Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study
Abstract
:1. Introduction
2. Materials and Methods
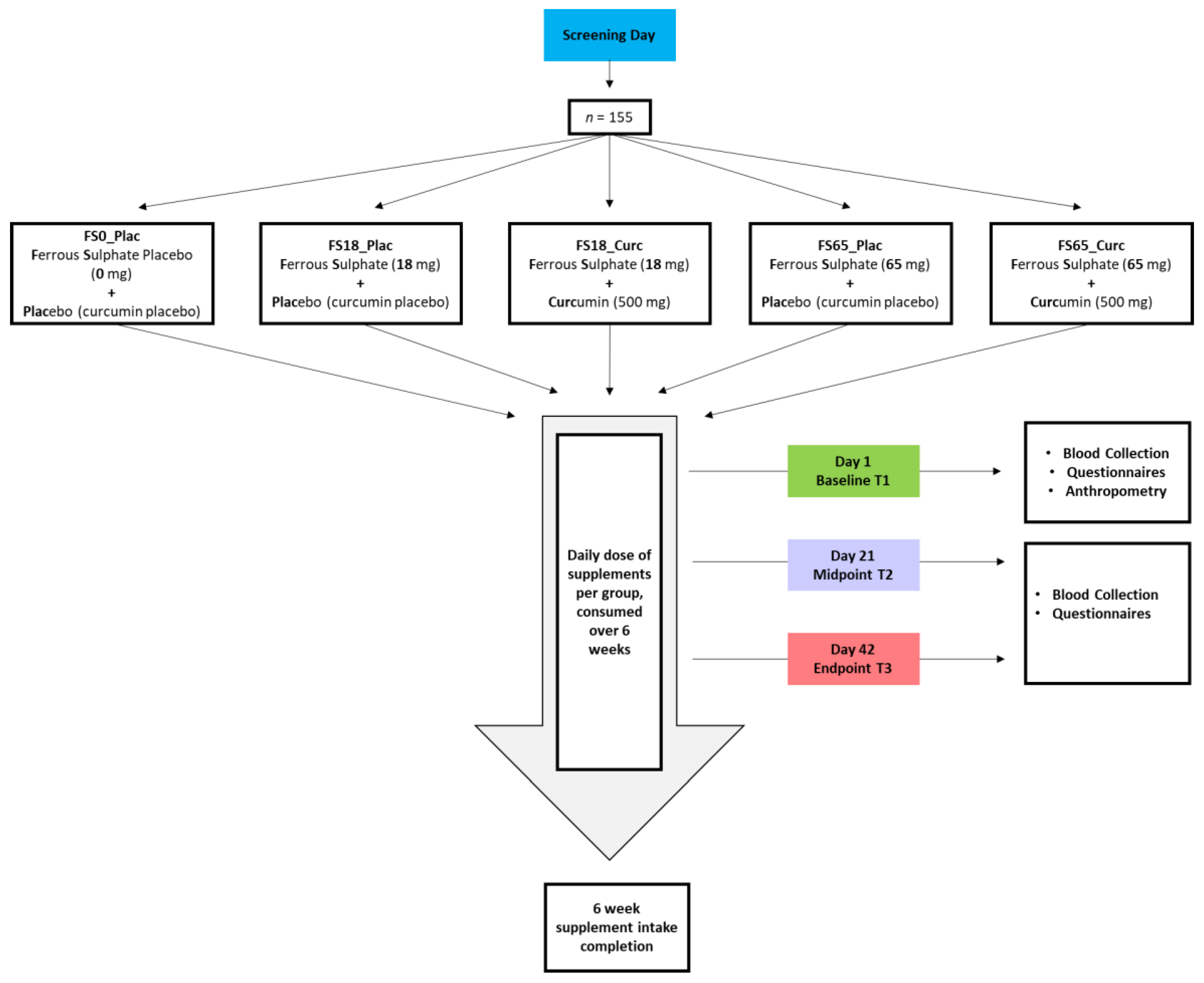
2.1. Study Design
2.2. Ethics Approval and Study Registration
2.3. Inclusion and Exclusion Criteria for Participants
2.4. Safety Screening
2.5. Treatment Groups
2.6. Supplements
2.7. Physical Examination
2.8. Blood Sample Collection and Processing
2.9. Analysis of Serum Ferritin Concentration and C-Reactive Protein (CRP)
2.10. Analysis of Serum Iron Profile
2.11. Analysis of Whole Blood Haemoglobin (HGB) Concentration
2.12. Analysis of Interleukin 6 (IL-6), Interleukin 10 (IL-10), Interleukin 1 Beta (IL-1β), and Tumour Necrosis Factor (TNF)
2.13. Analysis of Thiobarbituric Acid Reactive Substances (TBARS)
2.14. Fatigue Severity Scale (FSS)
2.15. Fatigue Visual Analogue Scale (F-VAS)
2.16. Questionnaire to Assess GI Symptoms after Oral Ferrous Iron Supplementation
2.17. Study Compliance and Adverse Reactions
2.18. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Inflammatory Biomarkers
3.2.1. Effect of Supplementation on Plasma CRP Levels
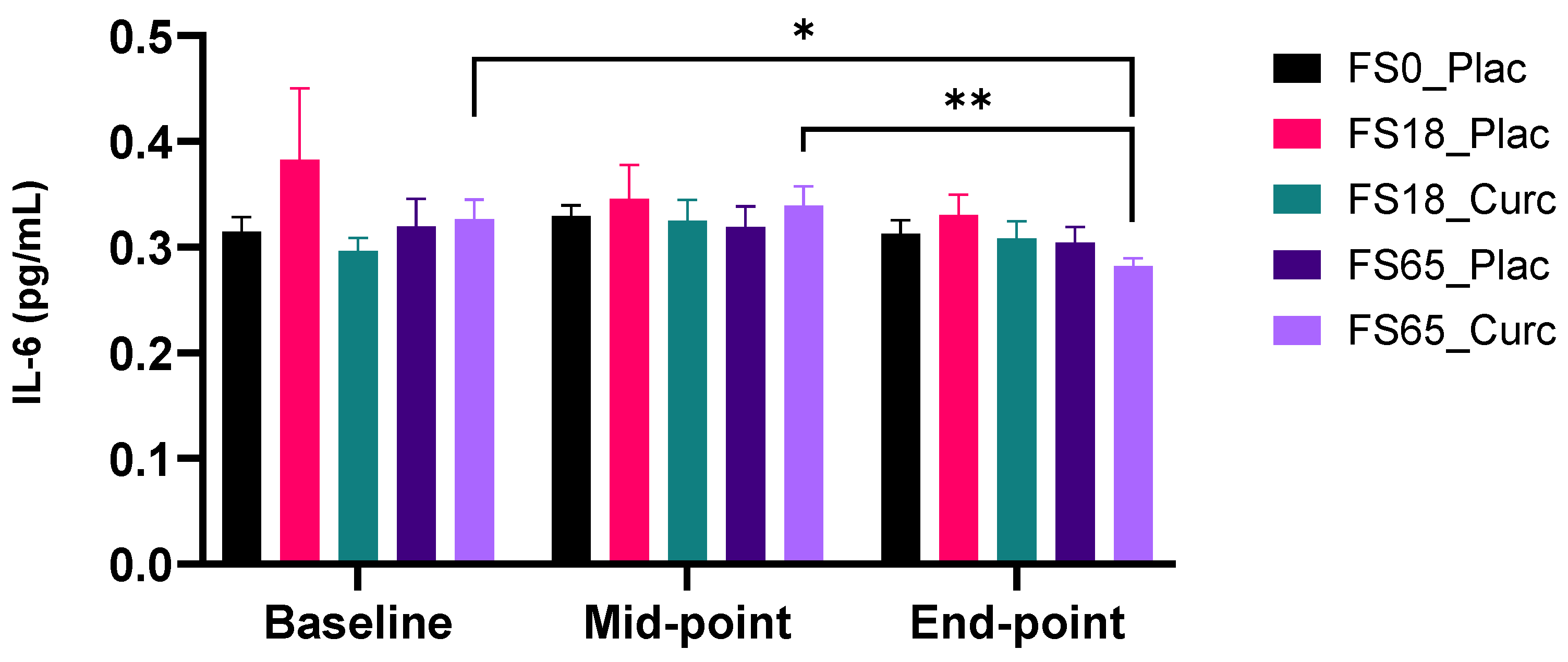
3.2.2. Effect of Supplementation on Plasma IL-6 Levels
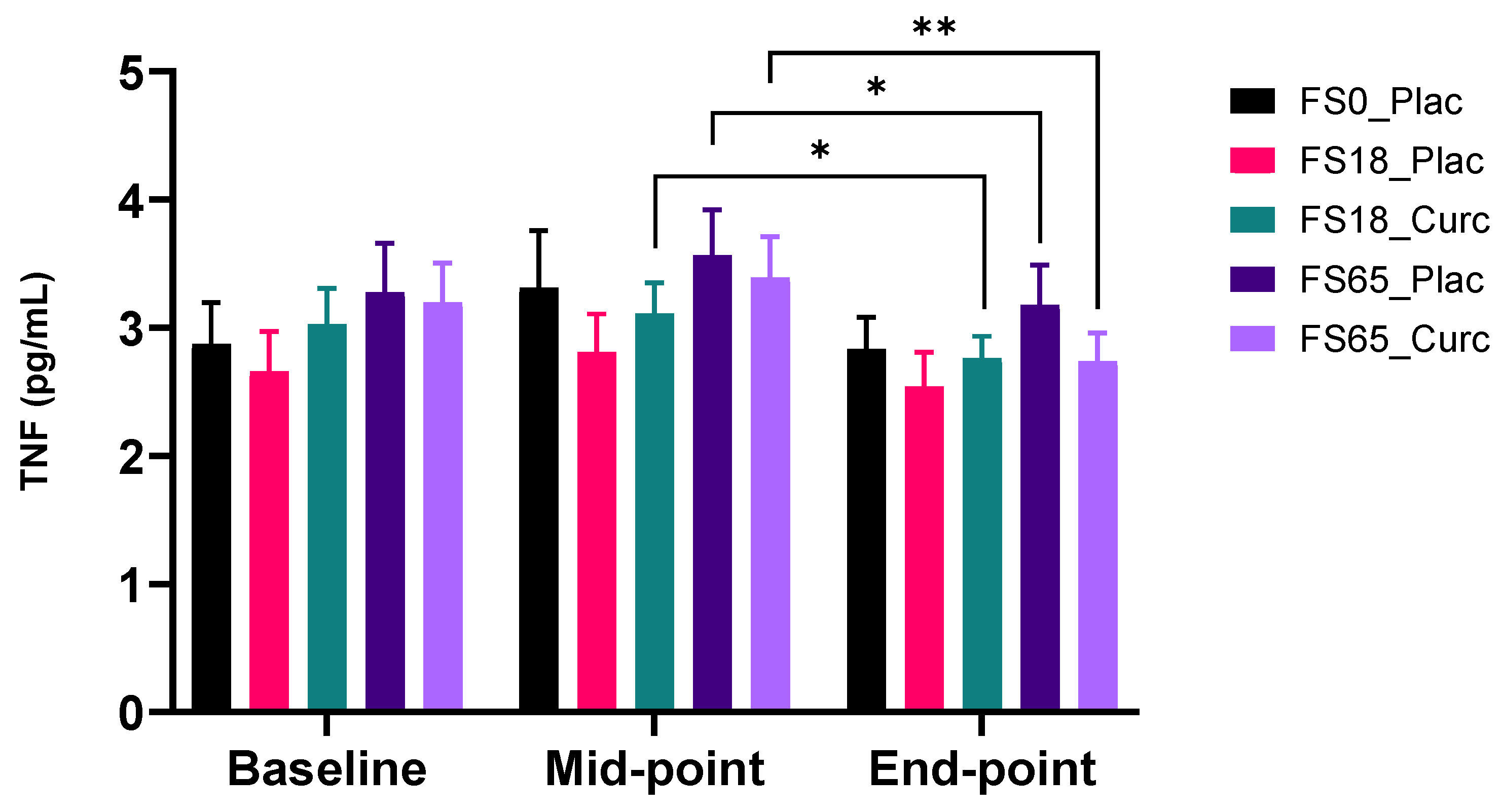
3.2.3. Effect of Supplementation on Plasma TNF
3.2.4. Effect of Supplementation on Plasma IL-1β
3.2.5. Effect of Supplementation on Plasma IL-10 Levels
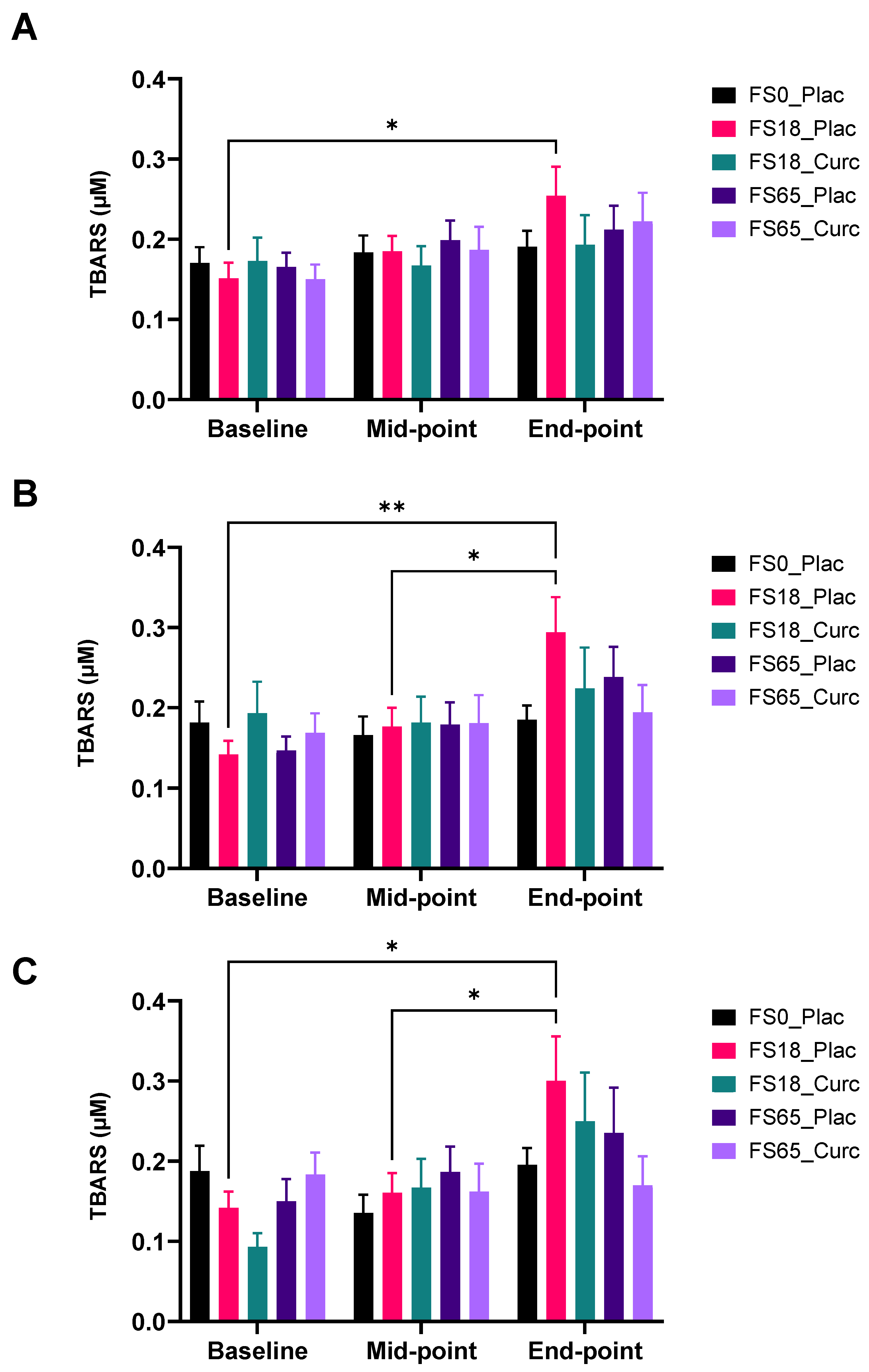
3.3. Oxidative Stress Marker
Effect of Supplementation on Serum (TBARS) Levels
3.4. Iron Status Markers
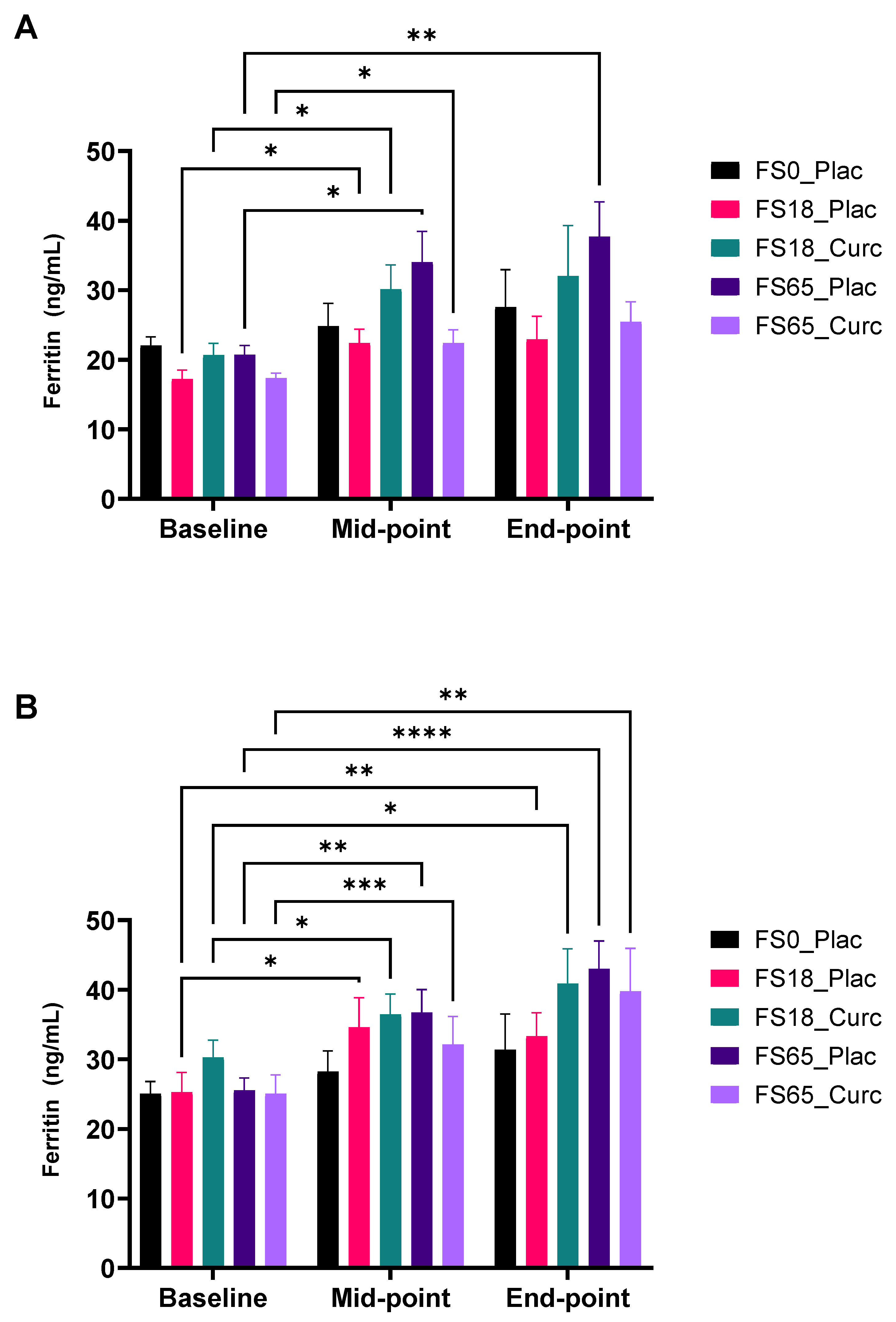
3.4.1. Effect of Supplementation on Serum Ferritin Levels
3.4.2. Effect of Supplementation on Serum Fe Levels
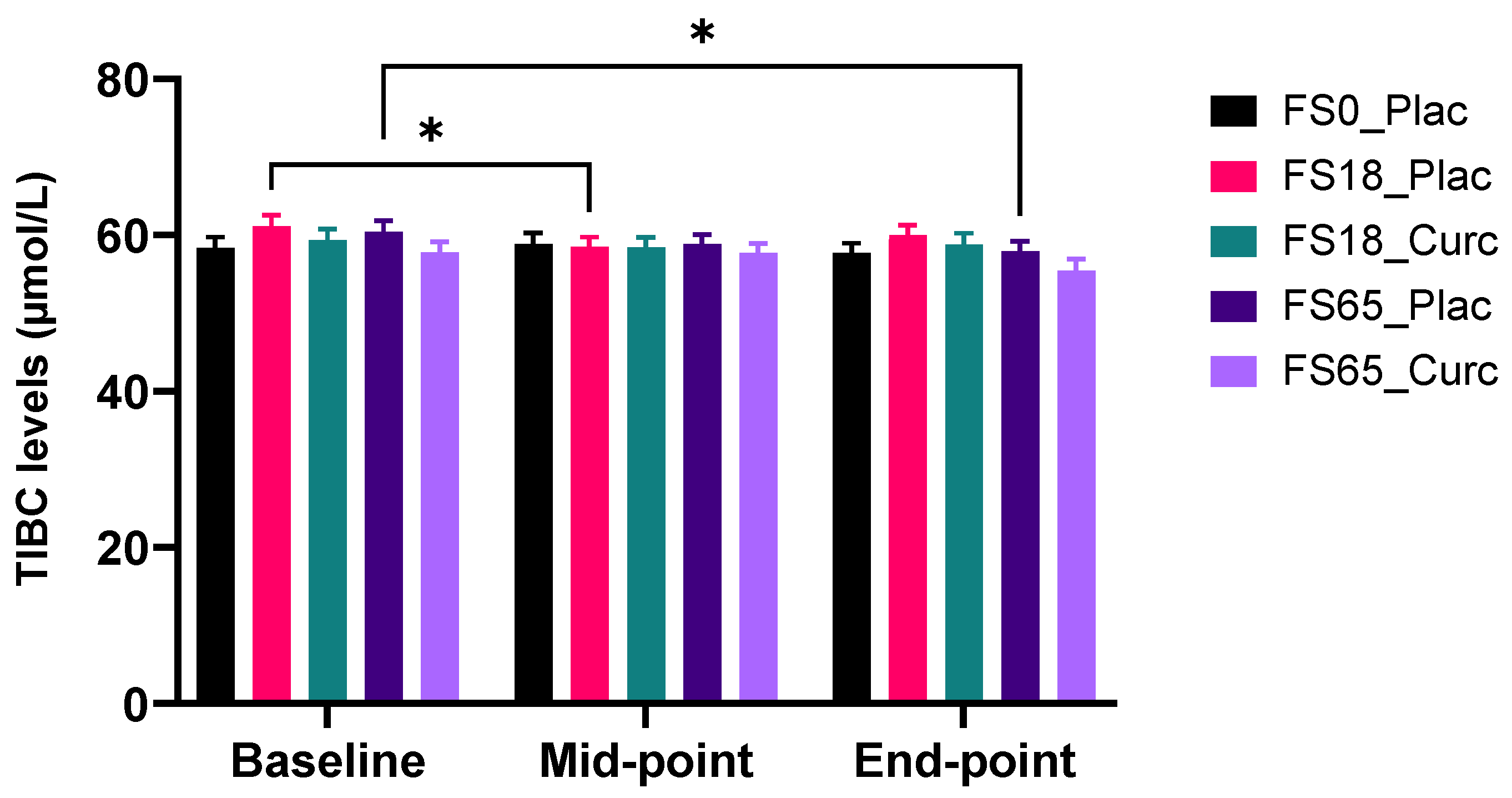
3.4.3. Effect of Supplementation on Serum TIBC Levels
3.4.4. Effect of Supplementation on Serum UIBC Levels
3.4.5. Effect of Supplementation on Serum TS%
3.5. Subjective Markers
3.5.1. Gastrointestinal Side-Effects: Frequency of Darker and Black Bowel Movements
3.5.2. Effect of Supplementation on Other Symptoms
3.5.3. Effect of Supplementation on Subjective Fatigue Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pasricha, S.R.S.; De-Regil, L.M. Daily iron supplementation for improving iron status and health among menstruating women (Protocol). Cochrane Database Syst. Rev. 2012, 4, CD009747. [Google Scholar]
- Gasche, C.; Lomer, M.C.; Cavill, I.; Weiss, G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasche, C.; Berstad, A.; Befrits, R.; Beglinger, C.; Dignass, A.; Erichsen, K.; Gomollon, F.; Hjortswang, H.; Koutroubakis, I.; Kulnigg, S.; et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm. Bowel Dis. 2007, 13, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Brownlie, T., IV. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [Green Version]
- Brownlie, T., IV; Utermohlen, V.; Hinton, P.S.; Giordano, C.; Haas, J.D. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am. J. Clin. Nutr. 2002, 75, 734–742. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.J.; Brown, W.J.; Powers, J.R.; Roberts, D.C.K. Iron deficiency, general health and fatigue: Results from the australian longitudinal study on women’s health. Qual. Life Res. 2000, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [Green Version]
- Carrier, J.; Aghdassi, E.; Cullen, J.; Allard, J.P. Iron supplementation increases disease activity and vitamin e ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2002, 132, 3146–3150. [Google Scholar] [CrossRef] [Green Version]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I. Oral ferrous sulfate supplements increase the free radical–generating capacity of feces from healthy volunteers. Am. J. Clin. Nutr. 1999, 69, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Orozco, M.N.; Solomons, N.W.; Schumann, K.; Friel, J.K.; de Montenegro, A.L.M. Antioxidant-rich oral supplements attenuate the effects of oral iron on in situ oxidation susceptibility of human feces. J. Nutr. 2010, 140, 1105–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Kortman, G.A.M.; Boleij, A.; Swinkels, D.W.; Tjalsma, H. Iron availability increases the pathogenic potential of salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS ONE 2012, 7, e29968. [Google Scholar] [CrossRef]
- Werner, T.; Wagner, S.J.; Martínez, I.; Walter, J.; Chang, J.S.; Clavel, T.; Kisling, S.; Schuemann, K.; Haller, D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 2011, 60, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Asperti, M.; Gryzik, M.; Brilli, E.; Castagna, A.; Corbella, M.; Gottardo, R.; Girelli, D.; Tarantino, G.; Arosio, P.; Poli, M. Sucrosomial® iron supplementation in mice: Effects on blood parameters, hepcidin, and inflammation. Nutrients 2018, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Toblli, J.E.; Cao, G.; Angerosa, M. Ferrous sulfate, but not iron polymaltose complex, aggravates local and systemic inflammation and oxidative stress in dextran sodium sulfate-induced colitis in rats. Drug Des. Dev. Ther. 2015, 9, 2585–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. BioMetals 2010, 23, 411–417. [Google Scholar] [CrossRef]
- Provenzano, R.; Schiller, B.; Rao, M.; Coyne, D.; Brenner, L.; Pereira, B.J. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadiiska, M.B.; Burkitt, M.J.; Xiang, Q.H.; Mason, R.P. Iron supplementation generates hydroxyl radical in vivo. An ESR spin-trapping investigation. J. Clin. Investig. 1995, 96, 1653–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldenburg, B.; Henegouwen, V.B.; Rennick, D.; Asbeck, V.; Koningsberger, J.C. Iron supplementation affects the production of pro-inflammatory cytokines in IL-10 deficient mice. Eur. J. Clin. Investig. 2000, 30, 505–510. [Google Scholar] [CrossRef]
- Reifen, R.; Matas, Z.; Zeidel, L.; Berkovitch, Z.; Bujanover, Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Am. J. Dig. Dis. 2000, 45, 394–397. [Google Scholar] [CrossRef]
- Coplin, M.; Schuette, S.; Leichtmann, G.; Lashner, B. Tolerability of iron: A comparison of bis-glycino iron II and ferrous sulfate. Clin. Ther. 1991, 13, 606–612. [Google Scholar]
- Assunção, M.; Santos-Marques, M.J.; Carvalho, F.; Andrade, J.P. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic. Biol. Med. 2010, 48, 831–838. [Google Scholar] [CrossRef]
- Bich, V.T.; Thuy, N.T.; Binh, N.T.; Huong, N.T.M.; Yen, P.N.D.; Luong, T.T. Structural and Spectral Properties of Curcumin and Metal-Curcumin Complex Derived from Turmeric (Curcuma Longa). In Physics and Engineering of New Materials; Springer Proceedings in Physics; Springer Science and Business Media, LLC.: Berlin, Germany, 2009; Volume 127, pp. 271–278. [Google Scholar]
- Reddy, A.C.P.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hjorth-Tønnesen, H.; Smistad, G.; Ågren, T.; Karlsen, J. Studies on curcumin and curcuminoids. XXIII: Effects of curcumin on liposomal lipid peroxidation. Int. J. Pharm. 1993, 90, 221–228. [Google Scholar] [CrossRef]
- Borsari, M.; Ferrari, E.; Grandi, R.; Saladini, M. Curcuminoids as potential new iron-chelating agents: Spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorg. Chim. Acta 2002, 328, 61–68. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.A.; González-Vergara, E.; Rojas-Hernández, A. Spectrophotometric and Electrochemical Determination of the Formation Constants of the Complexes Curcumin-Fe(III)-Water and Curcumin-Fe(II)-Water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1105–1113. [Google Scholar] [CrossRef]
- Ferrari, E.; Benassi, R.; Sacchi, S.; Pignedoli, F.; Asti, M.; Saladini, M. Curcumin derivatives as metal-chelating agents with potential multifunctional activity for pharmaceutical applications. J. Inorg. Biochem. 2014, 139, 38–48. [Google Scholar] [CrossRef]
- Srichairatanakool, S.; Thephinlap, C.; Phisalaphong, C.; Porter, J.; Fucharoen, S. Curcumin contributes to in vitro removal of non-transferrin bound iron by deferiprone and desferrioxamine in thalassemic plasma. Med. Chem. 2007, 3, 469–474. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Samba-Mondonga, M.; Constante, M.; Fragoso, G.; Calvé, A.; Santos, M.M. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLoS ONE 2019, 14, e0208677. [Google Scholar] [CrossRef] [Green Version]
- Tuntipopipat, S.; Zeder, C.; Siriprapa, P.; Charoenkiatkul, S. Inhibitory effects of spices and herbs on iron availability. Int. J. Food Sci. Nutr. 2009, 60, 43–55. [Google Scholar] [CrossRef]
- Lorinczova, H.; Begum, G.; Renshaw, D.; Zariwala, M. Acute Administration of Bioavailable Curcumin Alongside Ferrous Sulphate Supplements Does Not Impair Iron Absorption in Healthy Adults in a Randomised Trial. Nutrients 2021, 13, 2300. [Google Scholar] [CrossRef]
- Briskey, D.; Sax, A.; Mallard, A.; Rao, A. Increased bioavailability of curcumin using a novel dispersion technology system (LipiSperse®). Eur. J. Nutr. 2019, 58, 2087–2097. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin Modulates Nuclear Factor KB (Nf-KB)-Mediated Inflammation in Human Tenocytes in Vitro: Role of the Phosphatidylinositol 3-Kinase/Akt Pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [Green Version]
- Sung, B.; Pandey, M.K.; Ahn, K.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B. Anacardic Acid (6-Nonadecyl Salicylic Acid), an Inhibitor of Histone Acetyltransferase, Suppresses Expression of Nuclear Factor-κB-Regulated Gene Products Involved in Cell Survival, Proliferation, Invasion, and Inflammation through Inhibition of the Inhibitory Subunit of Nuclear Factor-κBα Kinase, Leading to Potentiation of Apoptosis. Blood J. Am. Soc. Hematol. 2008, 111, 4880–4891. [Google Scholar]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Study Randomizer App. Studyrandomizer.Com. Available online: https://app.studyrandomizer.com (accessed on 12 May 2021).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, J.; Worwood, M. Guidelines on Diagnosis and Therapy: Genetic Haemochromatosis; British Committee for Standards in Haematology: London, UK, 2000; pp. 1–33. [Google Scholar]
- Fitzsimons, E.J.; Cullis, J.O.; Thomas, D.W.; Tsochatzis, E.; Griffiths, W.J.H. Diagnosis and therapy of genetic haemochromatosis (review and 2017 update). Br. J. Haematol. 2018, 181, 293–303. [Google Scholar] [CrossRef]
- Blood Pressure UK. Available online: http://www.bloodpressureuk.org/your-blood-pressure/understanding-your-bloodpressure/what-do-the-numbers-mean/ (accessed on 12 May 2021).
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Hyland, M.E.; Bacon, A.M.; Lanario, J.W.; Davies, A.F. Symptom frequency and development of a generic functional disorder symptom scale suitable for use in studies of patients with irritable bowel syndrome, fibromyalgia syndrome or chronic fatigue syndrome. Chronic Dis. Transl. Med. 2019, 5, 129–138. [Google Scholar] [CrossRef]
- Lorinczova, H.T.; Fitzsimons, O.; Mursaleen, L.; Renshaw, D.; Begum, G.; Zariwala, M.G. Co-Administration of Iron and a Bioavailable Curcumin Supplement Increases Serum BDNF Levels in Healthy Adults. Antioxidants 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Coudène, P.; Marson, B.; Badiou, S.; Flavier, S.; Anelli, S.; Cristol, J.P.; Dupuy, A.M. Evaluation of the ABX Pentra 400: A newly available clinical chemistry analyser. Clin. Chem. Lab. Med. 2005, 43, 782–792. [Google Scholar] [CrossRef] [PubMed]
- .Simó, J.M.; Joven, J.; Clivillé, X.; Sans, T. Automated Latex Agglutination Immunoassay of Serum Ferritin with a Centrifugal Analyzer. Clin. Chem. 1994, 40, 625–629. [Google Scholar] [CrossRef]
- Elsayed, M.E.; Sharif, M.U.; Stack, A.G. Transferrin Saturation: A Body Iron Biomarker. In Advances in Clinical Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 75, pp. 71–97. [Google Scholar]
- Van Dievoet, M.A.; Louagie, H.; Ghys, T. Performance evaluation of the Sysmex®XP-300 in an oncology setting: Evaluation and comparison of hematological parameters with the Sysmex®XN-3000. Int. J. Lab. Hematol. 2016, 38, 490–496. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Lerdal, A.; Wahl, A.K.; Rustoen, T.; Hanestad, B.R.; Moum, T. Fatigue in the general population: A translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand. J. Public Health 2005, 33, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the Fatigue Severity Scale in a Swiss Cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Tseng, B.Y.; Gajewski, B.J.; Kluding, P.M. Reliability, Responsiveness, and Validity of the Visual Analog Fatigue Scale to Measure Exertion Fatigue in People with Chronic Stroke: A Preliminary Study. Stroke Res. Treat. 2010, 2010, 412964. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-R.; Han, A.-L. Improved Chronic Fatigue Symptoms after Removal of Mercury in Patient with Increased Mercury Concentration in Hair Toxic Mineral Assay: A Case. Korean J. Fam. Med. 2012, 33, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.I.; Irving, S.S.C.; Lomer, M.C.; Powell, J.J. A rapid, simple questionnaire to assess gastrointestinal symptoms after oral ferrous sulphate supplementation. BMC Gastroenterol. 2014, 14, 103. [Google Scholar] [CrossRef] [Green Version]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Educ. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Harwell, M.R.; Rubinstein, E.N.; Hayes, W.S.; Olds, C.C. Summarizing Monte Carlo results in methodological research: The one-and two-factor fixed effects ANOVA cases. J. Educ. Stat. 1992, 17, 315–339. [Google Scholar] [CrossRef]
- Lix, L.M.; Keselman, J.C.; Keselman, H.J. Consequences of assumption violations revisited: A quantitative review of alternatives to the one-way analysis of variance F test. Rev. Educ. Res. 1996, 66, 579–619. [Google Scholar]
- Mast, A.E.; Blinder, M.A.; Gronowski, A.M.; Chumley, C.; Scott, M.G. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin. Chem. 1998, 44, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Clénin, G.E. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med. Wkly. 2017, 147, w14434. [Google Scholar] [CrossRef] [PubMed]
- Soppi, E. Iron Deficiency Without Anemia—Common, Important, Neglected. Clin. Case Rep. Rev. 2019, 5, 1000456. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.2). 2011. Available online: https://www.who.int/vmnis/indicators/serum_ferritin.pdf (accessed on 9 July 2020).
- Koulaouzidis, A.; Cottier, R.; Bhat, S.; Said, E.; Linaker, B.D.; Saeed, A.A. A ferritin level >50 μg/L is frequently consistent with iron deficiency. Eur. J. Intern. Med. 2009, 20, 168–170. [Google Scholar] [CrossRef]
- Lerdal, A.; Kottorp, A. Psychometric properties of the Fatigue Severity Scale—Rasch analyses of individual responses in a Norwegian stroke cohort. Int. J. Nurs. Stud. 2011, 48, 1258–1265. [Google Scholar] [CrossRef]
- Tsai, N.-W.; Chang, Y.-T.; Huang, C.-R.; Lin, Y.-J.; Lin, W.-C.; Cheng, B.-C.; Su, C.-M.; Chiang, Y.-F.; Chen, S.-F.; Huang, C.-C.; et al. Association between Oxidative Stress and Outcome in Different Subtypes of Acute Ischemic Stroke. BioMed Res. Int. 2014, 2014, 256879. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E. TNF Trafficking to Human Mast Cell Granules: Mature Chain-Dependent Endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Haraoui, B.; Bykerk, V. Etanercept in the treatment of rheumatoid arthritis. Ther. Clin. Risk Manag. 2007, 3, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, C.; Pedersen, B.K. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J. Biomed. Biotechnol. 2010, 2010, 520258. [Google Scholar] [CrossRef] [PubMed]
- Fäldt, J.; Wernstedt, I.; Fitzgerald, S.M.; Wallenius, K.; Bergström, G.; Jansson, J.O. Reduced Exercise Endurance in Interleukin-6-Deficient Mice. Endocrinology 2004, 145, 2680–2686. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Kosicka, A.; Cunliffe, A.D.; Mackenzie, R.; Zariwala, M.G.; Perretti, M.; Flower, R.J.; Renshaw, D. Attenuation of plasma annexin A1 in human obesity. FASEB J. 2013, 27, 368–378. [Google Scholar] [CrossRef]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M. Chapter 14: Manipulation of the immune response. In Immunobiology: The Immune System in Health & Disease, 6th ed.; Janeway, C.A., Travers, P., Walport, M., Shlomchik, M., Eds.; Garland Science Publishing: New York, NY, USA, 2005; pp. 613–662. [Google Scholar]
- Blrgegård, G.; Hålxgren, R.; Killander, A.; Strömberg, A.; Venge, P.; Wide, L. Serum Ferritin during Infection. A longitudinal study. Scand. J. Haematol. 1978, 21, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Wikman, O.; Befrits, R.; Blom, H.; Eriksson, A.; Grännö, C.; Ung, K.-A.; Hjortswang, H.; Lindgren, A.; Unge, P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand. J. Gastroenterol. 2009, 44, 838–845. [Google Scholar] [CrossRef] [PubMed]
| Age (y) | Height (m) | Weight (kg) | BMI (kg/m2) | Body Fat (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| FS0_Plac | 25.87 ± 1.18 | 26.73 ± 1.31 | 1.77 ± 0.02 | 1.67 ± 0.02 | 75.22 ± 3.15 | 66.36 ± 3.25 | 24.06 ± 0.83 | 23.72 ± 0.85 | 18.37 ± 1.68 | 32.16 ± 1.57 |
| (n = 31) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||||||
| FS18_Plac | 26.11 ± 1.27 | 25.08 ± 1.47 | 1.76 ± 0.01 | 1.64 ± 0.01 | 80.70 ± 3.70 | 60.08 ± 3.07 | 25.94 ± 1.06 | 22.38 ± 1.21 | 21.97 ± 2.02 | 29.45 ± 2.44 |
| (n = 31) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||||||
| FS18_Curc | 25.60 ± 1.13 | 23.53 ± 1.24 | 1.77 ± 0.02 | 1.59 ± 0.02 | 74.58 ± 2.78 | 57.76 ± 2.64 | 23.71 ± 0.79 | 22.93 ± 1.03 | 18.35 ± 1.86 | 30.29 ± 2.07 |
| (n = 31) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||||||
| FS65_Plac | 26.92 ± 1.25 | 27.06 ± 1.14 | 1.78 ± 0.01 | 1.63 ± 0.02 | 77.13 ± 2.27 | 60.27 ± 2.89 | 24.32 ± 0.56 | 22.46 ± 0.82 | 19.13 ± 1.51 | 27.95 ± 1.52 |
| (n = 30) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||||||
| FS65_Curc | 27.12 ± 1.35 | 26.36 ± 1.08 | 1.78 ± 0.02 | 1.65 ± 0.02 | 75.24 ± 2.26 | 58.54 ± 1.91 | 23.95 ± 0.77 | 21.48 ± 0.65 | 19.44 ± 1.85 | 28.18 ± 1.25 |
| (n = 31) | (n = 31) | (n = 31) | (n = 30) | (n = 31) | ||||||
| Total | 26.32 ± 0.55 | 25.81 ± 0.56 | 1.77 ± 0.01 | 1.64 ± 0.01 | 76.69 ± 1.32 | 60.64 ± 1.28 | 24.44 ± 0.38 | 22.61 ± 0.41 | 19.56 ± 0.82 | 29.59 ± 0.79 |
| (n = 154) | (n = 151) | (n = 151) | (n = 151) | (n = 151) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiekou Lorinczova, H.; Begum, G.; Temouri, L.; Renshaw, D.; Zariwala, M.G. Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients 2022, 14, 712. https://doi.org/10.3390/nu14030712
Tiekou Lorinczova H, Begum G, Temouri L, Renshaw D, Zariwala MG. Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients. 2022; 14(3):712. https://doi.org/10.3390/nu14030712
Chicago/Turabian StyleTiekou Lorinczova, Helena, Gulshanara Begum, Lina Temouri, Derek Renshaw, and Mohammed Gulrez Zariwala. 2022. "Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study" Nutrients 14, no. 3: 712. https://doi.org/10.3390/nu14030712
APA StyleTiekou Lorinczova, H., Begum, G., Temouri, L., Renshaw, D., & Zariwala, M. G. (2022). Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients, 14(3), 712. https://doi.org/10.3390/nu14030712






