J.H.; Mattina, R.; Parvizi, J. Oral–gut microbiota and arthritis: Is there an evidence-based axis? J. Clin. Med. 2019, 8, 1753. [Google Scholar] [CrossRef]
- Jung, H.; Jung, S.M.; Rim, Y.A.; Park, N.; Nam, Y.; Lee, J.; Park, S.-H.; Ju, J.H. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS ONE 2017, 12, e0188698. [Google Scholar] [CrossRef]
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 2012, 189, 3178–3187. [Google Scholar] [CrossRef]
- Salaffi, F.; Farah, S.; Di Carlo, M. Frailty syndrome in rheumatoid arthritis and symptomatic osteoarthritis: An emerging concept in rheumatology. Acta Bio-Med. Atenei Parm. 2020, 91, 274–296. [Google Scholar] [CrossRef]
- Han, T.; Tajar, A.; Lean, M.E.J. Obesity and weight management in the elderly. Br. Med Bull. 2011, 97, 169–196. [Google Scholar] [CrossRef]
- Han, T.; Wu, F.; Lean, M. Obesity and weight management in the elderly: A focus on men. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 509–525. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-α and obesity. TNF Pathophysiol. 2010, 11, 145–156. [Google Scholar] [CrossRef]
- Paul, A.K.; Hossain, K.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Pereira, M.D.L.; et al. Does oxidative stress management help alleviation of COVID-19 symptoms in patients experiencing diabetes? Nutrients 2022, 14, 321. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef]
- Liu, Y.; Hazlewood, G.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of obesity on remission and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2016, 69, 157–165. [Google Scholar] [CrossRef]
- Moroni, L.; Farina, N.; Dagna, L. Obesity and its role in the management of rheumatoid and psoriatic arthritis. Clin. Rheumatol. 2020, 39, 1039–1047. [Google Scholar] [CrossRef]
- Dar, L.; Tiosano, S.; Watad, A.; Bragazzi, N.L.; Zisman, D.; Comaneshter, D.; Cohen, A.; Amital, H. Are obesity and rheumatoid arthritis interrelated? Int. J. Clin. Pr. 2017, 72, e13045. [Google Scholar] [CrossRef]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar]
- Coggon, D.; Reading, I.; Croft, P.; McLaren, M.; Barrett, D.; Cooper, C. Knee osteoarthritis and obesity. Int. J. Obes. 2001, 25, 622–627. [Google Scholar] [CrossRef]
- Kaeley, G.S.; MacCarter, D.K.; Pangan, A.L.; Wang, X.; Kalabic, J.; Ranganath, V.K. Clinical Responses and synovial vascularity in obese rheumatoid arthritis patients treated with adalimumab and methotrexate. J. Rheumatol. 2018, 45, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Stebbings, S.; Treharne, G.J. Fatigue in rheumatic disease: An overview. Int. J. Clin. Rheumatol. 2010, 5, 487. [Google Scholar] [CrossRef]
- Vlietstra, L.; Stebbings, S.; Meredith-Jones, K.; Abbott, J.H.; Treharne, G.; Waters, D.L. Sarcopenia in osteoarthritis and rheumatoid arthritis: The association with self-reported fatigue, physical function and obesity. PLoS ONE 2019, 14, e0217462. [Google Scholar] [CrossRef]
- Tournadre, A.; Pereira, B.; Gossec, L.; Soubrier, M.; Dougados, M. Impact of comorbidities on fatigue in rheumatoid arthritis patients: Results from a nurse-led program for comorbidities management (COMEDRA). Jt. Bone Spine 2018, 86, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Van Beers-Tas, M.H.; Turk, S.A.; Van Schaardenburg, D. How does established rheumatoid arthritis develop, and are there possibilities for prevention? Best Pr. Res. Clin. Rheumatol. 2015, 29, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.; Kondrup, J.; Høie, L.H.; Andersen, V.; Kristensen, J.H.; Heitmann, B.L. Weight reduction in obese patients with rheumatoid arthritis, with preservation of body cell mass and improvement of physical fitness. Clin. Exp. Rheumatol. 1996, 14, 289–293. [Google Scholar] [PubMed]
- Gudbergsen, H.; Boesen, M.; Lohmander, S.; Christensen, R.; Henriksen, M.; Bartels, E.; Rindel, L.; Aaboe, J.; Danneskiold-Samsøe, B.; Riecke, B.; et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthr. Cartil. 2012, 20, 495–502. [Google Scholar] [CrossRef]
- NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Prescription Medications to Treat Overweight & Obesity. Available online: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 15 August 2021).
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Lei, X.; Ruan, J.; Lai, C.; Sun, Z.; Yang, X. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: A systematic review and meta-analysis. Obesity 2021, 29, 985–994. [Google Scholar] [CrossRef]
- Siebenhofer, A.; Winterholer, S.; Jeitler, K.; Horvath, K.; Berghold, A.; Krenn, C.; Semlitsch, T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst. Rev. 2021, 2021, CD007654. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-term efficacy and safety of anti-obesity treatment: Where do we stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Di Nicolantonio, J.J.; Chatterjee, S.; O’Keefe, J.H.; Meier, P. Lorcaserin for the treatment of obesity? A closer look at its side effects. Open Heart 2014, 1, e000173. [Google Scholar] [CrossRef]
- Paul, A.; Gueven, N.; Dietis, N. Profiling the effects of repetitive morphine administration on motor behavior in rats. Molecules 2021, 26, 4355. [Google Scholar] [CrossRef]
- Paul, A.K.; Gueven, N.; Dietis, N. Data on prolonged morphine-induced antinociception and behavioral inhibition in older rats. Data Brief 2018, 19, 183–188. [Google Scholar] [CrossRef]
- Paul, A.K.; Gueven, N.; Dietis, N. Age-dependent antinociception and behavioral inhibition by morphine. Pharmacol. Biochem. Behav. 2018, 168, 8–16. [Google Scholar] [CrossRef]
- Andreasen, P.B.; Hutters, L. Paracetamol (acetaminophen) clearance in patients with cirrhosis of the liver. Acta Med. Scand. 2009, 205, 99–105. [Google Scholar] [CrossRef]
- Ochs, H.R.; Schuppan, U.; Greenblatt, D.J.; Abernethy, D.R. Reduced distribution and clearance of acetaminophen in patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1983, 5, 697–699. [Google Scholar] [CrossRef]
- Verpeut, J.L.; Bello, N.T. Drug safety evaluation of naltrexone/bupropion for the treatment of obesity. Expert Opin. Drug Saf. 2014, 13, 1–11. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Paron, E.; Burrows, M.; Blavignac, J.; Gould, E.; Camacho, F.; Barakat, M. Psychiatric Safety and Weight loss efficacy of naltrexone/bupropion as add-on to antidepressant therapy in patients with obesity or overweight. J. Affect. Disord. 2021, 289, 167–176. [Google Scholar] [CrossRef]
- Onge, E.S.; Miller, S.A.; Motycka, C. Liraglutide (Saxenda®) as a treatment for obesity. Food Nutr. Sci. 2016, 07, 227–235. [Google Scholar] [CrossRef][Green Version]
- Therapeutic Guidelines Limited. Principles of Nonsteroidal Anti-Inflammatory Drug Use for Musculoskeletal Conditions in Adults. In eTG Complete Melbourne; Therapeutic Guidelines Limited: West Melbourne, Australia. Available online: https://tgldcdp.tg.org.au/index (accessed on 29 December 2020).
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Bramming, M.; Becker, U.; Jørgensen, M.B.; Neermark, S.; Bisgaard, T.; Tolstrup, J.S. Bariatric surgery and risk of alcohol use disorder: A register-based cohort study. Int. J. Epidemiol. 2020, 49, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2016, 7, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Chopra, A. Ayurvedic medicine and arthritis. Rheum. Dis. Clin. N. Am. 2000, 26, 133–144. [Google Scholar] [CrossRef]
- Basnyat, S.; Kolasinski, S.L. Ayurvedic medicine for rheumatoid arthritis. Curr. Rheumatol. Rep. 2014, 16, 1–6. [Google Scholar] [CrossRef]
- Ali, A.M.T.; Agrawal, A.; Lulu, S.S.; Priya, A.M.; Vino, S. RAACFDb: Rheumatoid arthritis ayurvedic classical formulations database. J. Ethnopharmacol. 2017, 197, 87–91. [Google Scholar] [CrossRef]
- Prasad, S.; Kulshreshtha, A.; Lall, R.; Gupta, S.G. Inflammation and ROS in arthritis: Management by Ayurvedic Medicinal Plants. Food Funct. 2021, 12, 8227–8247. [Google Scholar] [CrossRef]
- Kumar, G.; Srivastava, A.; Sharma, S.K.; Gupta, Y.K. Safety evaluation of mercury based Ayurvedic formulation (Sidh Makardhwaj) on brain cerebrum, liver & kidney in rats. Indian J. Med. Res. 2014, 139, 610–618. [Google Scholar]
- Kessler, C.S.; Pinders, L.; Michalsen, A.; Cramer, H. Ayurvedic interventions for osteoarthritis: A systematic review and meta-analysis. Rheumatol. Int. 2014, 35, 211–232. [Google Scholar] [CrossRef]
- Park, J.; Ernst, E. Ayurvedic medicine for rheumatoid arthritis: A systematic review. Semin. Arthritis Rheum. 2005, 34, 705–713. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Srivastava, A.; Sharma, S.K.; Kumar, G.; Rao, T.D. Efficacy & safety evaluation of Ayurvedic treatment (Ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: A pilot prospective study. Indian J. Med. Res. 2015, 141, 100–106. [Google Scholar] [CrossRef]
- Wang, M.Z.; Jones, G.; Winzenberg, T.; Cai, M.G.; Laslett, L.L.; Aitken, D.; Hopper, I.; Singh, M.A.; Jones, R.; Fripp, J.; et al. Effectiveness of Curcuma longa Extract for the treatment of symptoms and effusion–synovitis of knee osteoarthritis. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef]
- Raj, J.P.; Venkatachalam, S.; Racha, P.; Bhaskaran, S.; Amaravati, R.S. Effect of Turmacin supplementation on joint discomfort and functional outcome among healthy participants—A randomized placebo-controlled trial. Complement. Ther. Med. 2020, 53, 102522. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Henrotin, Y.; Malaise, M.; Wittoek, R.; De Vlam, K.; Brasseur, J.-P.; Luyten, F.P.; Jiangang, Q.; Berghe, M.V.D.; Uhoda, R.; Bentin, J.; et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: A double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res. Ther. 2019, 21, 179. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia Serrata, A Potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Kulkarni, P.D.; Damle, N.D.; Singh, S.; Yadav, K.S.; Ghante, M.R.; Bhaskar, V.H.; Hingorani, L.; Gota, V.S. Double-blind trial of solid lipid Boswellia serrata particles (SLBSP) vs. standardized Boswellia serrata gum extract (BSE) for osteoarthritis of knee. Drug Metab. Pers. Ther. 2020, 35. [Google Scholar] [CrossRef]
- Chopra, A.; Saluja, M.; Tillu, G.; Venugopalan, A.; Narsimulu, G.; Handa, R.; Bichile, L.; Raut, A.; Sarmukaddam, S.; Patwardhan, B. Comparable efficacy of standardized Ayurveda formulation and hydroxychloroquine sulfate (HCQS) in the treatment of rheumatoid arthritis (RA): A randomized investigator-blind controlled study. Clin. Rheumatol. 2011, 31, 259–269. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kumar, K.; Kumar, A.; Mishra, H. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi)—Validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010, 1, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Saluja, M.; Tillu, G.; Sarmukkaddam, S.; Venugopalan, A.; Narsimulu, G.; Handa, R.; Sumantran, V.; Raut, A.; Bichile, L.; et al. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: A randomized, double-blind, controlled equivalence drug trial. Rheumatology 2013, 52, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, S.J.; Kim, H. A 12 week, randomized, double-blind, placebo-controlled clinical trial for the evaluation of the efficacy and safety of HT083 on mild osteoarthritis. Medicine 2020, 99, e20907. [Google Scholar] [CrossRef] [PubMed]
- Sairkar, P.K.; Sharma, A.; Shukla, N.P. SCAR marker for identification and discrimination of Commiphora wightii and C. myrrha. Mol. Biol. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef][Green Version]
- Paramdeep, G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of knee. Indian J. Physiol. Pharmacol. 2014, 57, 177–183. [Google Scholar]
- Bode, A.M.; Dong, Z. The amazing and mighty ginger. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Karegar, S.J. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Ricci, M.; Micheloni, G.M.; Berti, M.; Perusi, F.; Sambugaro, E.; Vecchini, E.; Magnan, B. Clinical comparison of oral administration and viscosupplementation of hyaluronic acid (HA) in early knee osteoarthritis. Musculoskelet. Surg. 2016, 101, 45–49. [Google Scholar] [CrossRef]
- Kizhakkedath, R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol. Med. Rep. 2013, 8, 1542–1548. [Google Scholar] [CrossRef]
- Pereira, L.M.S.; Gomes, S.T.M.; Ishak, R.; Vallinoto, A.C.R. Regulatory T cell and forkhead box protein 3 as modulators of immune homeostasis. Front. Immunol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Wang, J.; Fathman, J.W.; Lugo-Villarino, G.; Scimone, L.; Von Andrian, U.; Dorfman, D.M.; Glimcher, L.H. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J. Clin. Investig. 2006, 116, 414–421. [Google Scholar] [CrossRef]
- Ecoeur, F.; Weiss, J.; Kaupmann, K.; Hintermann, S.; Orain, D.; Guntermann, C. Antagonizing retinoic acid-related-orphan receptor gamma sctivity blocks the T helper 17/interleukin-17 pathway leading to attenuated pro-inflammatory human keratinocyte and skin responses. Front. Immunol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Kulkarni, R.; Patki, P.; Jog, V.; Gandage, S.; Patwardhan, B. Treatment of osteoarthritis with a herbomineral formulation: A double-blind, placebo-controlled, cross-over study. J. Ethnopharmacol. 1991, 33, 91–95. [Google Scholar] [CrossRef]
- Paul, A.K.; Al Arif, H.; Seraj, S.; Nahar, A.; Nasrin, D.; Chowdhury, M.H.; Islam, F.; Jahan, R.; Anwarul Bashar, A.B.M.; Freedman, R.; et al. A survey of plant items eaten by the low income groups of the rural population of Talbunia village in Bagerhat district, Bangladesh with an account of their folk medicinal applications. Am. Eurasian J. Sustain. Agric. 2011, 5, 132–144. [Google Scholar]
- Ali, K.; Ashraf, A.; Biswas, N.N. Analgesic, anti-inflammatory and anti-diarrheal activities of ethanolic leaf extract of Typhonium trilobatum L. Schott. Asian Pac. J. Trop. Biomed. 2012, 2, 722–726. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Cao, Y.; Huang, T.; Song, D.-X.; Tao, H.-R. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int. J. Mol. Med. 2018, 42, 2604–2614. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Sampath, V.; Pai, S.; Babu, U.; Lobo, R. Antiarthritic medicinal plants: A review. Res. J. Pharm. Technol. 2019, 12, 375–381. [Google Scholar] [CrossRef]
- Vetal, S.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Sci. Hum. Wellness 2013, 2, 59–67. [Google Scholar] [CrossRef]
- Rathi, B.; Bodhankar, S.; Mohan, V.; Thakurdesai, P. Ameliorative effects of a polyphenolic fraction of Cinnamomum zeylanicum L. bark in animal models of inflammation and arthritis. Sci. Pharm. 2013, 81, 567–589. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Tan, J.-P.; Wang, L.-L.; Li, Q.-H. Andrographolide benefits rheumatoid arthritis via inhibiting MAPK pathways. Inflammation 2017, 40, 1599–1605. [Google Scholar] [CrossRef]
- Burgos, R.A.; Hancke, J.L.; Bertoglio, J.C.; Aguirre, V.; Arriagada, S.; Calvo, M.; Cáceres, D.D. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: A prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009, 28, 931–946. [Google Scholar] [CrossRef]
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, T.; Muthulakshmi, V.; Sachdanandam, P. Effect of the milk extract of Semecarpus anacardium nut on adjuvant arthritis—A dose-dependent study in wistar albino rats. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1223–1226. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Shanthi, P.; Sachdanandam, P. Anti-inflammatory Effect of Semecarpus anacardium Linn. nut extract in acute and chronic inflammatory conditions. Biol. Pharm. Bull. 2004, 27, 2028–2031. [Google Scholar] [CrossRef][Green Version]
- Ramprasath, V.R.; Shanthi, P.; Sachdanandam, P. Evaluation of antioxidant effect of Semecarpus anacardium Linn. nut extract on the components of immune system in adjuvant arthritis. Vasc. Pharmacol. 2005, 42, 179–186. [Google Scholar] [CrossRef]
- Singh, D.; Aggarwal, A.; Mathias, A.; Naik, S. Immunomodulatory activity of Semecarpus anacardium extract in mononuclear cells of normal individuals and rheumatoid arthritis patients. J. Ethnopharmacol. 2006, 108, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rotpenpian, N.; Arayapisit, T.; Roumwong, A.; Pakaprot, N.; Tantisira, M.; Wanasuntronwong, A. A standardized extract of Centella asiatica (ECa 233) prevents temporomandibular joint osteoarthritis by modulating the expression of local inflammatory mediators in mice. J. Appl. Oral Sci. 2021, 29, e20210329. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Mannelli, L.D.C.; Mattoli, L.; Tamimi, S.; Flamini, E.; Garetto, S.; Lucci, J.; Giovagnoni, E.; Cinci, L.; D’Ambrosio, M.; et al. Intra-articular route for the system of molecules 14G1862 from Centella asiatica: Pain relieving and protective effects in a rat model of osteoarthritis. Nutrients 2020, 12, 1618. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, R.; Thakur, S.C. Attenuation of collagen induced arthritis by Centella asiatica methanol fraction via modulation of cytokines and oxidative stress. Biomed. Environ. Sci. 2014, 27, 926–938. [Google Scholar] [CrossRef]
- Nair, V.; Singh, S.; Gupta, Y. Evaluation of disease modifying activity of Coriandrum sativum in experimental models. Indian J. Med. Res. 2012, 135, 240–245. [Google Scholar]
- Singh, S.; Gupta, Y.; Nair, V. Anti-granuloma activity of Coriandrum sativum in experimental models. J. Ayurveda Integr. Med. 2013, 4, 13–18. [Google Scholar] [CrossRef]
- Arya, V.; Gupta, V.K.; Kaur, R. A review on plants having anti-arthritic potential. Int. J. Pharm Sci. Rev. Res. 2011, 7, 131–136. [Google Scholar]
- Mehta, A.; Sethiya, N.K.; Mehta, C.; Shah, G. Anti–arthritis activity of roots of Hemidesmus indicus R.Br. (Anantmul) in rats. Asian Pac. J. Trop. Med. 2012, 5, 130–135. [Google Scholar] [CrossRef]
- Jung, H.-J.; Nam, J.H.; Choi, J.; Lee, K.-T.; Park, H.-J. Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund’s complete adjuvant-induced rats. J. Ethnopharmacol. 2005, 97, 359–367. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, M.-E.; Ko, S.-Y. n-Butanol extracts of Panax notoginseng suppress LPS-induced MMP-2 expression in periodontal ligament fibroblasts and inhibit osteoclastogenesis by suppressing MAPK in LPS-activated RAW264.7 cells. Arch. Oral Biol. 2011, 56, 1319–1327. [Google Scholar] [CrossRef]
- Wei, C.C.; Yue, L.F.; You, F.T.; Tao, C. Panax notoginseng saponins alleviate osteoporosis and joint destruction in rabbits with antigen-induced arthritis. Exp. Ther. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Han, G.; Zhou, S.; Li, J.; Chen, M.; Li, H. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int. J. Mol. Med. 2020, 45, 1225–1236. [Google Scholar] [CrossRef]
- Kim, H.A.; Kim, S.; Chang, S.H.; Hwang, H.J.; Choi, Y.-N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int. Immunopharmacol. 2007, 7, 1286–1291. [Google Scholar] [CrossRef]
- Piwowar, A.; Rembiałkowska, N.; Rorbach-Dolata, A.; Garbiec, A.; Ślusarczyk, S.; Dobosz, A.; Długosz, A.; Marchewka, Z.; Matkowski, A.; Saczko, J. Anemarrhenae asphodeloides rhizoma extract enriched in mangiferin protects PC12 cells against a neurotoxic agent-3-nitropropionic acid. Int. J. Mol. Sci. 2020, 21, 2510. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.-Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.-S.; et al. The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Dixit, P.K. In-vivo anti-inflammatory and anti-arthritic activity of Asparagus racemosus roots. Int. J. Pharm. Sci. Res. 2013, 4, 2652. [Google Scholar]
- Joshi, G.; Rawat, M.; Bisht, V.; Negi, J.S.; Singh, P. Chemical constituents of Asparagus. Pharmacogn. Rev. 2010, 4, 215–220. [Google Scholar] [CrossRef]
- Pecio, Ł.; Alilou, M.; Orhan, I.E.; Eren, G.; Deniz, F.S.S.; Stuppner, H.; Oleszek, W. Yuccalechins A–C from the Yucca schidigera Roezl ex ortgies bark: Elucidation of the relative and absolute configurations of three new spirobiflavonoids and their cholinesterase inhibitory activities. Molecules 2019, 24, 4162. [Google Scholar] [CrossRef]
- Cheeke, P.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 6. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.-M.; Oleszek, W.; Stochmal, A.; Kunert, O.; Bauer, R. Influence of Phenolic Constituents from Yucca schidigera bark on Arachidonate metabolism in Vitro. J. Agric. Food Chem. 2008, 56, 8885–8890. [Google Scholar] [CrossRef]
- Bhagwat, D.; Kharya, M.D.; Bani, S.; Kaul, A.; Kour, K.; Chauhan, P.S.; Suri, K.; Satti, N. Immunosuppressive properties of Pluchea lanceolata leaves. Indian J. Pharmacol. 2010, 42, 21–26. [Google Scholar] [CrossRef]
- Srivastava, P.; Shanker, K. Pluchea lanceolata (Rasana): Chemical and biological potential of Rasayana herb used in traditional system of medicine. Fitoterapia 2012, 83, 1371–1385. [Google Scholar] [CrossRef]
- Sivanesan, S.; Mundugaru, R.; Udaykumar, P.; Rao, N.; Chandra, N. Protective effect of Pluchea lanceolata against aluminum chloride-induced neurotoxicity in Swiss albino mice. Pharmacogn. Mag. 2017, 13, S567–S572. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Xu, Y.; Chen, K.; Yu, X.; Sun, G. Efficacy of Xixiancao (Herba siegesbeckiae orientalis) on interactions between nuclear factor kappa-B and inflammatory cytokines in inflammatory reactions of rat synovial cells induced by sodium urate. J. Tradit. Chin. Med. = Chung I Tsa Chih Ying Wen Pan 2020, 40, 774–781. [Google Scholar]
- Wang, J.-P.; Zhou, Y.-M.; Ye, Y.-J.; Shang, X.-M.; Cai, Y.-L.; Xiong, C.-M.; Wu, Y.-X.; Xu, H.-X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011, 137, 1089–1094. [Google Scholar] [CrossRef]
- Végh, K.; Riethmüller, E.; Tóth, A.; Alberti, A.; Béni, S.; Balla, J.; Kéry, A. Convergence chromatographic determination of camphor in the essential oil of Tanacetum parthenium L. Biomed. Chromatogr. 2016, 30, 2031–2037. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Parada-Turska, J.; Mitura, A.; Brzana, W.; Jabłoński, M.; Majdan, M.; Rzeski, W. Parthenolide inhibits proliferation of fibroblast-like synoviocytes in vitro. Inflammation 2008, 31, 281–285. [Google Scholar] [CrossRef]
- Xie, G.; Schepetkin, I.A.; Quinn, M.T. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int. Immunopharmacol. 2007, 7, 1639–1650. [Google Scholar] [CrossRef]
- Juan-Badaturuge, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial parts. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar] [CrossRef]
- Lin, B.; Zhao, Y.; Han, P.; Yue, W.; Ma, X.-Q.; Rahman, K.; Zheng, C.-J.; Qin, L.-P.; Han, T. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund׳s adjuvant induced arthritis in rats. J. Ethnopharmacol. 2014, 155, 248–255. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A Review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef]
- Hossen, M.J.; Cho, J.Y.; Kim, D. PDK1 in NF-κB signaling is a target of Xanthium strumarium methanolic extract-mediated anti-inflammatory activities. J. Ethnopharmacol. 2016, 190, 251–260. [Google Scholar] [CrossRef]
- Ghorbani, N.; Sahebari, M.; Mahmoudi, M.; Rastin, M.; Zamani, S.; Zamani, M. Berberine inhibits the gene expression and production of proinflammatory cytokines by mononuclear cells in rheumatoid arthritis and healthy individuals. Curr. Rheumatol. Rev. 2021, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, N.; Philipov, S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996, 18, 553–561. [Google Scholar] [CrossRef]
- Fan, H.-Y. Effectiveness of a hydroxynaphthoquinone fraction from Arnebia euchromain rats with experimental colitis. World J. Gastroenterol. 2013, 19, 9318–9327. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yang, M.; Che, X.; Zhang, Z.; Xu, H.; Liu, K.; Meng, Q. Activity study of a hydroxynaphthoquinone fraction from Arnebia euchroma in experimental arthritis. Fitoterapia 2012, 83, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Taussig, S.J.; Batkin, S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J. Ethnopharmacol. 1988, 22, 191–203. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Tabata, K.; Nakamura, Y.; Nishimura, R.; Kimura, Y.; Suzuki, T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J. Ethnopharmacol. 2006, 107, 249–253. [Google Scholar] [CrossRef]
- Blain, E.J.; Ali, A.Y.; Duance, V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phytother. Res. 2009, 24, 905–912. [Google Scholar] [CrossRef]
- Murugananthan, G.; Kumar, G.S.; Chethan, P.S.; Mohan, S. Anti-arthritic and anti-inflammatory constituents from medicinal plants. J. Appl. Pharm. Sci. 2013, 3, 161–164. [Google Scholar] [CrossRef]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef]
- Mishra, N.; Bstia, S.; Mishra, G.; Chowdary, K.; Patra, S. Anti-arthritic activity of Glycyrrhiza glabra, Boswellia serrata and their synergistic activity in combined formulation studied in freund’s adjuvant induced arthritic rats. J. Pharm. Educ. Res. 2011, 2, 92. [Google Scholar]
- Yun, X.; Chen, X.M.; Wang, J.Y.; Lu, W.; Zhang, Z.H.; Kim, Y.H.; Zong, S.C.; Li, C.H.; Gao, J.M. Cassane diterpenoids from Caesalpinia pulcherrima and their anti-inflammatory and α-glycosidase inhibitory activities. Nat Prod Res. 2021, 1–9, online ahead of print. [Google Scholar] [CrossRef]
- Rajaram, C.; Kandula, R.R. Evaluation of anti-arthritic activity of Caesalpinia pulcherrima in freund’s complete adjuvant induced arthritic rat model. J. Young Pharm. 2015, 7, 132. [Google Scholar] [CrossRef]
- Costa, B.; Colleoni, M.; Conti, S.; Parolaro, D.; Franke, C.; Trovato, A.E.; Giagnoni, G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2004, 369, 294–299. [Google Scholar] [CrossRef]
- Berg, M.V.D.; John, M.; Black, M.; Semprini, A.; Oldfield, K.; Glass, M.; Braithwaite, I. Cannabis-based medicinal products in arthritis, a painful conundrum. N. Z. Med. J. 2020, 133, 35–45. [Google Scholar]
- Baron, E.P.; Lucas, P.; Eades, J.; Hogue, O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J. Headache Pain 2018, 19, 1–28. [Google Scholar] [CrossRef]
- Ageel, A.M.; Parmar, N.S.; Mossa, J.S.; Al-Yahya, M.A.; Al-Said, M.S.; Tariq, M. Anti-inflammatory activity of some Saudi Arabian medicinal plants. Agents Actions 1986, 17, 383–384. [Google Scholar] [CrossRef]
- Feng, X.; Lu, J.; Xin, H.; Zhang, L.; Wang, Y.; Tang, K. Anti-arthritic active fraction of Capparis spinosa L. fruits and its chemical constituents. Yakugaku Zasshi 2011, 131, 423–429. [Google Scholar] [CrossRef]
- Maresca, M.; Micheli, L.; Mannelli, L.D.C.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Deng, W.; Sun, C.; Wei, Q.; Adu-Frimpong, M.; Shi, J.; Yu, J.; Xu, X. Anti-hyperuricemic and anti-gouty arthritis activities of polysaccharide purified from Lonicera japonica in model rats. Int. J. Biol. Macromol. 2018, 123, 801–809. [Google Scholar] [CrossRef]
- Cheng, B.C.Y.; Yu, H.; Guo, H.; Su, T.; Fu, X.-Q.; Li, T.; Cao, H.-H.; Tse, A.K.-W.; Wu, Z.-Z.; Kwan, H.-Y.; et al. A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos, attenuates collagen-induced arthritis and inhibits TLR4 signalling in rats. Sci. Rep. 2016, 6, 20042. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.; Greten, H.J.; Efferth, T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Comput. Biol. Med. 2021, 133, 104359. [Google Scholar] [CrossRef]
- Huh, J.-E.; Lee, W.-I.; Seo, B.-K.; Baek, Y.-H.; Lee, J.-D.; Choi, D.-Y.; Park, N.-S. Gastroprotective and safety effects of WIN-34B, a novel treatment for osteoarthritis, compared to NSAIDs. J. Ethnopharmacol. 2011, 137, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, X.; Li, W.; Chen, W.; Wang, X.; Ma, Z.; Lin, N. Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev. 2020, 41, 1337–1374. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.H. Tripterygium wilfordii Hook F. versus sulfasalazine in the treatment of rheumatoid arthritis: A well-designed clinical trial of a botanical demonstrating effectiveness. Fitoterapia 2011, 82, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.-Y.; Tan, H.-W.; Jia, Y.-F.; Li, D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J. Ethnopharmacol. 2008, 118, 479–484. [Google Scholar] [CrossRef]
- Tao, X.; Lipsky, P.E. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii hook F. Rheum. Dis. Clin. N. Am. 2000, 26, 29–50. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Gao, W. Tripterygium wilfordii inhibiting angiogenesis for rheumatoid arthritis treatment. J. Natl. Med. Assoc. 2017, 109, 142–148. [Google Scholar] [CrossRef]
- Narendhirakannan, R.; Subramanian, S.; Kandaswamy, M. Anti-inflammatory and lysosomal stability actions of Cleome gynandra L. studied in adjuvant induced arthritic rats. Food Chem. Toxicol. 2007, 45, 1001–1012. [Google Scholar] [CrossRef]
- Karlapudi, V.; Mungara, A.V.V.P.; Sengupta, K.; Davis, B.A.; Raychaudhuri, S.P. A placebo-controlled double-blind study demonstrates the clinical efficacy of a novel herbal formulation for relieving joint discomfort in human subjects with osteoarthritis of Knee. J. Med. Food 2018, 21, 511–520. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Erusappan, T.; Srinivasan, A. Hydrolysable tannin-rich fraction from Terminalia chebula Retz. fruits ameliorates collagen-induced arthritis in BALB/c mice. Inflammopharmacology 2019, 28, 275–287. [Google Scholar] [CrossRef]
- Seo, J.-B.; Jeong, J.-Y.; Park, J.-Y.; Jun, E.-M.; Lee, S.-I.; Choe, S.-S.; Park, D.-Y.; Choi, E.-W.; Seen, D.-S.; Lim, J.-S.; et al. Anti-arthritic and analgesic effect of NDI10218, a standardized extract of Terminalia chebula, on arthritis and pain model. Biomol. Ther. 2012, 20, 104–112. [Google Scholar] [CrossRef]
- Nair, V.; Singh, S.; Gupta, Y.K. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J. Pharm. Pharmacol. 2010, 62, 1801–1806. [Google Scholar] [CrossRef]
- Muhammad, S.; Khan, B.A.; Akhtar, N.; Mahmood, T.; Rasul, A.; Hussain, I.; Khan, H.; Badshah, A. The morphology, extractions, chemical constituents and uses of Terminalia chebula: A review. J. Med. Plants Res. 2012, 6, 4772–4775. [Google Scholar] [CrossRef]
- Bag, A.; Bhattacharyya, S.K.; Pal, N.K.; Chattopadhyay, R.R. Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruits. Pharm. Biol. 2013, 51, 1515–1520. [Google Scholar] [CrossRef]
- Pan, R.; Li, Y.; Dai, Y.; Gao, X.-H.; Xia, Y.-F. Anti-arthritic effect of scopoletin, a coumarin compound occurring in Erycibe obtusifolia Benth stems, is associated with decreased angiogenesis in synovium. Fundam. Clin. Pharmacol. 2009, 24, 477–490. [Google Scholar] [CrossRef]
- Pan, R.; Dai, Y.; Gao, X.; Xia, Y. Scopolin isolated from Erycibe obtusifolia Benth stems suppresses adjuvant-induced rat arthritis by inhibiting inflammation and angiogenesis. Int. Immunopharmacol. 2009, 9, 859–869. [Google Scholar] [CrossRef]
- Marzouk, B.; Marzouk, Z.; Fenina, N.; Bouraoui, A.; Aouni, M. Anti-inflammatory and analgesic activities of Tunisian Citrullus colocynthis Schrad. immature fruit and seed organic extracts. Eur. Rev. Med Pharmacol. Sci. 2011, 15, 665–672. [Google Scholar]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, D.; Di, L.; Xu, T.; Lin, X.; Yang, B.; Zhou, X.; Yang, X.; Liu, Y. The analgesic and anti-rheumatic effects of Thladiantha dubia fruit crude polysaccharide fraction in mice and rats. J. Ethnopharmacol. 2011, 137, 1381–1387. [Google Scholar] [CrossRef]
- Vennila, V.; Anitha, R. In vitro evaluation of anti-arthritic activity in different solvent extracts from Cuscuta reflexa. World J. Pharm. Pharm. Sci. 2015, 4, 1340–1350. [Google Scholar]
- Kumaraswamy, D.; Puchchakayala, G.; Yatla, P. Evaluation of anti-rheumatoid activity of Cuscuta reflexa in Freund’s adjuvant induced arthritic rats. Int. J. Pharm. Technol. 2016, 8, 13515–13530. [Google Scholar]
- Saini, P.; Mithal, R.; Menghani, E. A parasitic medicinal plant Cuscuta reflexa: An overview. Int. J. Sci. Eng. Res. 2015, 6, 951–959. [Google Scholar]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, X.; Kang, X.; Huang, H.; Yu, J.; Pan, J.; Zhang, X. The protective effect of sophocarpine in osteoarthritis: An in vitro and in vivo study. Int. Immunopharmacol. 2018, 67, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Ratheesh, M.; Shyni, G.; Nambisan, B.; Helen, A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012, 12, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Shyni, G.L.; Pushpan, C.K.; Nambisan, B.; Helen, A. Evaluation of anti-arthritic potential of Trigonella foenum graecum L. (Fenugreek) mucilage against rheumatoid arthritis. Prostaglandins Other Lipid Mediat. 2018, 138, 48–53. [Google Scholar] [CrossRef]
- Suresh, P.; Kavitha, C.N.; Babu, S.M.; Reddy, V.P.; Latha, A.K. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund’s adjuvant-induced arthritis in Albino rats. Inflammation 2012, 35, 1314–1321. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Tammar, S.; Hammami, M.; Saharkhiz, M.J.; Debiche, N.; Limam, F.; Marzouk, B. Essential oil composition of Lavandula dentata, L. stoechas and L. multifida cultivated in Tunisia. J. Essent. Oil Bear. Plants 2012, 15, 1030–1039. [Google Scholar] [CrossRef]
- Augustine, B.B.; Dash, S.; Lahkar, M.; Sarma, U.; Samudrala, P.K.; Thomas, J.M. Leucas aspera inhibits the Dalton′s ascitic lymphoma in Swiss albino mice: A preliminary study exploring possible mechanism of action. Pharmacogn. Mag. 2014, 10, 118–124. [Google Scholar] [CrossRef]
- Kripa, K.; Chamundeeswari, D.; Thanka, J.; Reddy, C.U.M. Modulation of inflammatory markers by the ethanolic extract of Leucas aspera in adjuvant arthritis. J. Ethnopharmacol. 2011, 134, 1024–1027. [Google Scholar] [CrossRef]
- Gonçalves, G.D.A.; de Sá-Nakanishi, A.B.; Comar, J.F.; Bracht, L.; Dias, M.I.; Barros, L.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Water soluble compounds of Rosmarinus officinalis L. improve the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct. 2018, 9, 2328–2340. [Google Scholar] [CrossRef]
- González-Trujano, M.; Peña, E.; Martinez, A.L.; Moreno, J.; Guevara-Fefer, P.; Déciga-Campos, M.; López-Muñoz, F. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, X.; Zhou, L.; Liu, Z.; Yuan, J.; Cheng, J.; Zhao, J.; Wu, L.; Li, H.; Qiu, H.; et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats. J. Cell. Physiol. 2018, 233, 6291–6303. [Google Scholar] [CrossRef]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppression by regulating ROS-dependent p38 pathway. Free Radic. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Jiang, W.-Y.; Jeon, B.-H.; Kim, Y.-C.; Lee, S.H.; Sohn, D.H.; Seo, G.S. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int. Immunopharmacol. 2013, 16, 160–164. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Luo, X.-Y.; Jiang, H.; Xing, Y.; Yang, M.-H.; Yuan, G.-H.; Tang, Z.; Wang, H. Salvia miltiorrhiza injection restores apoptosis of fibroblast-like synoviocytes cultured with serum from patients with rheumatoid arthritis. Mol. Med. Rep. 2014, 11, 1476–1482. [Google Scholar] [CrossRef][Green Version]
- Jiang, R.; Zhang, X.; Li, Y.; Zhou, H.; Wang, H.; Wang, F.; Ma, H.; Cao, L. Identification of the molecular mechanisms of Salvia miltiorrhiza relevant to the treatment of osteoarthritis based on network pharmacology. Discov. Med. 2021, 30, 83–95. [Google Scholar]
- Zheng, C.-J.; Zhao, X.-X.; Ai, H.-W.; Lin, B.; Han, T.; Jiang, Y.-P.; Xing, X.; Qin, L.-P. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine 2014, 21, 838–846. [Google Scholar] [CrossRef]
- Jing, R.; Ban, Y.; Xu, W.; Nian, H.; Guo, Y.; Geng, Y.; Zang, Y.; Zheng, C. Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 2019, 58, 152825. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Tong, B.; Xia, Y.-F.; Lu, Q.; Chou, G.-X.; Wang, Z.-T.; Dai, Y. Norisoboldine suppresses osteoclast differentiation through preventing the accumulation of TRAF6-TAK1 complexes and activation of MAPKs/NF-κB/c-Fos/NFATc1 pathways. PLoS ONE 2013, 8, e59171. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-F.; Lv, Q.; Xia, Y.; Yue, M.-F.; Shi, C.; Xia, Y.-F.; Chou, G.-X.; Wang, Z.-T.; Dai, Y. Norisoboldine, an anti-arthritis alkaloid isolated from Radix Linderae, Attenuates osteoclast differentiation and inflammatory bone erosion in an aryl hydrocarbon receptor-dependent manner. Int. J. Biol. Sci. 2015, 11, 1113–1126. [Google Scholar] [CrossRef]
- Tong, B.; Dou, Y.; Wang, T.; Yu, J.; Wu, X.; Lu, Q.; Chou, G.; Wang, Z.; Kong, L.; Dai, Y.; et al. Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. Toxicol. Appl. Pharmacol. 2015, 282, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.A.B.S.; Klein-Júnior, L.C.; Cruz, S.M.; Cáceres, A.; Quintão, N.L.M.; Monache, F.D.; Cechinel-Filho, V. Anti-inflammatory and anti-hyperalgesic evaluation of the condiment laurel (Litsea guatemalensis Mez.) and its chemical composition. Food Chem. 2012, 132, 1980–1986. [Google Scholar] [CrossRef]
- Patil, K.R.; Patil, C.R.; Jadhav, R.B.; Mahajan, V.K.; Patil, P.R.; Gaikwad, P.S. Anti-arthritic activity of bartogenic acid isolated from fruits of Barringtonia racemosa Roxb. (Lecythidaceae). Evid.-Based Complement. Altern. Med. 2011, 2011, 785245. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Z.; Liu, J.; Hu, J.; Yang, C. Therapeutic effect of nux vomica total alkali gel on adjuvants arthritis rats. China J. Chin. Mater. Med. 2012, 37, 1434–1439. [Google Scholar]
- Patel, K.; Laloo, D.; Singh, G.K.; Gadewar, M.; Patel, D.K. A review on medicinal uses, analytical techniques and pharmacological activities of Strychnos nux-vomica Linn.: A concise report. Chin. J. Integr. Med. 2017, 1–13. [Google Scholar] [CrossRef]
- Yin, W.; Wang, T.-S.; Yin, F.-Z.; Cai, B.-C. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnos nux-vomica. J. Ethnopharmacol. 2003, 88, 205–214. [Google Scholar] [CrossRef]
- Gautam, R.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of antiarthritic activity of butanol fraction of Punica granatum Linn. Rind extract against Freund’s complete adjuvant-induced arthritis in rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 53–62. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 128–144. [Google Scholar]
- Mahdavi, A.M.; Seyedsadjadi, N.; Javadivala, Z. Potential effects of pomegranate (Punica granatum) on rheumatoid arthritis: A systematic review. Int. J. Clin. Pr. 2021, 75, e13999. [Google Scholar] [CrossRef]
- Bhajipale, N.S. Anti-Arthritic Activity of Abutilon hirtum. Int. J. Pharm. Biol. Arch. 2014, 5, 99–101. [Google Scholar]
- Sharma, A.; Sharma, R.; Singh, H. Phytochemical and pharmacological profile of Abutilon indicum L. Sweet: A review. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 120–127. [Google Scholar]
- Gupta, S.; Nirmal, S.; Patil, R.; Asane, G. Anti-arthritic activity of various extracts of Sida rhombifolia aerial parts. Nat. Prod. Res. 2009, 23, 689–695. [Google Scholar] [CrossRef]
- Narendhirakannan, R.; Limmy, T. Anti-inflammatory and anti-oxidant properties of Sida rhombifolia stems and roots in adjuvant induced arthritic rats. Immunopharmacol. Immunotoxicol. 2011, 34, 326–336. [Google Scholar] [CrossRef]
- Bharate, S.B.; Vishwakarma, R.A. Cyclin-dependent kinase inhibition by flavoalkaloids. Mini-Rev. Med. Chem. 2012, 12, 632–649. [Google Scholar] [CrossRef]
- Gelmini, F.; Ruscica, M.; Macchi, C.; Bianchi, V.; Facino, R.M.; Beretta, G.; Magni, P. Unsaponifiable fraction of unripe fruits of Olea europaea: An interesting source of anti-inflammatory constituents. Planta Medica 2015, 82, 273–278. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, C.; Shin, K.K.; Choi, E.; Hwang, S.-H.; Jang, Y.-J.; Taamalli, A.; Yum, J.; Kim, J.-H.; Kim, E.; et al. Tunisian Olea europaea L. leaf extract suppresses Freund’s complete adjuvant-induced rheumatoid arthritis and lipopolysaccharide-induced inflammatory responses. J. Ethnopharmacol. 2020, 268, 113602. [Google Scholar] [CrossRef]
- Wardhana, E.E.S.; Datau, E.A. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med. Indones. 2011, 43, 138–143. [Google Scholar] [PubMed]
- Flemmig, J.; Kuchta, K.; Arnhold, J.; Rauwald, H. Olea europaea leaf (Ph.Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine 2011, 18, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Koike, T.; Taniguchi, I.; Tanaka, K. Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 2013, 20, 861–864. [Google Scholar] [CrossRef]
- Sahu, A.N.; Bharati, A.C. Ethnobotany, phytochemistry and pharmacology of Biophytum sensitivum DC. Pharmacogn. Rev. 2012, 6, 68–73. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, Y.; Hou, X.; Xu, J.; Mu, X.; Chen, W. Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol. 2011, 137, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, S.-M. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int. Immunopharmacol. 2012, 14, 27–31. [Google Scholar] [CrossRef]
- Pradit, W.; Chomdej, S.; Nganvongpanit, K.; Ongchai, S. Chondroprotective potential of Phyllanthus amarus Schum. & Thonn. in experimentally induced cartilage degradation in the explants culture model. In Vitro Cell. Dev. Biol.-Anim. 2014, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Pinkaew, D.; Kiattisin, K.; Wonglangka, K.; Awoot, P. Phonophoresis of Phyllanthus amarus nanoparticle gel improves functional capacity in individuals with knee osteoarthritis: A randomized controlled trial. J. Bodyw. Mov. Ther. 2019, 24, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.M.; Sinnathambi, A.; Kapase, C.U.; Bodhankar, S.L.; Mahadik, K.R. Anti-arthritic activity of standardised extract of Phyllanthus amarus in Freund’s complete adjuvant induced arthritis. Biomed. Aging Pathol. 2011, 1, 185–190. [Google Scholar] [CrossRef]
- Alam, J.; Jantan, I.; Kumolosasi, E.; Nafiah, M.A.; Mesaik, M.A. Suppressive effects of the standardized extract of Phyllanthus amarus on type II collagen-induced rheumatoid arthritis in Sprague Dawley rats. Curr. Pharm. Biotechnol. 2019, 19, 1156–1169. [Google Scholar] [CrossRef]
- Yende, S.R.; Sannapuri, V.D.; Vyawahare, N.S.; Harle, U.N. Antirheumatoid activity of aqueous extract of Piper longum on freunds adjuvant-induced arthritis in rats. Int. J. Pharm. Sci. Res. 2010, 1, 129–133. [Google Scholar]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2019, 247, 112255. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Malhotra, H.; Singh, S. Medicinal plants with potential anti-arthritic activity. J. Intercult. Ethnopharmacol. 2015, 4, 147–179. [Google Scholar] [CrossRef]
- Puente, R.A.; Illnait, J.; Mas, R.M.; Carbajal, D.M.; Mendoza, S.; Ceballos, A.; Fernández, J.C.; Mesa, M.; Reyes, P.; Ruiz, D. Effects of a combined therapy with D-002 (Beeswax alcohols) plus D-003 (Sugarcane Wax acids) on osteoarthritis symptoms. Altern. Ther. Health Med. 2016, 22, 15–23. [Google Scholar]
- Ledón, N.; Casacó, A.; Remirez, D.; González, A.; Cruz, J.; Capote, A.; Tolón, Z.; Rojas, E.; Rodríguez, V.; Merino, N.; et al. Effects of a mixture of fatty acids from sugar cane (Saccharum officinarum L.) wax oil in two models of inflammation: Zymosan-induced arthritis and mice tail test of psoriasis. Phytomedicine 2007, 14, 690–695. [Google Scholar] [CrossRef]
- Ríos, J.-L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Yesilada, E.; Küpeli, E. Clematis vitalba L. aerial part exhibits potent anti-inflammatory, antinociceptive and antipyretic effects. J. Ethnopharmacol. 2007, 110, 504–515. [Google Scholar] [CrossRef]
- Meng, F.-C.; Wu, Z.-F.; Yin, Z.-Q.; Lin, L.-G.; Wang, R.; Zhang, Q.-W. Coptidis rhizoma and its main bioactive components: Recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 2018, 13, 13. [Google Scholar] [CrossRef]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-inflammatory, anti-arthritic and anti-nociceptive activities of Nigella sativa oil in a rat model of arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kashani, L.M.T.; Mahboubi, M. Nigella sativa fixed oil as alternative treatment in management of pain in arthritis rheumatoid. Phytomedicine 2018, 46, 69–77. [Google Scholar] [CrossRef]
- Hadi, V.; Kheirouri, S.; Alizadeh, M.; Khabbazi, A.; Hosseini, H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Avicenna J. Phytomed. 2016, 6, 34–43. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, W. Effects and mechanisms of glucosides of Chaenomeles speciosa on collagen-induced arthritis in rats. Int. Immunopharmacol. 2003, 3, 593–608. [Google Scholar] [CrossRef]
- Dai, M.; Wei, W.; Shen, Y.; Zheng, Y.-Q. Glucosides of Chaenomeles speciosa remit rat adjuvant arthritis by inhibiting synoviocyte activities. Acta Pharmacol. Sin. 2003, 24, 1161–1166. [Google Scholar]
- Li, X.; Yang, Y.-B.; Yang, Q.; Sun, L.-N.; Chen, W.-S. Anti-inflammatory and analgesic activities of Chaenomeles speciosa fractions in laboratory animals. J. Med. Food 2009, 12, 1016–1022. [Google Scholar] [CrossRef]
- Cerri, G.C.; Lima, L.C.F.; Lelis, D.D.F.; Barcelos, L.D.S.; Feltenberger, J.D.; Mussi, S.V.; Monteiro-Junior, R.S.; dos Santos, R.A.S.; Ferreira, L.A.M.; Santos, S.H.S. Sclareol-loaded lipid nanoparticles improved metabolic profile in obese mice. Life Sci. 2019, 218, 292–299. [Google Scholar] [CrossRef]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina—Rose hip pharmacological ingredients and molecular mechanics counteracting osteoarthritis—A systematic review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Kirkeskov, B.; Christensen, R.; Bügel, S.; Bliddal, H.; Danneskiold-Samsøe, B.; Christensen, L.P.; Andersen, J.R. The effects of rose hip (Rosa canina) on plasma antioxidative activity and C-reactive protein in patients with rheumatoid arthritis and normal controls: A prospective cohort study. Phytomedicine 2011, 18, 953–958. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.-M.; Luo, Y.-M. A new sesquiterpene lactone from the roots of Lasianthus acuminatissimus. Yao Xue Xue Bao Acta Pharm. Sin. 2006, 41, 426–430. [Google Scholar]
- Li, B.; Zhang, D.-M.; Luo, Y.-M.; Chen, X.-G. Three new and antitumor anthraquinone glycosides from Lasianthus acuminatissimus MERR. Chem. Pharm. Bull. 2006, 54, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.C.Y.C.; Kim, S.-Y.K.S.-Y.; Hyung, C.-G.H.C.-G. 8-Methoxycoumarin enhances melanogenesis via the MAPKase signaling pathway. Die Pharm. Int. J. Pharm. Sci. 2019, 74, 529–535. [Google Scholar] [CrossRef]
- Ratheesh, M.; Shyni, G.L.; Sindhu, G.; Helen, A. Protective effects of isolated polyphenolic and alkaloid fractions of Ruta graveolens L. on acute and chronic models of inflammation. Inflammation 2009, 33, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Shyni, G.L.; Helen, A. Methanolic extract of Ruta graveolens L. inhibits inflammation and oxidative stress in adjuvant induced model of arthritis in rats. Inflammopharmacology 2009, 17, 100–105. [Google Scholar] [CrossRef]
- Amina, K.; Mahmudur, R.; Lutfun, N.; Chung Yeng, L.; Won Fen, W.; Hazrina, H.; Mohamad Azrul bin, M.; Shaikh Jamal, U.; Khalijah, A.; Jamil Ahmad, S. Anti-inflammatory and NF-κB inhibitory activity of aerial parts of Cestrum diurnum (preprint v1). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Khan, B.; Bani, S.; Suri, K.; Satti, N.; Qazi, G. Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol. Res. 2006, 53, 233–240. [Google Scholar] [CrossRef]
- Grover, A.; Shandilya, A.; Punetha, A.; Bisaria, V.S.; Sundar, D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genom. 2010, 11, S25. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm. Biol. 2013, 52, 308–320. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmed, R.S.; Chandra, N.; Arora, V.K.; Ali, A. In vivo, Extract from Withania somnifera root ameliorates arthritis via regulation of key immune mediators of inflammation in experimental model of arthritis. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 55–70. [Google Scholar] [CrossRef]
- Basu, S.; Hazra, B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytother. Res. 2006, 20, 896–900. [Google Scholar] [CrossRef]
- Wu, P.; Song, Z.; Wang, X.; Li, Y.; Li, Y.; Cui, J.; Tuerhong, M.; Jin, D.-Q.; Abudukeremu, M.; Lee, D.; et al. Bioactive triterpenoids from Lantana camara showing anti-inflammatory activities in vitro and in vivo. Bioorg. Chem. 2020, 101, 104004. [Google Scholar] [CrossRef]
- Sathish, R.; Vyawahare, B.; Natarajan, K. Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. J. Ethnopharmacol. 2011, 134, 195–197. [Google Scholar] [CrossRef]
- Kore, K.; Shete, R.; Desai, N. Anti-arthritic activity of hydroalcoholic extract of Lawsonia Innermis. Int. J. Drug Dev. Res. 2011, 3, 217–224. [Google Scholar]
- Ziaei, A.; Sahranavard, S.; Gharagozlou, M.J.; Faizi, M. Preliminary investigation of the effects of topical mixture of Lawsonia inermis L. and Ricinus communis L. leaves extract in treatment of osteoarthritis using MIA model in rats. DARU J. Pharm. Sci. 2016, 24, 12. [Google Scholar] [CrossRef]
- Anand, S.; Muthusamy, V.; Sujatha, S.; Sangeetha, K.; Raja, R.B.; Sudhagar, S.; Devi, N.P.; Lakshmi, B. Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 2010, 584, 3170–3178. [Google Scholar] [CrossRef]
- Cowan, D. Oral Aloe vera as a treatment for osteoarthritis: A summary. Br. J. Community Nurs. 2010, 15, 280–282. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.A.; Jeong, S.; Lee, S.; Park, H.J.; Kim, N.J.; Lim, S. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2009, 126, 258–264. [Google Scholar] [CrossRef]
- Huang, G.; Xu, Z.; Huang, Y.; Duan, X.; Gong, W.; Zhang, Y.; Fan, J.; He, F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J. Clin. Immunol. 2012, 33, 550–557. [Google Scholar] [CrossRef]
- Nonose, N.; Pereira, J.A.; Machado, P.R.M.; Rodrigues, M.R.; Sato, D.T.; Martinez, C.A.R. Oral administration of curcumin (Curcuma longa) can attenuate the neutrophil inflammatory response in zymosan-induced arthritis in rats. Acta Cir. Bras. 2014, 29, 727–734. [Google Scholar] [CrossRef]
- Arora, R.; Kuhad, A.; Kaur, I.; Chopra, K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain 2014, 19, 940–952. [Google Scholar] [CrossRef]
- Nievergelt, A.; Marazzi, J.; Schoop, R.; Altmann, K.-H.; Gertsch, J. Ginger phenylpropanoids inhibit IL-1β and prostanoid secretion and disrupt Arachidonate-phospholipid remodeling by targeting phospholipases A2. J. Immunol. 2011, 187, 4140–4150. [Google Scholar] [CrossRef] [PubMed]
- Al Nahain, A.; Jahan, R.; Rahmatullah, M. Zingiber officinale: A potential plant against rheumatoid arthritis. Arthritis 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.N.; Ndikum, V.; Tabi, O.Y.; Jiofack, R.B.; Ngameni, B.; Guedje, N.M.; Tembe-Fokunang, E.A.; Tomkins, P.; Barkwan, S.; Kechia, F.; et al. Traditional medicine: Past, present and future research and development prospects and integration in the National Health System of Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef]
- Anusha, D.; Chaly, P.E.; Junaid, M.; Nijesh, J.; Shivashankar, K.; Sivasamy, S. Efficacy of a mouthwash containing essential oils and curcumin as an adjunct to nonsurgical periodontal therapy among rheumatoid arthritis patients with chronic periodontitis: A randomized controlled trial. Indian J. Dent. Res. 2019, 30, 506. [Google Scholar] [CrossRef]
- Brzeski, M.; Madhok, R.; Capell, H.A. Evening primrose oil in patients with rheumatoid arthritis and side-effects of non-steroidal anti-inflammatory drugs. Rheumatology 1991, 30, 370–372. [Google Scholar] [CrossRef]
- Nasiri, A.; Mahmodi, M.A.; Nobakht, Z. Effect of aromatherapy massage with Lavender essential oil on pain in patients with osteoarthritis of the knee: A randomized controlled clinical trial. Complement. Ther. Clin. Pr. 2016, 25, 75–80. [Google Scholar] [CrossRef]
- Nasiri, A.; Mahmodi, M.A. Aromatherapy massage with lavender essential oil and the prevention of disability in ADL in patients with osteoarthritis of the knee: A randomized controlled clinical trial. Complement. Ther. Clin. Pr. 2018, 30, 116–121. [Google Scholar] [CrossRef]
- Paixão, V.L.B.; de Carvalho, J.F. Essential oil therapy in rheumatic diseases: A systematic review. Complement. Ther. Clin. Pr. 2021, 43, 101391. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Tseng, H.-C.; Lin, S.-Y.; Sheen, L.-Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef]
- Hong, S.J.; Cho, J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.-C. Inhalation of Patchouli (Pogostemon Cablin Benth.) essential oil improved metabolic parameters in obesity-induced Sprague Dawley rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Kammoun, J.; Hlila, M.B.; Louati, H.; Hammami, S.; Flamini, G.; Achour, L.; Selmi, B. Rhaponticum acaule (L) DC essential oil: Chemical composition, in vitro antioxidant and enzyme inhibition properties. BMC Complement. Altern. Med. 2018, 18, 79. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger essential oil ameliorates hepatic injury and lipid accumulation in high fat diet-induced nonalcoholic fatty liver disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.-J.; Jeong, S.-J.; Lee, M.-H.; Kim, S.-H. Essential oil of Pinus koraiensis leaves exerts antihyperlipidemic effects via up-regulation of low-density lipoprotein receptor and inhibition of acyl-coenzyme A: Cholesterol acyltransferase. Phytother. Res. 2012, 26, 1314–1319. [Google Scholar] [CrossRef]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Essential oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef]
- Hwang, D.I.; Won, K.-J.; Kim, D.-Y.; Yoon, S.W.; Park, J.-H.; Kim, B.; Lee, H.M. Anti-adipocyte differentiation activity and chemical composition of essential oil from Artemisia annua. Nat. Prod. Commun. 2016, 11, 539–542. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Abu-Zaitoun, S.Y.; Dudai, N.; Jamous, R.M. Downy Lavender oil: A promising source of antimicrobial, antiobesity, and anti-Alzheimer’s disease agents. Evid.-Based Complement. Altern. Med. 2020, 2020, 5679408. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Safi, S.; Nadjarzadeh, A.; Fallahzadeh, H.; Abdollahi, N.; Mazaheri, M.; Nazari, M.; Salehi-Abargouei, A. The effect of Nigella sativa supplementation on cardiovascular risk factors in obese and overweight women: A crossover, double-blind, placebo-controlled randomized clinical trial. Eur. J. Nutr. 2020, 60, 1863–1874. [Google Scholar] [CrossRef]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: A randomized double-blind, placebo-controlled clinical trial. J. Clin. Lipidol. 2016, 10, 1203–1211. [Google Scholar] [CrossRef]
- Grube, B.; Chong, P.; Lau, K.; Orzechowski, H. A natural fiber complex reduces body weight in the overweight and obese: A double-blind, randomized, placebo-controlled study. Obesity 2013, 21, 58–64. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Gomes, A.P.O.; de Moraes, A.P.G.; Stringhini, M.L.F.; Mota, J.F.; Coelho, A.; Botelho, P.B. Green tea extract outperforms metformin in lipid profile and glycaemic control in overweight women: A double-blind, placebo-controlled, randomized trial. Clin. Nutr. ESPEN 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Azar, P.S.; Hadi, A.; Ostadrahimi, A. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int. J. Clin. Pr. 2021, 75, e14334. [Google Scholar] [CrossRef]
- Odunsi, S.T.; Vázquez-Roque, M.I.; Camilleri, M.; Papathanasopoulos, A.; Clark, M.M.; Wodrich, L.; Lempke, M.; McKinzie, S.; Ryks, M.; Burton, D.; et al. Effect of alginate on satiation, appetite, gastric function, and selected gut satiety hormones in overweight and obesity. Obesity 2010, 18, 1579–1584. [Google Scholar] [CrossRef]
- Amagase, H.; Nance, D.M. Lycium barbarum increases caloric expenditure and decreases waist circumference in healthy overweight men and women: Pilot study. J. Am. Coll. Nutr. 2011, 30, 304–309. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Azar, M.R.M.H.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef]
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of daily ingestion of quercetin-rich onion powder for 12 weeks on visceral fat: A randomised, double-blind, placebo-controlled, parallel-group study. Nutrients 2019, 12, 91. [Google Scholar] [CrossRef]
- Maheu, E.; Cadet, C.; Marty, M.; Moyse, D.; Kerloch, I.; Coste, P.; Dougados, M.; Mazières, B.; Spector, T.D.; Halhol, H.; et al. Randomised, controlled trial of avocado–soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: The ERADIAS study. Ann. Rheum. Dis. 2013, 73, 376–384. [Google Scholar] [CrossRef]
- May, L.S.; Sanip, Z.; Shokri, A.A.; Kadir, A.A.; Lazin, R.M. The effects of Momordica charantia (bitter melon) supplementation in patients with primary knee osteoarthritis: A single-blinded, randomized controlled trial. Complement. Ther. Clin. Pr. 2018, 32, 181–186. [Google Scholar] [CrossRef]
- Nash, R.; Azantsa, B.; Kuate, D.; Singh, H.; Oben, J. The use of a stem and leaf aqueous extract of Cissus quadrangularis (CQR-300) to reduce body fat and other components of metabolic syndrome in overweight participants. J. Altern. Complement. Med. 2019, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K. Potential role of flavonoids against SARS-CoV-2 induced diarrhea. Trop. Biomed. 2021, 38, 360–365. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L.; Raju, C.S.; Mahboob, T.; Kayesth, S.; Gupta, K.K.; Jain, G.K.; Dhobi, M.; Nawaz, M.; Wilairatana, P.; Pereira, M.D.L.; et al. Precision and advanced nano-phytopharmaceuticals for therapeutic applications. Nanomaterials 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Jannat, K.; Paul, A.K.; Bondhon, T.A.; Hasan, A.; Nawaz, M.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Pereira, M.D.L.; et al. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics 2021, 13, 1895. [Google Scholar] [CrossRef]
- Romanová, D.; Vachálková, A.; Cipák, L.; Ovesná, Z.; Rauko, P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma 2001, 48, 104–107. [Google Scholar]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Khitka, B.; Kupittayanant, S.; Rangsriwatananon, K.; Manakasem, Y. Antioxidant properties of puerarin and genistein from White Kwao Krua induced by elicitors and their antihyperglycemic effect on rats. Suranaree J. Sci. Technol. 2010, 17, 27–37. [Google Scholar]
- Henning, S.M.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem. 2005, 16, 610–616. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Chang, X.; He, H.; Zhu, L.; Gao, J.; Wei, T.; Ma, Z.; Yan, T. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB pathway. Chem. Interact. 2015, 236, 41–46. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Bai, J.-Y.; Xia, S.; Mao, M.; Li, X.; Li, N.; Chen, L. The roles of synovial hyperplasia, angiogenesis and osteoclastogenesis in the protective effect of apigenin on collagen-induced arthritis. Int. Immunopharmacol. 2019, 73, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Z.; Zhu, Y.; Shen, T.; Wang, H.; Shui, G.; Loor, J.J.; Fang, Z.; Chen, M.; Wang, X.; et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. J. Cereb. Blood Flow Metab. 2020, 177, 3591–3607. [Google Scholar] [CrossRef]
- Thielecke, F.; Rahn, G.; Böhnke, J.; Adams, F.; Birkenfeld, A.L.; Jordan, J.; Boschmann, M. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.E.; Van Boekschoten, M.; De Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, J.R.E.; Vasconcelos-Ulloa, J.J.; González-Mendoza, D.; Beltrán-González, G.; Díaz-Molina, R. Efecto de una intervención dietética con un producto alimenticio a base de leguminosas sobre los niveles de malondialdehído, índice HOMA y perfil de lípidos. Endocrinol. Diabetes Nutr. 2019, 67, 235–244. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive ffects of kaempferol on high-fat diet-induced obesity complications in C57BL/6 mice. BioMed. Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.; Kim, S.; Hong, M.J.; Jeong, N.; Kim, M. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J. Food Biochem. 2020, 44, e13358. [Google Scholar] [CrossRef]
- Li, W.; Hu, H.; Zou, G.; Ma, Z.; Liu, J.; Li, F. Therapeutic effects of puerarin on polycystic ovary syndrome. Medicine 2021, 100, e26049. [Google Scholar] [CrossRef]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2016, 36, 9–15. [Google Scholar] [CrossRef]
- Nyakudya, T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.; Ndhlala, A.R. The Ppotential therapeutic value of medicinal plants in the management of metabolic disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants—An update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2018, 110, 224–238. [Google Scholar] [CrossRef]
- Verma, R.K.; Paraidathathu, T. Herbal medicines used in the traditional Indian medicinal system as a therapeutic treatment option for overweight and obesity management: A review. Int. J. Pharm. Pharm. Sci. 2014, 6, 40–47. [Google Scholar]
- Sun, N.-N.; Wu, T.-Y.; Chau, C.-F. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- García-Barrado, M.J.; Iglesias-Osma, M.C.; Pérez-García, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M.; Carretero, J. Role of Flavonoids in the interactions among obesity, inflammation, and autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef]
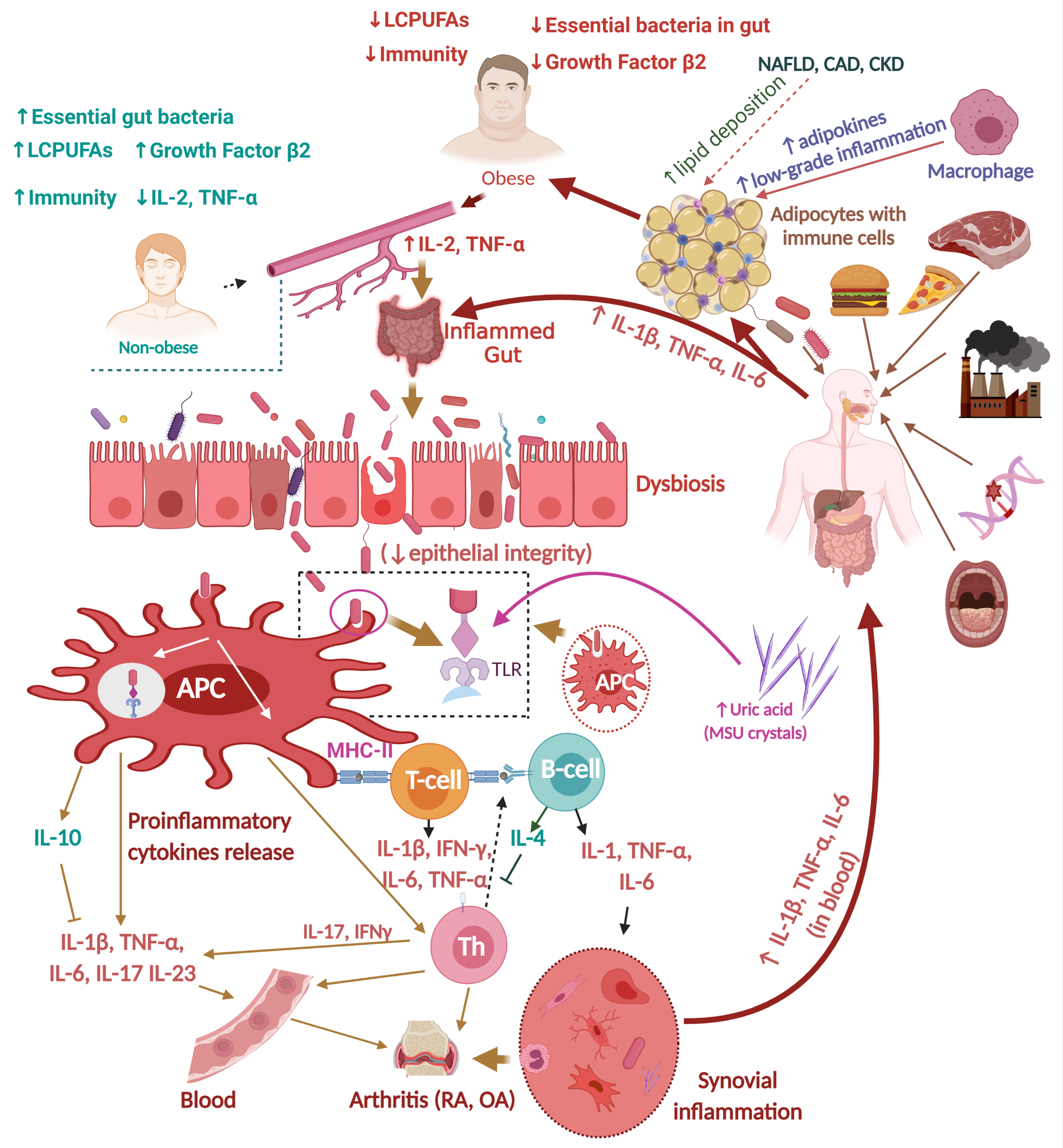
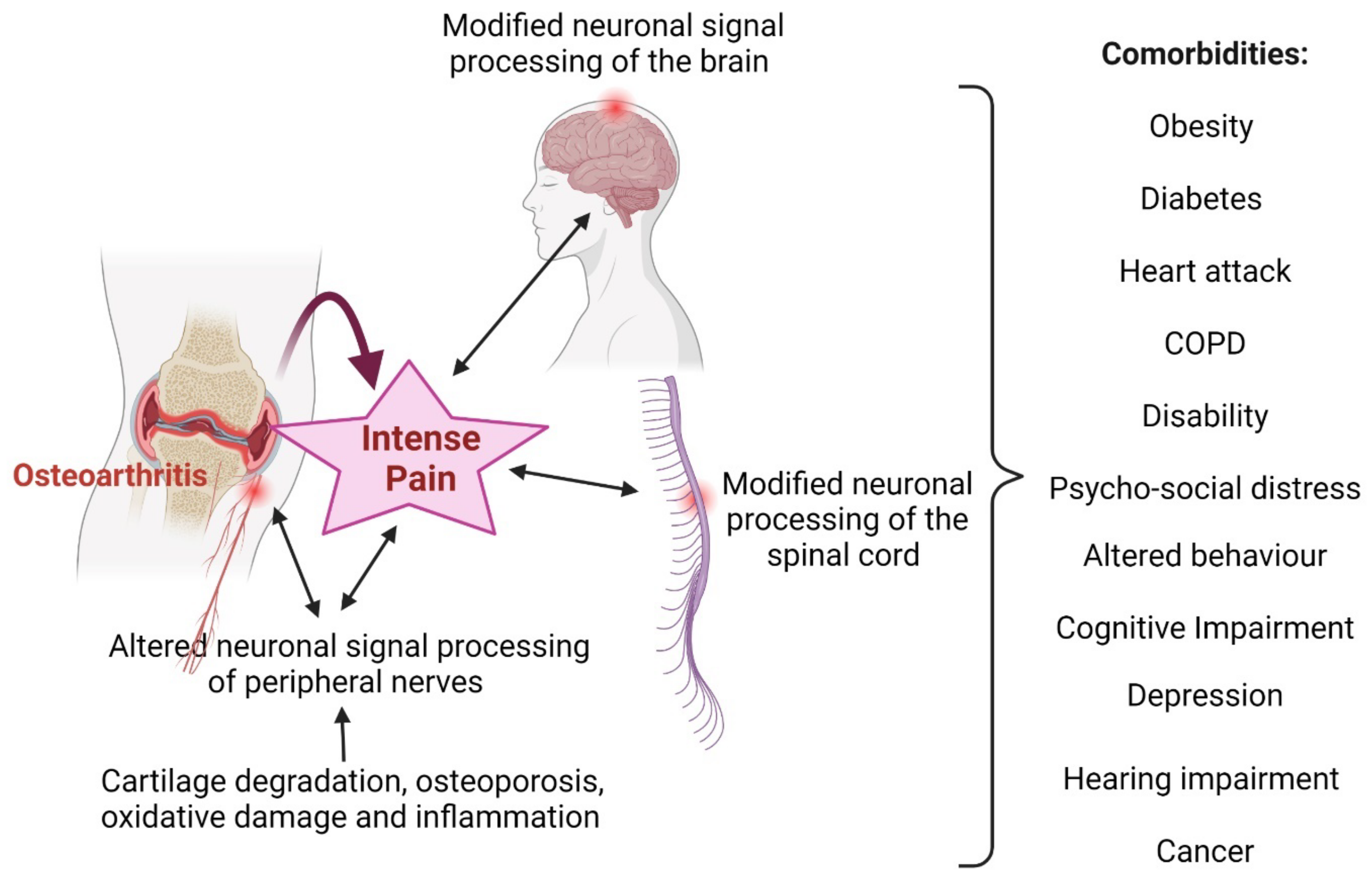
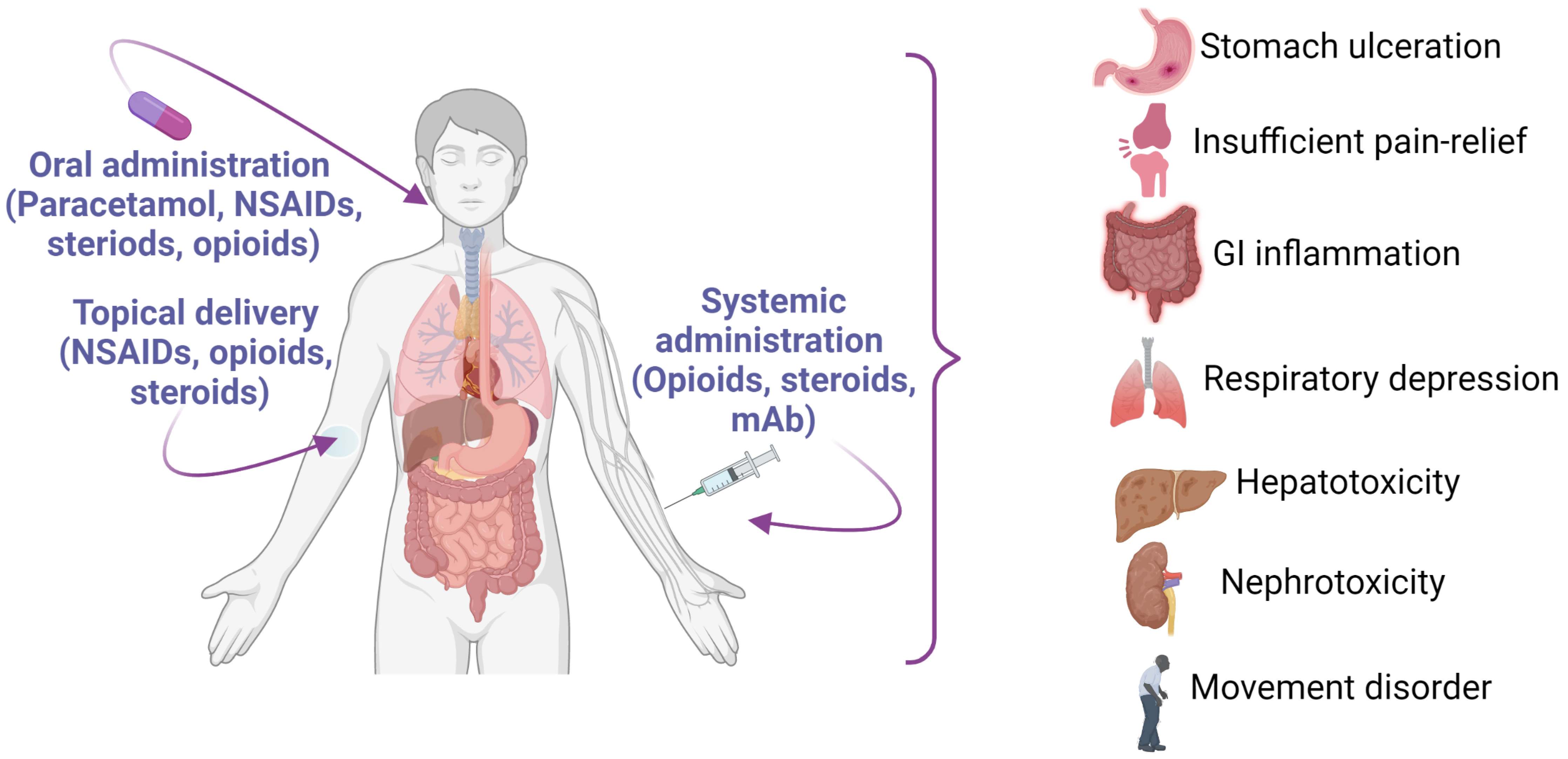
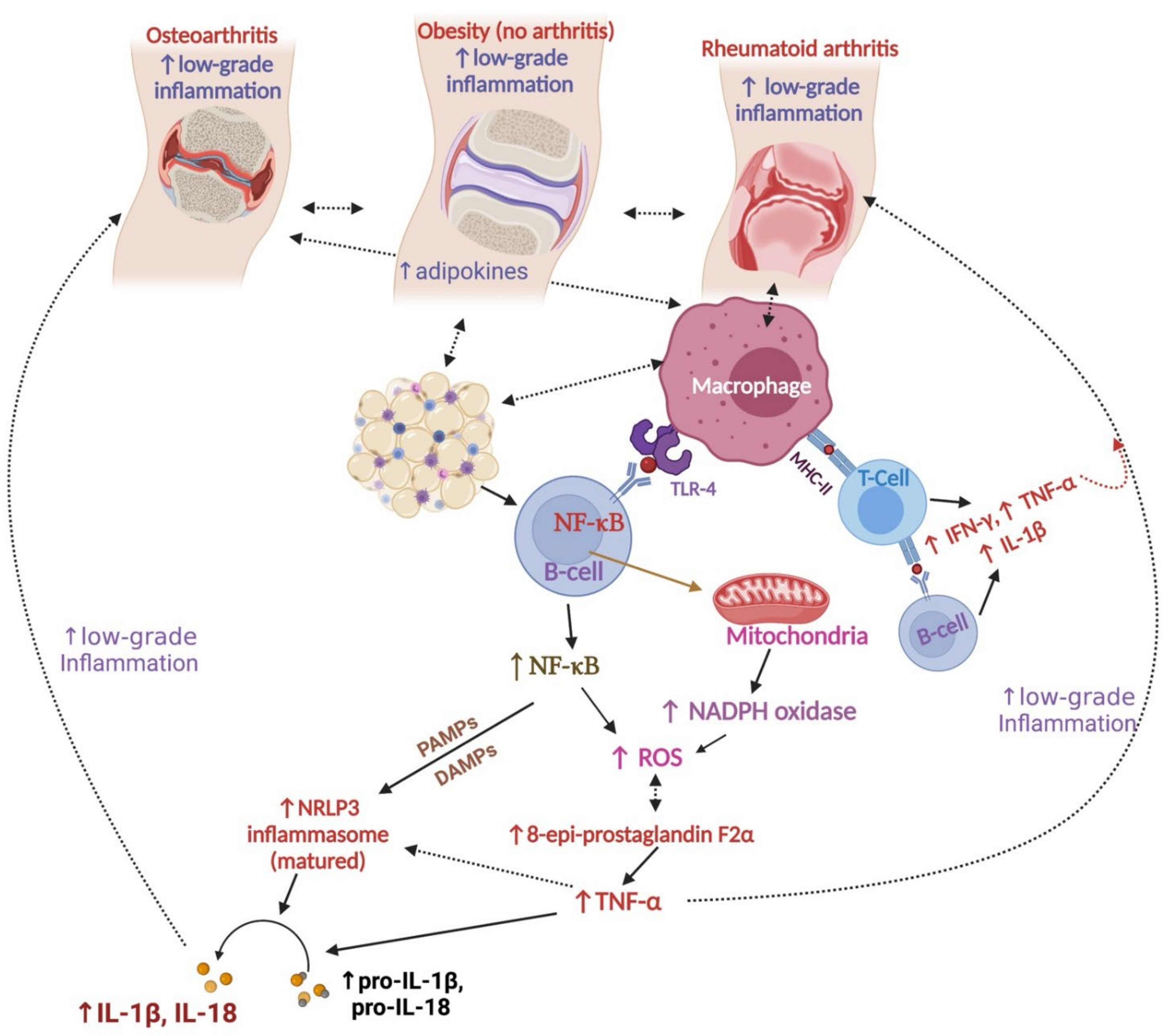
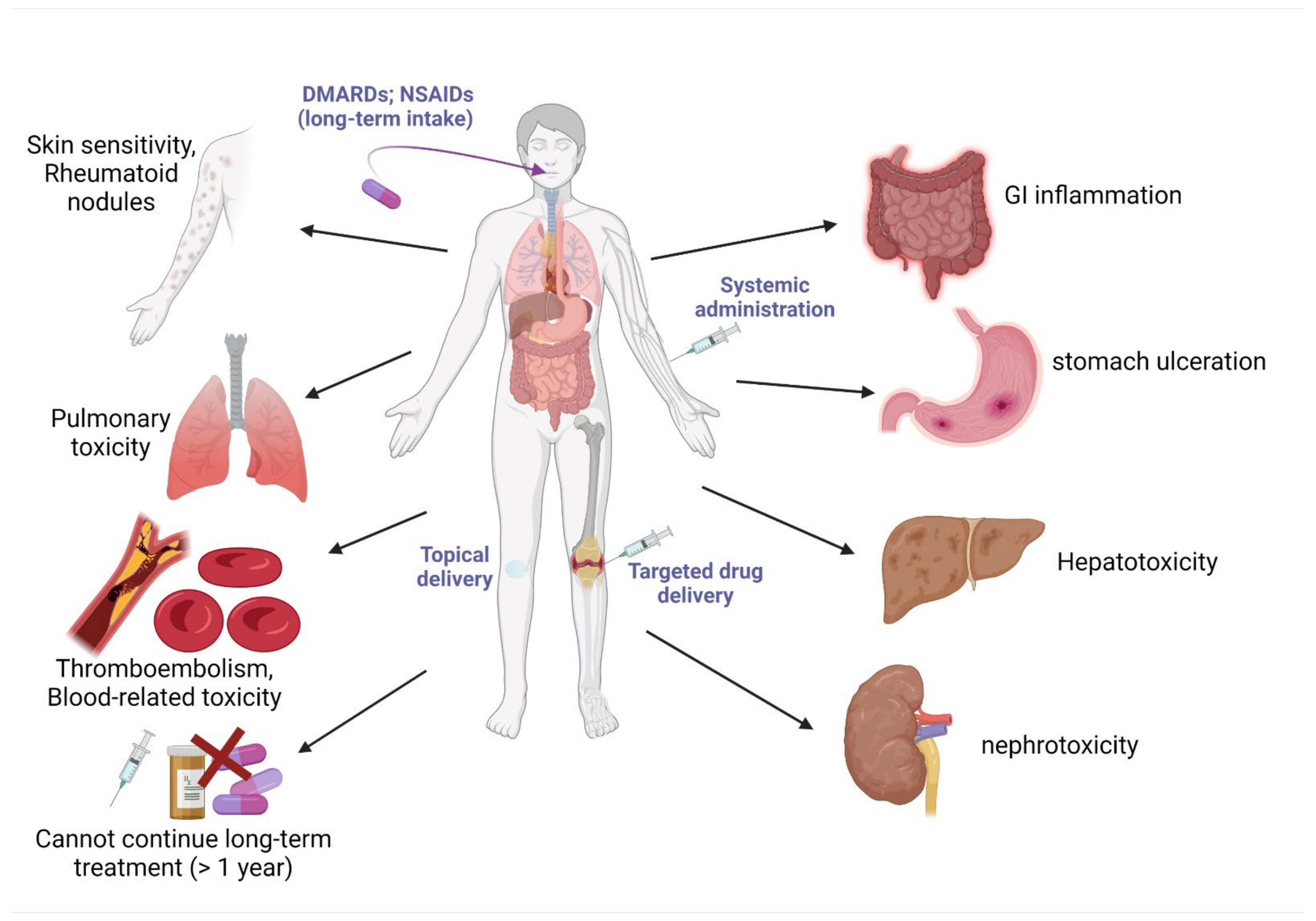
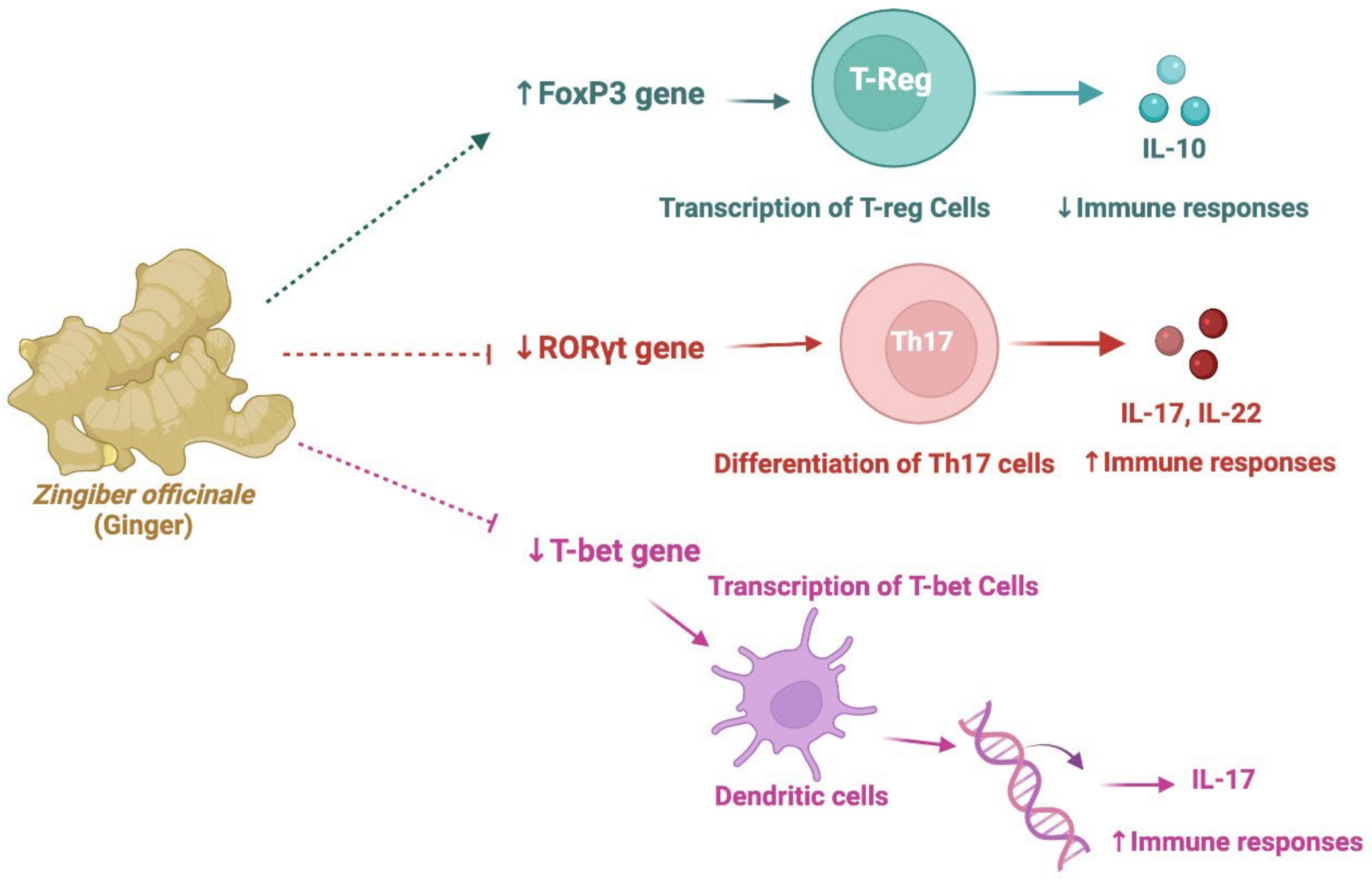
| Name | Traditional Use(s) | Clinical Evidence (Total n, n in Each Group) | Formulation (Treatment Duration, Days) | Arthritis Measurement Parameters | Arthritic Pain Measurement Parameters | Reference |
|---|---|---|---|---|---|---|
| Curcuma longa | Against asthma, allergies, food poisoning, rheumatism, liver disorders, and inflammation (rhizome) | Improved RA (total 90, n ≅ 30) | 2 or 4 caps/day for 84 days (0.25 g/cap turmacin) | Stair mill test | VAS | [152,153] |
| Curcuma longa | - | Decreased knee OA (total 150 n ≅ 50) | 2 or 3 caps/day for 90 days (46.7 mg turmeric extract) | PGADA; serum sColl2-1 | VAS; KOOS | [154] |
| Boswellia serrata | Rheumatism | Decreased knee OA (total 48, n ≅ 24) | 169.33 mg/cap for 120 days (87.3 mg β-boswellic acids) twice daily | MRI to inspect changes in knee joint gap and osteophytes | Pain and stiffness | [155,156] |
| Boswellia serrata | - | Decreased knee OA (total 43 n = 20 (BSE); 23(SLBSP)) | SLBSP; BSE: three times daily for 60 days | CTX-II (in urine); IL-2, IL-4, IL-6, TNF-α, and IFN-γ (in serum) | WOMAC, VAS | [157] |
| Tinospora cordifolia (formulation of: T. cordifolia, Zingiber officinale, W. somnifera, and T. terrestris) | Leprosy, fever, asthma, anorexia, jaundice, gout, skin infections, diabetes, chronic diarrhea, and dysentery | Reduced knee OA (total 121, n ≅ 40 per group) | 4 caps/day for 168 days (water extracts: 750 mg daily) | Joint counts, global disease assessments, and health assessment questionnaires; plasma inflammatory cytokines. | VAS | [158,159] |
| Tinospora cordifolia (Formulation of: T. cordifolia, Boswellia serrata, Emblica officinalis, Zingiber officinale) | - | Reduced knee OA (total 440, n = 110 per group) | 6 caps for 168 days (2 caps three times daily) | Functional difficulty Likert score | VAS; Modified WOMAC | [160] |
| Commiphora myrrha | Anti-inflammatory, hepatoprotective, muscle relaxing, anti-arthritic, anti-obesity, and anti-malarial | Reduced knee OA (total 100, n = 50 per group) | 0.5 g (Commiphora myrrha) tab twice daily for 84 days | KSF-36, personal evaluation, and laboratory analysis | VAS | [161,162] |
| Zingiber officinale | Colds, nausea, arthritis, migraines, and hypertension | Improved OA (total 60, n = 20/group) | Ginger (750 mg cap daily); ginger plus Diclofenac tab (750 mg + 50 mg) for 84 days | WOMAC | VAS | [163,164] |
| Zingiber officinale | - | Improved RA (total 70, n = 35/group) | 2 caps/day (750 mg ginger/cap) for 84 days | Gene expression of FoxP3, RORγt, and T-bet. Disease Activity Score-28 | - | [165] |
| Piper nigrum (mixed with: Curcuma longa, and Zingiber officinale) | Improved knee OA (total 60, n = 30/group) (compared against Naproxen) | 2 caps/day for 28 days (ingredients: 300 mg curcumin, 7.5 mg gingerols, and 3.75 mg piperine) | Reduced prostaglandin E2 levels | Beck’s International Questionnaire | [166] |
| Family | Name | Parts Used | Potential Ingredient(s) | Reference |
|---|---|---|---|---|
| Acanthaceae | Andrographis paniculata | Leaves | Andrographolide | [180,181] |
| Amaryllidaceae | Allium sativum | Essential oil | Diallyl disulfide, diallyl trisulfide, diallyl tetrasulfide | [182] |
| Anacardiaceae | Semecarpus anacardium | Nut, milk extract (as per Siddha formulary) | Bioflavonoids | [183,184,185,186] |
| Apiaceae | Centella asiatica | Leaves (alcoholic extract) | Madecassoside, triterpenoid glycoside, asiaticoside | [187,188,189] |
| Apiaceae | Coriandrum sativum | Herb, fruit, seed, essential oils, hydroalcoholic extract | Cineole | [190,191] |
| Apocynaceae | Calotropis procera | Leaves, seeds, roots | Benzoyllineolone, benzolisolineolone | [192] |
| Apocynaceae | Hemidesmus indicus | Roots | Terpenoids | [193] |
| Araliaceae | Acanthopanax chiisanensis | Leaves | Chiisanoside, chiisanogenin | [194] |
| Araliaceae | Panax notoginseng | Ethanol extract, n-butanol extract | Ginsenoside | [195,196,197,198] |
| Asparagaceae | Anemarrhena asphodeloides | Roots | Mangiferin, polysaccharides, fructan | [199,200] |
| Asparagaceae | Asparagus racemosus | Hydroalcoholic extract | Shatavarin, saponin | [201,202] |
| Asparagaceae | Yucca schidigera | Bark, methanolic extract | Resveratrol, trans-3,3′,5,5′-tetrahydroxy -4′- methoxystilbene, yuccaols, spirobiflavonoids | [203,204,205] |
| Asteraceae | Pluchea lanceolata | Root, hydroalcoholic extract | Sorghumol acetate, boehmerol acetate | [206,207,208] |
| Asteraceae | Siegesbeckia orientalis | Ethanolic extract | Kirenol | [209,210] |
| Asteraceae | Tanacetum parthenium | Inflorescence | Parthenolide | [211,212,213] |
| Asteraceae | Tanacetum vulgare | Aerial parts, methanolic extract, hydroalcoholic extract | 3,5-O-dicaffeoylquinic acid (3,5-DCQA) | [214,215] |
| Asteraceae | Xanthium strumarium | Fruits, methanolic extract | Sesquiterpenoids, phenylpropanoids, lignanoids, coumarins, steroids, glycosides, flavonoids, thiazides, anthraquinones, naphthoquinones | [216,217,218] |
| Berberidaceae | Berberis vulgaris | Root extract | Berberine | [219,220] |
| Boraginaceae | Arnebia euchroma | Entire herb (alcoholic extract) | Hydroxy naphthaquinone | [221,222] |
| Bromeliaceae | Ananas comosus | Fruit | Bromelain | [223,224] |
| Burseraceae | Boswellia carteri | Resin | Boswellic acids | [156,225] |
| Burseraceae | Boswellia frereana | Resin | Boswellic acid, epi-lupeol | [226,227] |
| Burseraceae | Boswellia serrata | Resin | 3-Oacetyl-11-keto-β-boswellic acid, boswellic acid | [228,229] |
| Caesalpiniaceae | Caesalpinia pulcherrima | Plant, alcoholic extract | ß-Amyrin, glucose, aspartic acid, glycine, proline, caesalpulcherrins | [230,231] |
| Cannabaceae | Cannabis sativa Cannabis indica | Leaves | Cannabidiol | [232,233,234] |
| Capparaceae | Capparis spinosa | Ethanol extract, water extract | P-hydroxy benzoic acid, 5-(hydroxymethyl) furfural; bis(5- formylfurfuryl) ether, daucosterol; α-dfructofuranosides, uracil, stachydrine | [235,236,237] |
| Caprifoliaceae | Lonicera japonica | Dried leaves, dried flowers, water extract | Chlorogenic acid, ioniflavone, polysaccharides | [200,238,239,240,241] |
| Celastraceae | Tripterygium wilfordii | Entire herb, flower, ethyl acetate extracts | Celastrol, macrocyclic dilactone, valerian-type sesquiterpenes, triptolide (diterpene), alkaloids (celabazine, celacinnine, celafurine, and celallocinnine) | [242,243,244,245,246] |
| Cleomaceae | Cleome gynandra | Ethanolic extract | Triterpenes, tannins, anthroquinones, flavonoids, saponins, steroids | [247] |
| Combretaceae | Terminalia chebula | Fruits, hydroalcoholic extract | Chebulic acid, chebulagic acid, chebulinic acid, ellagic acid | [248,249,250,251,252,253] |
| Convolvulaceae | Erycibe obtusifolia | Stems | Scopoletin | [254,255] |
| Cucurbitaceae | Citrullus colocynthis | Herb, aqueous extract | Alkaloids, glycosides, flavonoids, tannins, sterols | [177,256,257] |
| Cucurbitaceae | Thladiantha dubia | Fruit | Polysaccharides | [258] |
| Cuscutaceae | Cuscuta reflexa | Alcoholic extract | Dulcitol, mannitol, sitosterol, lycopene, apigenin-7-β-rutinoside, 6-7 dimethoxy coumarin, quercetin, hyperoside, propenamide, reflexin, lutein, cuscutin, cuscutalin, kaempferol, kaempferol-3-O-glucoside | [259,260,261] |
| Fabaceae | Bauhinia tarapotensis | Leaves (chloroform extract) | Triterpenic acids of ursane and oleanane | [262] |
| Fabaceae | Sophora flavescens | Rhizomes | Kurarinone, kuraridin, isoxanthohumol | [263,264] |
| Fabaceae | Trigonella foenum-graecum | Seeds, alcoholic extract, | Choline, mucilage, trigonelline | [177,265,266,267] |
| Lamiaceae | Lavandula multifida | Aerial parts, essential oils | Linalool, camphene, linalyl acetate, α-thujene, bornyl acetate, β-caryophellene | [262,268] |
| Lamiaceae | Leucas aspera | Ethanolic extract | Epicatechin, β-epicatechin, procyanidin, β-sitosterol | [269,270] |
| Lamiaceae | Rosmarinus officinalis | Aerial parts, water extract, ethanol extract, essential oils | Carnosic acid, α-pinene, camphene, β-pinene, myrcene | [271,272,273,274] |
| Lamiaceae | Salvia miltiorrhiza | Flower, hydroalcoholic extracts | Tanshinone, cryptotanshinone | [275,276,277] |
| Lamiaceae | Vitex negundo | Seeds, leaves, | Lignans (e.g., vitexdoins), Tris(2,4-di-tert-butylphenyl) phosphate | [278,279] |
| Lauraceae | Cinnammomum zeylicanium | Bark, essential oil | Cinnamaldehyde, eugenol, cymene, caryophyllene | [177,178,179] |
| Lauraceae | Lindera aggregata | Dry roots | Norisoboldine | [280,281,282] |
| Lauraceae | Litsea guatemalensis | Etanolic extract, essential oils | 5,7,3′,4′-Tetrahydroxy-isoflavone, pinocembrin, scopoletin | [283] |
| Lecythidaceae | Barringtonia racemosa | Fruits | Bartogenic acid | [284] |
| Loganiaceae | Strychnos nux-vomica | Seeds | Brucine, brucine n-oxide, strychnine | [285,286,287] |
| Lythraceae | Punica granatum | Seeds, leaves (juice), methanolic extract | Gallic acid, anthocyanins, ellagic acid, tannins, flavones, flavonoids, anthocyanidins, sterols | [288,289,290,291] |
| Malvaceae | Abutilon hirtum | Herb, essential oil | β-sitosterol, tocopherol, α-pinene, caryophyllene, caryophyllene oxide, endesmol, farnesol, borenol, geraniol, geranyl acetate, elemene and α-cineole | [292,293] |
| Malvaceae | Sida rhombifolia | Aerial parts, stems, roots, hydroalcoholic extract | Flavonoids, tannins, vitamin C | [294,295] |
| Meliaceae | Dysoxylum binectariferum | Seeds | Rohitukine | [296] |
| Oleaceae | Olea europaea | Leaves, fruit, compression-extracted oil | Omega-3 fatty acids, hydroxytyrosol | [297,298,299,300,301] |
| Oxalidaceae | Biophytum sensitivum | Inflorescence | Amentoflavone, polysaccharide | [227,302] |
| Paeoniaceae | Paeonia lactiflora | Flowers, roots, | Glucosides, gallic acid | [303,304] |
| Phyllanthaceae | Phyllanthus amarus | Aqueous extract | Phyllanthin, hypophyllanthin | [305,306,307,308] |
| Piperaceae | Piper longum | Seeds, aqueous extracts | Piperine, piperlongumine, piperlonguminine, methyl 3, 4, 5-trimehoxycinnamate | [309,310,311] |
| Poaceae | Saccharum officinarum | Whole plant, wax oil | Palmitic, oleic, linoleic, and linolenic acids | [312,313] |
| Polyporaceae | Poria cocos (saprophytic fungus) | Sclerotium | Triterpenoids | [314] |
| Ranunculaceae | Clematis vitalba | Aerial parts | Vitalboside | [315] |
| Ranunculaceae | Coptidis rhizoma | Roots and rhizomes | Berberine | [258,316] |
| Ranunculaceae | Nigella sativa | Seeds, compression-extracted oil | Thymoquinone | [317,318,319,320] |
| Rosaceae | Chaenomeles speciosa | Hydroalcoholic extract | Chlorogenic acid | [321,322,323,324] |
| Rosaceae | Rosa canina | Water extract | Terpenoids, galactolipids, carotenoids, fruit acids, fatty oils, phenolics, | [325,326,327] |
| Rubiaceae | Lasianthus acuminatissimus | Roots (methanolic and ethyly acetate extracts) | Anthraquinone glycosides, lasianthuoside, codonolactone | [328,329] |
| Rutaceae | Ruta graveolens | Methanolic extract | 8-Methoxycoumarin | [330,331,332] |
| Solanaceae | Cestrum diurnum | Leaves, alcoholic extract | Ursolic acid | [333,334] |
| Solanaceae | Withania somnifera | Roots, leaves, water extract | Withanolides (steroidal lactones) | [335,336,337] |
| Verbenaceae | Lantana camara | Leaves, methanolic extract | Triterpenoids | [338,339,340] |
| Verbenaceae | Lawsonia inermis | Leaves, hydroalcoholic extract | Lawsone, luteolins, apigenin, esculetin, scopletin | [341,342] |
| Xanthorrhoeaceae | Aloe vera | Gel from leaves | Anthroquinone glycosides | [343,344] |
| Zingiberaceae | Alpinia officinarum | Rhizomes | Diaryl heptanoids | [345] |
| Zingiberaceae | Curcuma longa | Rhizome | Curcumin | [346,347,348] |
| Zingiberaceae | Zingiber officinale | Rhizome, alcoholic extract | Gingerols, gingerdiols, phenylpropanoids, [6]-shogaol, shogaols | [349,350,351,352] |
| Oil Type | Key Findings | Reference |
|---|---|---|
| Evening primrose oil | Patients with RA (n = 40 total) and NSAID-induced GI lesions treated with γ-linolenic acid 540 mg/day (evening primrose oil 6 g/day) for 3 months slightly improved RA-related morning stiffness. | [355] |
| Lavender oil | Aromatherapy with lavender oil improved arthritic pain (against placebo) in patients (n = 30 each group) with knee osteoarthritis, but no proof of its long-term efficacy. | [356] |
| Lavender oil | Aromatherapy with lavender oil improved daily routine activities of patients (n = 30 each group) with knee osteoarthritis (against placebo), but no proof of its long-term efficacy. | [357] |
| Mouthwash with essential oils and curcumin | Gargling with mouthwash containing essential oils and curcumin (MEC) over 6 weeks reduced periodontal disease and RA-related parameters (n = 15 each group) | [354] |
| Essential Oil | Key Findings | Reference |
|---|---|---|
| Garlic essential oil | Daily consumption of garlic essential oil (25, 50, and 100 mg/kg) or diallyl disulfide (10 and 20 mg/kg) for 12 weeks in C57BL/6J mice prevented the development of non-alcoholic fatty liver disease. The oil and its major compound also significantly prevented the release of proinflammatory cytokines from murine livers. | [359] |
| Ginger essential oil | Ginger essential oil (28 mg/kg/day i.p. for 4 weeks) treatment improved joint inflammation caused by streptococcal cell-wall-induced arthritis in female Lewis rats. | [351] |
| Pogostemon cablin Benth. or patchouli essential oil | Inhalation of the oil reduced food intake, systolic blood pressure, and plasma low-density lipoprotein cholesterol levels in SD rats. | [360] |
| Rhaponticum acaule (L.) DC. | Treatment inhibited xanthine oxidase and turkey pancreatic lipase, thus reducing oxidative stress and pancreatitis. | [361] |
| Ginger essential oil (GEO) | Male C57BL/6J mice with a high-fat diet (HFD) mixed with GEO (12.5, 62.5, and 125 mg/kg) or citral (2.5 and 25 mg/kg) for 12 weeks showed improved HFD-induced obesity by reducing triglyceride and total cholesterol levels. In addition, the treatment reduced inflammatory response in murine liver. | [362] |
| Pinus koraiensis Siebold and Zucc. leaf essential oil | Treatment inhibited the level of cholesterol acyltransferase-1 and -2, as well as low-density lipoprotein (LDL) oxidation activity; thus, it may act against hyperlipidemia. | [363] |
| Citrus aurantifolia (Christm.) swingle essential oil | Forty-five days of treatment with this oil (125 mg/kg/day, s.c.) prevented ketotifen (32 mg/kg/day s.c.)-induced body weight gain and food intake in mice. | [364] |
| Artemisia annua L. essential oil | Treatment reduced obesity-related PPAR-γ, C/EBP-α, SREBP-1c, FAS, and ACC levels in vitro using 3T3-LI cells. | [365] |
| Lavandula pubescens Decne. essential oil | L. pubescens EO was assessed against pancreatic lipase inhibitory activity with an IC50 of 1.08 μL/mL (in vitro). | [366] |
| Material | Key Findings | Reference |
|---|---|---|
| Nigella sativa oil | Daily consumption of capsules with oil (2 mg/day over a period of 8 weeks) improved HDL-C and lowered LDL-C and TC/HDL-C ratio compared to placebo in obese and overweight women. | [367] |
| Nigella sativa oil | Patients were given a low-calorie diet supplemented with Nigella sativa oil (3 g/day for 8 weeks) (n = 45 each group), which reduced TNF-α and hepatic C-reactive protein levels, but no changes were observed in plasma IL-6 levels in obese (BMI: 30–35 kg/m2) women aged 25–50 years old. | [368] |
| Opuntia ficus-indica | Natural fiber complex (litramine) was 3 g/day with a low-calorie diet for 12 weeks, which reduced body weight compared to placebo in obese women (total n = 133) | [369] |
| Camellia sinensis | Green tea (n = 32; 1 g of dry green tea extract in capsule/day) reduced total cholesterol (TC) and LDL-C after 12 weeks of treatment in non-diabetic obese women. | [370] |
| Crocus sativus | Saffron reduced hyperglycaemia and hyperlipidaemia and improved liver function in patients with type 2 diabetes in an 8-week randomized clinical trial. | [371] |
| Laminaria digitata (brown seaweed) | Treatment with sodium alginate from Laminaria digitata over a period of 10 days showed no effects in an anti-obesity related trial. | [372] |
| Lycium barbarum (fruit juice) | A single-day bolus drink increased metabolic rate; 120 mL of fruit juice per day for 2 weeks reduced waist circumference in overweight men and women (n = 15, BMI = 29, age = 34 years). | [373] |
| Allium sativum | Consumption of 1.6 g of garlic powder (4 × 400 mg tablets daily, for 12 weeks) produced significant decreases in waist circumference and body fat percentage in patients with non-alcoholic fatty liver disease (n = 45). | [374] |
| Allium cepa | Onion powder (9 g per day for 12 weeks) did not cause any major changes between groups. | [375] |
| Persea americana | Avocados are a natural source of lutein. Daily oral consumption of 300 mg/day of ASU-E (Avocado–Soybean Unsaponifiables, Expanscience—a formula with a 1:3 ratio of avocado: soybean oil) for 3 years did not cause any changes in joint space width loss compared to the placebo group. | [376] |
| Momordica charantia | Oral consumption of Momordica charantia (3 × 500 mg per capsule daily for 3 months) taken thrice daily reduced body weight, body mass index, fasting blood glucose levels, and Knee Injury and Osteoarthritis Outcome scores. | [377] |
| Cissus quandrangularis | Consumption of (n = 35) aqueous extract of Cissus quandrangularis (300 mg/day, over 8 weeks) reduced body fat and improved blood parameters related to metabolic syndrome in overweight patients. | [378] |
| Flavonoid’s Name | Role against Obesity or Arthritis | Reference |
|---|---|---|
| Apigenin | RA was induced by 0.1 mL Freund’s complete adjuvant (FCA) injections in the palmar surface of paws of Sprague–Dawley (SD) rats. Apigenin suppressed the expressions of P2X7/NF-κB signaling and associated RA-related inflammatory reactions (e.g., reduced IL-1β, Il-6 and TNF-α) | [388] |
| Apigenin | RA was induced in a murine collagen-induced arthritis (CIA) model. Apigenin inhibited CIA by repressing synovial hyperplasia (by reducing the multiplication of fibroblast-like synoviocytes), causing the growth of new blood vessels and osteoclastogenesis. | [389] |
| Cyanidin | The effects of cyanidin-3-O-glucoside were investigated in a murine high-fat-diet-induced non-alcoholic fatty liver disease (NAFLD) model. Treatment with this flavone reduced NLRP3 inflammasome activation, oxidative stress, and steatosis in mice. | [390] |
| (-)-Epigallocatechin-3-O-gallate (EGCG) | Over a period of 3 days, 300 mg of EGCG drink increased postprandial fat oxidation in obese men similarly to 200 mg of caffeine, but the effect was not observed with 600 mg of EGCG drink. Limitation: total n = 10, pilot study. | [391] |
| (-)-Epigallocatechin-3-O-gallate (EGCG) | Consumption of EGCG and resveratrol (282 mg and 80 mg/day over a period of 12-week accordingly) increased oxidative capacity in permeabilized muscle fibers, but showed reduced plasma triacylglycerol concentration in a high-fat mixed-meal assay in obese men (n = 18). | [392] |
| Genistein | Consumption of 15 g of genistein for 3 months (5 days of daily administration per week plus 2 days without treatment) in adult patients (53% men) reduced blood glucose and malondialdehyde levels, but did not impact on lipid profile. | [393] |
| Kaempferol | Treatment with 200 mg/kg of kaempferol (over eight weeks) with a high-fat diet in C57BL/6 mice reduced the increases in body and liver weight, serum cholesterol, and triglyceride levels | [394] |
| Luteolin | Luteolin increased the expression of liver X receptor (LXR)-α (in vitro). Luteolin (0.05% w/w in high fat diet) reduced plasma cholesterol and low- and very-low-density lipoprotein cholesterols in male C57BL/6 mice. | [395] |
| Puerarin | Obese women with polycystic ovary syndrome (PCOS) took 150 mg/d of puerarin tablets for 3 months in addition to their standard treatment, and showed decreased total cholesterol and systolic blood pressure compared with their pre-treatment levels. | [396] |
| Quercetin | Quercetin (500 mg/day for 8 weeks) reduced RA symptoms (based on an assessment questionnaire) and high-sensitivity tumor necrosis factor α (hs-TNF-α) in women with RA. | [397] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes Pereira, M.; et al. The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review. Nutrients 2022, 14, 985. https://doi.org/10.3390/nu14050985
Paul AK, Jahan R, Paul A, Mahboob T, Bondhon TA, Jannat K, Hasan A, Nissapatorn V, Wilairatana P, de Lourdes Pereira M, et al. The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review. Nutrients. 2022; 14(5):985. https://doi.org/10.3390/nu14050985
Chicago/Turabian StylePaul, Alok K., Rownak Jahan, Anita Paul, Tooba Mahboob, Tohmina A. Bondhon, Khoshnur Jannat, Anamul Hasan, Veeranoot Nissapatorn, Polrat Wilairatana, Maria de Lourdes Pereira, and et al. 2022. "The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review" Nutrients 14, no. 5: 985. https://doi.org/10.3390/nu14050985
APA StylePaul, A. K., Jahan, R., Paul, A., Mahboob, T., Bondhon, T. A., Jannat, K., Hasan, A., Nissapatorn, V., Wilairatana, P., de Lourdes Pereira, M., Wiart, C., & Rahmatullah, M. (2022). The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review. Nutrients, 14(5), 985. https://doi.org/10.3390/nu14050985









