Unfavorable Dietary Quality Contributes to Elevated Risk of Ischemic Stroke among Residents in Southwest China: Based on the Chinese Diet Balance Index 2016 (DBI-16)
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Outcome Definition
2.3. Dietary Data Collection
2.4. Dietary Intake Assessment
2.5. Other Variables Collection
2.6. Statistical Analysis
3. Results
3.1. Descriptions of Study Population
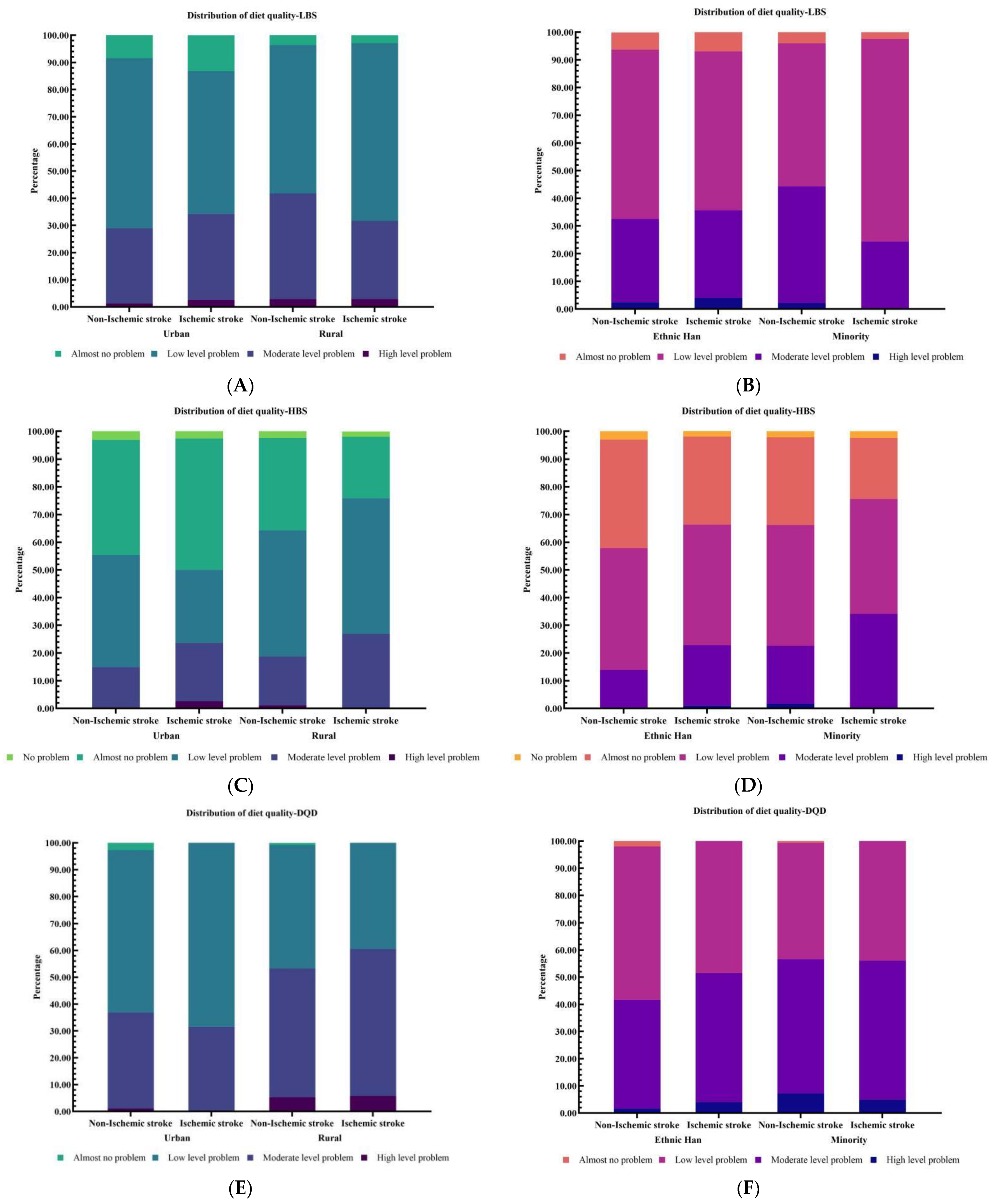
3.2. Assessments of Dietary Quality
3.3. Association Analyses of Ischemic Stroke with Dietary Quality Indicators and DBI-16 Components
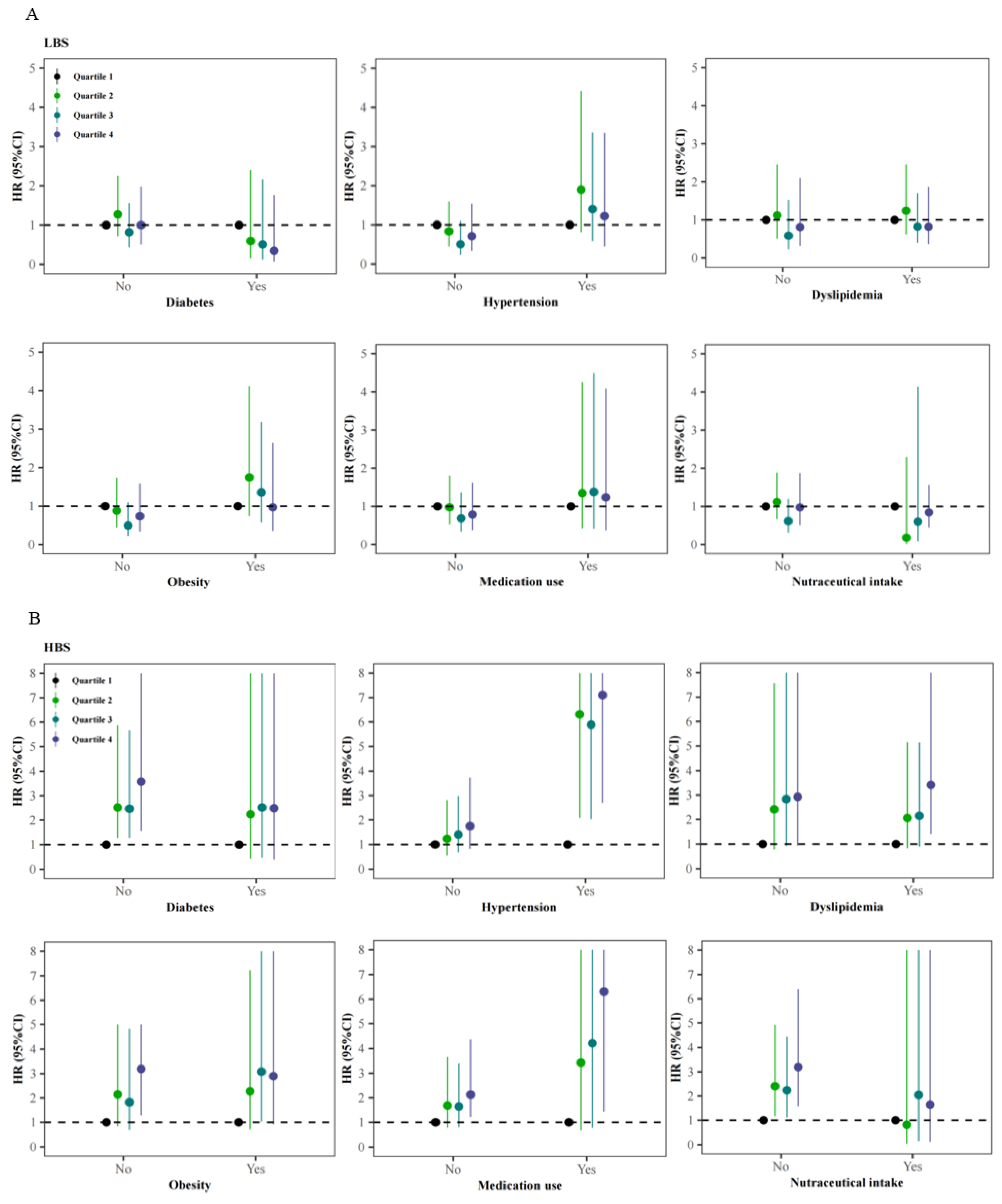
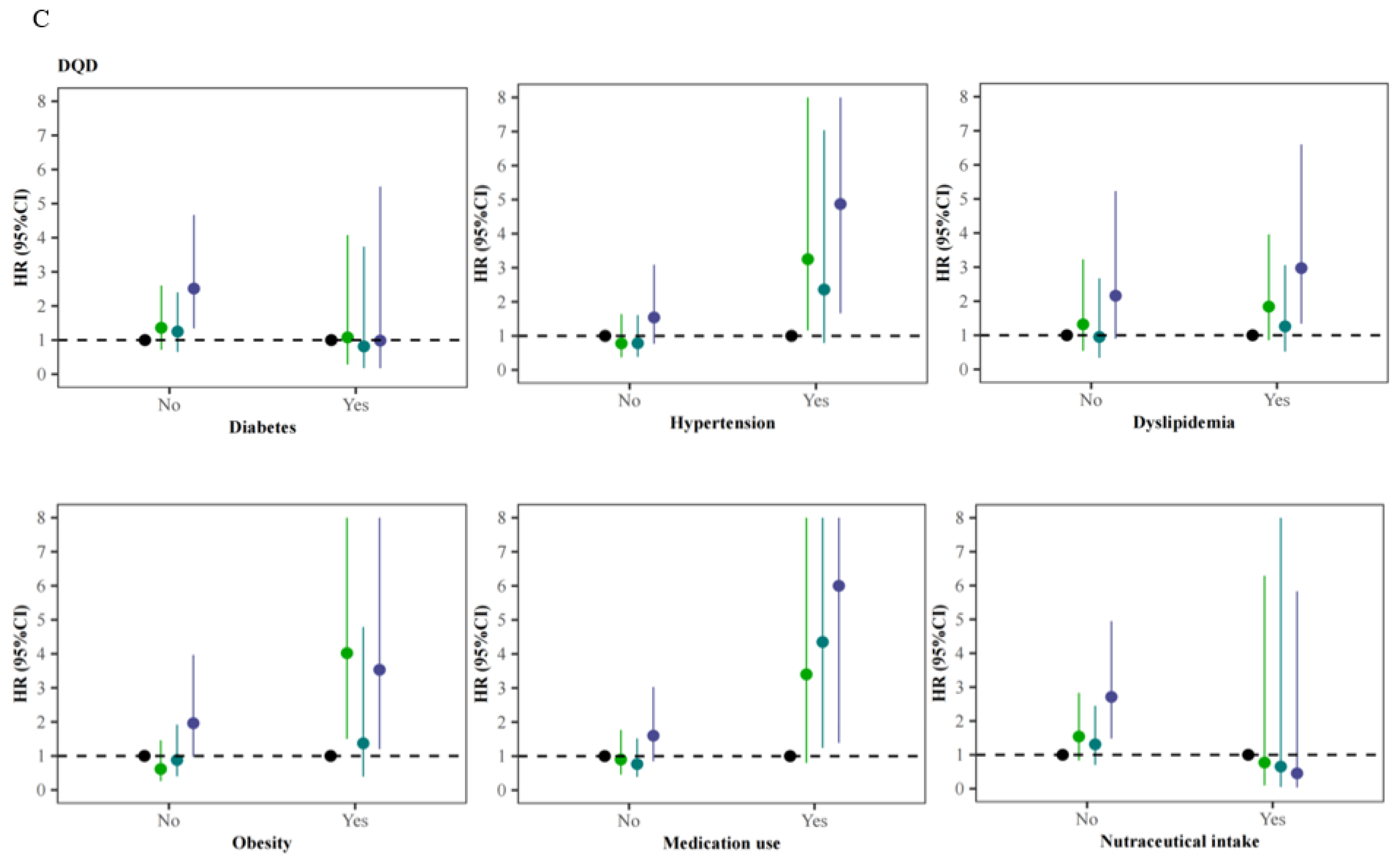
3.4. Stratified Analyses of the Association of Ischemic Stroke with Dietary Quality Indicators across Different Status of Comorbidities, Medication Use, and Nutraceutical Intake
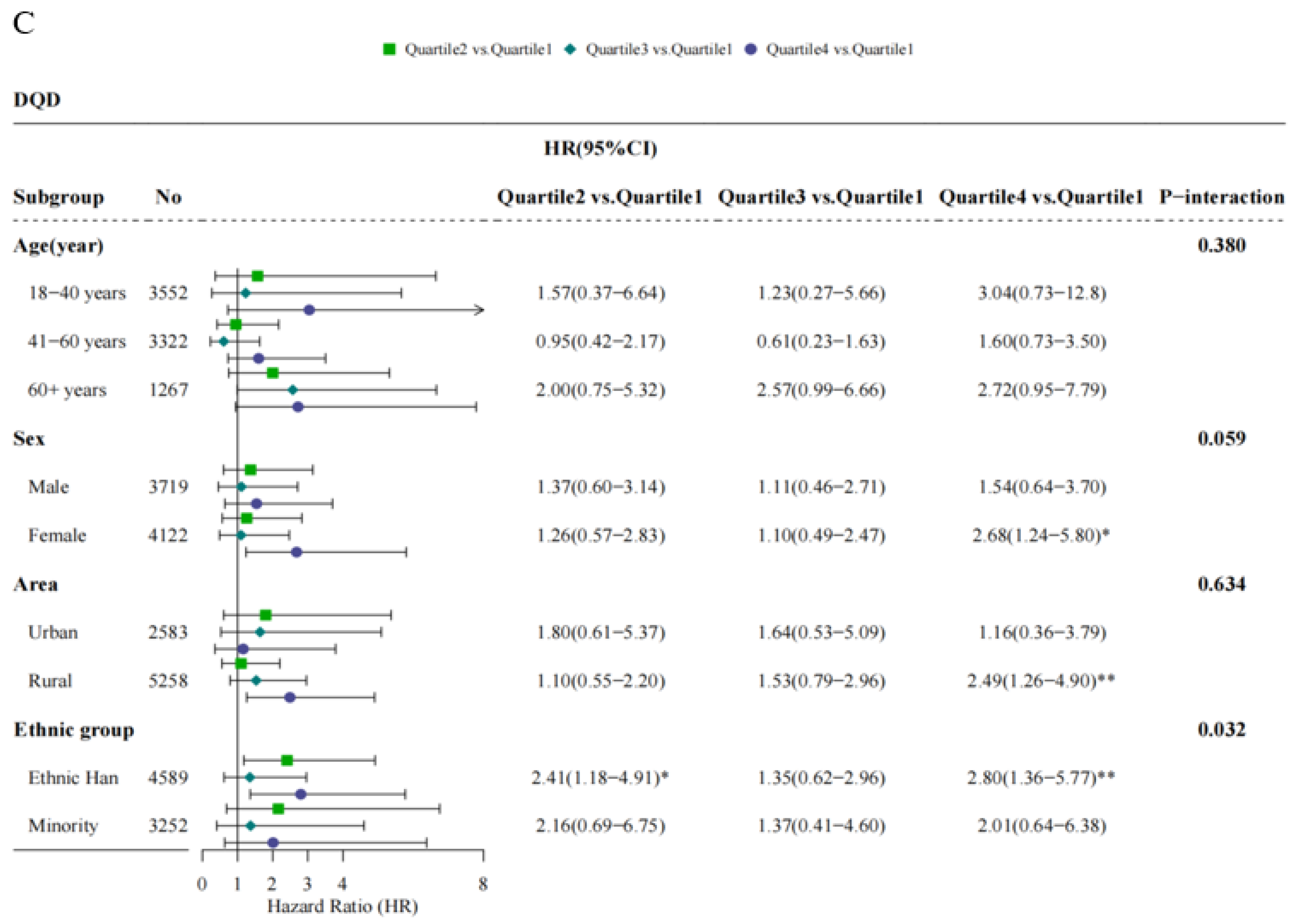
3.5. Stratified Analyses of the Associations of Ischemic Stroke with Dietary Quality Indicators across Baseline Demographic Factors
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Wang, Y.-J.; Li, Z.-X.; Gu, H.-Q.; Zhai, Y.; Jiang, Y.; Zhao, X.-Q.; Wang, Y.-L.; Yang, X.; Wang, C.-J.; Meng, X.; et al. China Stroke Statistics 2019: A Report From the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc. Neurol. 2020, 5, 211–239. [Google Scholar] [PubMed]
- Li, Z.; Jiang, Y.; Li, H.; Xian, Y.; Wang, Y. China’s response to the rising stroke burden. BMJ 2019, 364, l879. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- National Center for Cardiovascular Diseases. Annual Report on Cardiovascular Health and Diseases in China 2019; China Science Publishing House: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Tang, X.; Zhang, W.; Qian, Y.; Huang, Y.; et al. Adherence to Healthy Lifestyle and Cardiovascular Diseases in the Chinese Population. J. Am. Coll. Cardiol. 2017, 69, 1116–1125. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Ojo, O. Dietary Intake and Type 2 Diabetes. Nutrients 2019, 11, 2177. [Google Scholar] [CrossRef] [Green Version]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Sharon, R.D.; Kirkpatrick, I.; Lerman, J.L.; Tooze, J.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.D.; Park, S.-Y.; Shvetsov, Y.B.; Boushey, C.J.; Monroe, K.R.; Le Marchand, L.; Maskarinec, G. Diet Quality and Breast Cancer Incidence in the Multiethnic Cohort. Eur. J. Clin. Nutr. 2020, 74, 1743–1747. [Google Scholar] [CrossRef]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- He, Y.N.; Fang, Y.H.; Xia, J. Update of the Chinese Diet Balance Index: DBI-16. Acta Nutr. Sin. 2018, 40, 526–530. [Google Scholar]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents (2016); People’s Medical Publishing House: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Tan, S.; Lu, H.; Song, R.; Wu, J.; Xue, M.; Qian, Y.; Wang, W.; Wang, X. Dietary quality is associated with reduced risk of diabetes among adults in Northern China: A cross-sectional study. Br. J. Nutr. 2021, 126, 923–932. [Google Scholar] [CrossRef]
- Wang, X.; Liu, A.; Du, M.; Wu, J.; Wang, W.; Qian, Y.; Zheng, H.; Liu, D.; Nan, X.; Jia, L.; et al. Diet quality is associated with reduced risk of hypertension among Inner Mongolia adults in northern China. Public Health Nutr. 2020, 23, 1543–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Tian, Z.; Zhao, D.; Li, K.; Zhao, Y.; Xu, L. Associations between Adherence to Four A Priori Dietary Indexes and Cardiometabolic Risk Factors among Hyperlipidemic Patients. Nutrients 2021, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018, 61, 1249–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.N.; Zhai, F.Y.; Yang, X.G.; Ge, K.Y. The Chinese Diet Balance Index Revised. Acta Nutr. Sin. 2009, 31, 532–536. [Google Scholar]
- Writing group of Chinese Guideline for Cardiometabolic Diseases Prevention; Editorial board of Chinese Journal of Cardiometabolic Diseases. Chinese Guideline for Cardiometabolic Diseases Prevention (2017). Chin. J. Cardiometabolic Dis. 2018, 46, 10–25. [Google Scholar]
- National Health and Family Planning Commission of the People’s Republic of China. Health Standard of the People’s Republic of China; No. WS/T 428-2013; Criteria of Weight for Adults 2013; Standards Press of China: Beijing, China, 2013. (In Chinese)
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef]
- Johansson, A.; Drake, I.; Engström, G.; Acosta, S. Modifiable and Non-Modifiable Risk Factors for Atherothrombotic Ischemic Stroke among Subjects in the Malmö Diet and Cancer Study. Nutrients 2021, 13, 1952. [Google Scholar] [CrossRef]
- Han, Y.; Hu, Y.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Sun, D.; Pang, Y.; et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J. 2021, 42, 3374–3384. [Google Scholar] [CrossRef]
- Zhai, F.; Du, S.F.; Wang, Z.; Zhang, J.G.; Du, W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991-2011. Obes. Rev. 2014, 15 (Suppl. 1), 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Wang, Z.; Wang, H.; Zhao, L.; Jiang, H.; Zhang, B.; Ding, G. Nutrition transition and related health challenges over decades in China. Eur J. Clin. Nutr. 2021, 75, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, J.; Su, C.; Zhang, J.; Zhang, B.; Wang, H. Cooking oil and salt consumption among chinese adults aged 18-59 years in 2015. Acta Nutr. Sin. 2018, 40, 27–31. [Google Scholar]
- Zang, J.; Yu, H.; Zhu, Z.; Lu, Y.; Liu, C.; Yao, C.; Bai, P.; Guo, C.; Jia, X.; Zou, S.; et al. Does the Dietary Pattern of Shanghai Residents Change across Seasons and Area of Residence: Assessing Dietary Quality Using the Chinese Diet Balance Index (DBI). Nutrients 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; Qiao, Y.; Xiong, S.; Liu, S.; Ke, C.; Shen, Y. Association between Dietary Quality and Prediabetes based on the Diet Balance Index. Sci. Rep. 2020, 10, 3190. [Google Scholar] [CrossRef] [Green Version]
- Muto, M.; Ezaki, O. High Dietary Saturated Fat is Associated with a Low Risk of Intracerebral Hemorrhage and Ischemic Stroke in Japanese but not in Non-Japanese: A Review and Meta-Analysis of Prospective Cohort Studies. J. Atheroscler. Thromb. 2018, 25, 375–392. [Google Scholar] [CrossRef] [Green Version]
- Arsang-Jang, S.; Mansourian, M.; Mohammadifard, N.; Khosravi, A.; Oveis-Gharan, S.; Nouri, F.; Sarrafzadegan, N. Temporal trend analysis of stroke and salt intake: A 15-year population-based study. Nutr. Neurosci. 2021, 24, 384–394. [Google Scholar] [CrossRef]
- Guo, H.; Ban, Y.-H.; Cha, Y.; Kim, T.-S.; Lee, S.-P.; An, E.S.; Choi, J.; Seo, D.W.; Yon, J.-M.; Choi, E.-K.; et al. Comparative effects of plant oils and trans-fat on blood lipid profiles and ischemic stroke in rats. J. Biomed. Res. 2017, 31, 122–129. [Google Scholar]
- Seo, W.J.; Ahn, J.H.; Lee, T.-K.; Kim, B.; Lee, J.-C.; Park, J.H.; Yoo, Y.H.; Shin, M.C.; Cho, J.H.; Won, M.-H.; et al. High fat diet accelerates and exacerbates microgliosis and neuronal damage/death in the somatosensory cortex after transient forebrain ischemia in gerbils. Lab. Anim. Res. 2020, 36, 28. [Google Scholar] [CrossRef]
- Frisoli, T.M.; Schmieder, R.E.; Grodzicki, T.; Messerli, F.H. Salt and hypertension: Is salt dietary reduction worth the effort? Am. J. Med. 2012, 125, 433–439. [Google Scholar] [CrossRef]
- Mills, K.T.; Chen, J.; Yang, W.; Appel, L.J.; Kusek, J.W.; Alper, A.; Delafontaine, P.; Keane, M.G.; Mohler, E.; Ojo, A.; et al. Sodium Excretion and the Risk of Cardiovascular Disease in Patients With Chronic Kidney Disease. JAMA 2016, 315, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, C.; Lu, J.; Chen, B.; Li, Y.; Yang, Y.; Hu, S.; Li, J. Cardiovascular risk factors in China: A nationwide population-based cohort study. Lancet Public Health 2020, 5, e672–e681. [Google Scholar] [CrossRef]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; Ciccone, M.M. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, G.; Liu, Z.; Li, Z.; Li, J.; Varma, D.S.; Wan, Q.; Zhao, J.; Min, X.; Han, X.; et al. Dietary Balance Index-07 and the Risk of Anemia in Middle Aged and Elderly People in Southwest China: A Cross Sectional Study. Nutrients 2018, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, B.L.; Coyle, D.H.; Santos, J.A.; Burrows, T.; Rosewarne, E.; Peters, S.A.E. Investigating sex differences in the accuracy of dietary assessment methods to measure energy intake in adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 1241–1255. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | All (n = 7841) | Non-Ischemic Stroke (n = 7699) | Ischemic Stroke (n = 142) | p Value |
|---|---|---|---|---|
| Age (n, %) | <0.001 | |||
| 18–40 years | 3252 (41.5) | 3231 (42.0) | 21 (14.8) | |
| 41–60 years | 3322 (42.4) | 3256 (42.3) | 66 (46.5) | |
| ≥60 years | 1267 (16.1) | 1212 (15.7) | 55 (38.7) | |
| Sex (n, %) | 0.754 | |||
| Male | 3719 (47.4) | 3654 (47.5) | 65 (45.8) | |
| Female | 4122 (52.6) | 4045 (52.5) | 77 (54.2) | |
| Area (n,%) | 0.136 | |||
| Urban | 2583 (32.9) | 2545 (33.1) | 38 (26.8) | |
| Rural | 5258 (67.1) | 5154 (66.9) | 104 (73.2) | |
| Ethnic group (n,%) | 0.003 | |||
| Ethnic Han | 4589 (58.5) | 4488 (58.3) | 101 (71.1) | |
| Minority | 3252 (41.5) | 3211 (41.7) | 41 (28.9) | |
| Education (n,%) | 0.038 | |||
| No formal education | 1606 (20.5) | 1565 (20.3) | 41 (28.9) | |
| Junior middle school and below | 5193 (66.2) | 5111 (66.4) | 82 (57.7) | |
| Senior high school and above | 1042 (13.3) | 1023 (13.3) | 19 (13.4) | |
| Family income (n, %) | 0.010 | |||
| <3000 RMB/person | 1664 (32.6) | 1625 (32.6) | 39 (33.9) | |
| 3000–10,000 RMB/person | 2129 (41.8) | 2080 (41.7) | 49 (42.6) | |
| ≥10,000 RMB/person | 1306 (25.6) | 1279 (25.7) | 27 (23.5) | |
| Marriage (n,%) | 0.001 | |||
| Married/Cohabit | 6340 (80.9) | 6226 (80.9) | 114 (80.3) | |
| Unmarried/Single | 744 (9.5) | 740 (9.6) | 4 (2.8) | |
| Divorced/Widowed/Separated | 757 (9.7) | 733 (9.5) | 24 (16.9) | |
| Occupation (n, %) | 0.284 | |||
| Farmers | 4490 (57.3) | 4407 (57.2) | 83 (58.5) | |
| Others | 2092 (26.7) | 2061 (26.8) | 31 (21.8) | |
| Unemployed or retired | 1259 (16.1) | 1231 (16.0) | 28 (19.7) | |
| Smoking (n, %) | 0.375 | |||
| No | 5856 (74.7) | 5755 (74.7) | 101 (71.1) | |
| Yes | 1985 (25.3) | 1944 (25.3) | 41 (28.9) | |
| Alcohol drinking (n, %) | 0.403 | |||
| No | 6038 (77.0) | 5924 (76.9) | 114 (80.3) | |
| Yes | 1803 (23.0) | 1775 (23.1) | 28 (19.7) | |
| Diabetes (n, %) | 0.015 | |||
| No | 7162 (91.7) | 7042 (91.8) | 120 (85.7) | |
| Yes | 648 (8.3) | 628 (8.2) | 20 (14.3) | |
| Hypertension (n, %) | <0.001 | |||
| No | 5835 (74.4) | 5756 (74.8) | 79 (55.6) | |
| Yes | 2006 (25.6) | 1943 (25.2) | 63 (44.4) | |
| Dyslipidemia (n, %) | 0.581 | |||
| No | 3353 (42.8) | 3296 (42.8) | 57 (40.1) | |
| Yes | 4488 (57.2) | 4403 (57.2) | 85 (59.9) | |
| Obesity (n, %) | 0.376 | |||
| No | 4794 (64.1) | 4716 (64.1) | 78 (60.0) | |
| Yes | 2688 (35.9) | 2636 (35.9) | 52 (40.0) | |
| Medication use (n, %) | <0.001 | |||
| No | 6919 (88.2) | 6812 (88.5) | 107 (75.4) | |
| Yes | 922 (11.8) | 887 (11.5) | 35 (24.6) | |
| Nutraceutical intake (n, %) | 0.044 | |||
| No | 6944 (88.7) | 6810 (88.6) | 134 (94.4) | |
| Yes | 883 (11.3) | 875 (11.4) | 8 (5.6) | |
| MET (per day, mean ± SD) | 109.82 ± 122.62 | 109.87 ± 122.68 | 107.17 ± 120.00 | 0.795 |
| WC (cm, mean ± SD) | 7661 ± 9.46 | 76.57 ± 9.46 | 78.67 ± 9.63 | 0.013 |
| WHtR | 5.52 ± 10.15 | 5.50 ± 10.15 | 6.83 ± 9.76 | 0.182 |
| BMI (kg/m2, mean ± SD) | 22.90 ± 3.36 | 22.89 ± 3.36 | 23.26 ± 3.25 | 0.203 |
| FPG (mmol/L, mean ± SD) | 5.25 ± 1.26 | 5.25 ± 1.25 | 5.40 ± 1.51 | 0.158 |
| 2h-PG (mmol/L, mean ± SD) | 5.79 ± 2.25 | 5.79 ± 2.25 | 6.12 ± 2.52 | 0.088 |
| SBP (mmHg, mean ± SD) | 125.09 ± 20.87 | 124.90 ± 20.72 | 135.33 ± 25.97 | <0.001 |
| DBP (mmHg, mean ± SD) | 78.24 ± 11.90 | 78.16 ± 11.85 | 82.56 ± 13.88 | <0.001 |
| TG (mmol/L, mean ± SD) | 1.76 ± 1.57 | 1.75 ± 1.56 | 1.89 ± 1.92 | 0.324 |
| CHOL (mmol/L, mean ± SD) | 4.79 ± 1.32 | 4.79 ± 1.31 | 4.85 ± 1.55 | 0.64 |
| HDL-C (mmol/L, mean ± SD) | 1.45 ± 0.56 | 1.45 ± 0.56 | 1.41 ± 0.63 | 0.405 |
| LDL-C (mmol/L, mean ± SD) | 2.66 ± 1.18 | 2.66 ± 1.18 | 2.54 ± 1.30 | 0.239 |
| Components | Score Range a | Group | Distribution of Score (%) | p Value b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (−12)–(−11) | (−10)–(−9) | (−8)–(−7) | (−6)–(−5) | (−4)–(−3) | (−2)–(−1) | 0 | (1)–(2) | (3)–(4) | (5)–(6) | (7)–(8) | (9)–(10) | (11)–(12) | ||||
| Cereals | (−12)–(12) | Non-Ischemic stroke | 0.6 | 0.8 | 1.4 | 2.2 | 4.8 | 1.7 | 16.0 | 1.1 | 18.2 | 8.8 | 5.6 | 2.9 | 36.0 | 0.296 |
| Ischemic stroke | 0 | 1.4 | 0.7 | 0.7 | 5.6 | 1.4 | 16.2 | 2.8 | 13.4 | 5.6 | 7.7 | 2.1 | 42.3 | |||
| Vegetables | (−6)–(0) | Non-Ischemic stroke | 4.2 | 26.3 | 31.5 | 38.0 | 0.249 | |||||||||
| Ischemic stroke | 1.4 | 23.2 | 35.9 | 39.4 | ||||||||||||
| Fruits | (−6)–(0) | Non-Ischemic stroke | 49.0 | 39.4 | 7.1 | 4.5 | 0.325 | |||||||||
| Ischemic stroke | 46.5 | 45.8 | 4.9 | 2.8 | ||||||||||||
| Dairy | (−6)–(0) | Non-Ischemic stroke | 91.3 | 6.2 | 2.3 | 0.3 | 0.167 | |||||||||
| Ischemic stroke | 88.7 | 5.6 | 4.9 | 0.7 | ||||||||||||
| Soybeans | (−6)–(0) | Non-Ischemic stroke | 31.0 | 12.8 | 10.6 | 45.6 | 0.065 | |||||||||
| Ischemic stroke | 24.6 | 14.1 | 16.9 | 44.4 | ||||||||||||
| Red meats/products, Poultry/game | (−4)–(4) | Non-Ischemic stroke | 5.2 | 28.3 | 19.8 | 15.6 | 31.1 | 0.004 | ||||||||
| Ischemic stroke | 12.0 | 32.4 | 16.2 | 14.1 | 25.4 | |||||||||||
| Fish/shrimps | (−4)–(0) | Non-Ischemic stroke | 85.8 | 11.7 | 2.5 | 0.546 | ||||||||||
| Ischemic stroke | 88.7 | 9.9 | 1.4 | |||||||||||||
| Eggs | (−4)–(4) | Non-Ischemic stroke | 49.4 | 34.1 | 13.3 | 1.4 | 1.8 | 0.896 | ||||||||
| Ischemic stroke | 49.3 | 36.6 | 12.0 | 0.7 | 1.4 | |||||||||||
| Cooking oils | (0)–(6) | Non-Ischemic stroke | 36.2 | 22.4 | 12.4 | 29.0 | <0.001 | |||||||||
| Ischemic stroke | 19.7 | 19.0 | 16.2 | 45.1 | ||||||||||||
| Alcoholic beverages | (0)–(6) | Non-Ischemic stroke | 93.8 | 3.2 | 1.3 | 1.7 | 0.009 | |||||||||
| Ischemic stroke | 91.5 | 1.4 | 4.2 | 2.8 | ||||||||||||
| Addible sugar | (0)–(6) | Non-Ischemic stroke | 98.9 | 0.7 | 0.1 | 0.3 | 0.915 | |||||||||
| Ischemic stroke | 99.3 | 0.7 | 0 | 0 | ||||||||||||
| Salt | (0)–(6) | Non-Ischemic stroke | 39.5 | 41.0 | 5.1 | 14.4 | 0.004 | |||||||||
| Ischemic stroke | 27.5 | 55.6 | 3.5 | 13.4 | ||||||||||||
| Diet variety | (−12)–(0) | Non-Ischemic stroke | 0 | 0.1 | 4.2 | 9.3 | 21.1 | 50.0 | 15.3 | 0.644 | ||||||
| Ischemic stroke | 0 | 0 | 4.9 | 6.3 | 21.8 | 54.9 | 12.0 | |||||||||
| Diet Quality | Indicator | Score Range | Group | Mean ± SD | Distribution of Dietary Quality (%) a | ||||
|---|---|---|---|---|---|---|---|---|---|
| No Problem | Almost No Problem | Low Level Problem | Moderate Level Problem | High Level Problem | |||||
| Under intake | LBS | 0–60 | Non-Ischemic stroke | 22.68 ± 6.82 | 0 | 5.3 | 57.2 | 35.2 | 2.3 |
| Ischemic stroke | 22.42 ± 7.09 | 0 | 5.6 | 62.0 | 29.6 | 2.8 | |||
| Over intake | HBS | 0–40 | Non-Ischemic stroke | 11.84 ± 6.68 | 2.7 | 36.0 | 43.8 | 16.6 | 0.9 |
| Ischemic stroke | 13.30 ± 6.43 | 2.1 | 28.9 | 42.9 | 25.4 | 0.7 | |||
| Overall unbalance | DQD | 0–84 | Non-Ischemic stroke | 34.51 ± 8.35 | 0 | 1.4 | 50.7 | 44.0 | 3.9 |
| Ischemic stroke | 35.72 ± 7.81 | 0 | 0 | 47.2 | 48.6 | 4.2 | |||
| Indicators | No (n) | Cases (n) | Incident Density (Cases per 1000 PYs) | HR (95%CI) a | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2 | ||||
| LBS b | ||||||
| Quartile 1 (Q1) | 1761 | 34 | 2.78 | 1.00 | 1.00 | 1.00 |
| Quartile 2 (Q2) | 1907 | 41 | 3.06 | 1.04 (0.66–1.64) | 1.13 (0.68–1.89) | 1.13 (0.68–1.89) |
| Quartile 3 (Q3) | 2008 | 29 | 2.07 | 0.78 (0.48–1.29) | 0.79 (0.44–1.41) | 0.76 (0.43–1.36) |
| Quartile 4 (Q4) | 2165 | 38 | 2.46 | 0.92 (0.57–1.46) | 0.86 (0.46–1.59) | 0.84 (0.45–1.56) |
| HBS c | ||||||
| Quartile 1 (Q1) | 1619 | 14 | 1.19 | 1.00 | 1.00 | 1.00 |
| Quartile 2 (Q2) | 2042 | 37 | 2.57 | 2.24 (1.21–4.15) * | 2.38 (1.12–5.05)* | 2.38 (1.12–5.06) * |
| Quartile 3 (Q3) | 2168 | 44 | 2.89 | 2.48 (1.36–4.53) ** | 2.38 (1.14–5.00)* | 2.39 (1.14–5.01) * |
| Quartile 4 (Q4) | 2012 | 47 | 3.43 | 3.12 (1.72–5.68) *** | 3.15 (1.50–6.63)** | 3.31 (1.57–6.97) ** |
| DQD d | ||||||
| Quartile 1 (Q1) | 1853 | 26 | 1.98 | 1.00 | 1.00 | 1.00 |
| Quartile 2 (Q2) | 1797 | 32 | 2.50 | 1.28 (0.76–2.15) | 1.34 (0.75–2.37) | 1.33 (0.75–2.36) |
| Quartile 3 (Q3) | 2166 | 36 | 2.36 | 1.29 (0.78–2.13) | 1.11 (0.62–2.01) | 1.13 (0.63–2.04) |
| Quartile 4 (Q4) | 2025 | 48 | 3.44 | 1.99 (1.23–3.23) ** | 2.19 (1.24–3.86) ** | 2.26 (1.28–4.00) ** |
| Cereals | ||||||
| Score 0 | 1252 | 23 | 2.61 | 1.00 | 1.00 | 1.00 |
| Score (−12)–(−7) | 220 | 3 | 1.90 | 0.76 (0.23–2.51) | 1.06 (0.31–3.62) | 1.03 (0.30–3.53) |
| Score (−6)–(−1) | 680 | 11 | 2.26 | 0.85 (0.41–1.74) | 0.69 (0.29–1.65) | 0.65 (0.27–1.55) |
| Score (1)–(6) | 2192 | 31 | 2.01 | 0.74 (0.43–1.26) | 0.55 (0.29–1.04) | 0.54 (0.28–1.02) |
| Score (7)–(12) | 3497 | 74 | 3.02 | 1.08 (0.67–1.72) | 0.95 (0.56–1.61) | 0.94 (0.55–1.59) |
| Vegetables | ||||||
| Score 0 | 2982 | 56 | 2.67 | 1.00 | 1.00 | 1.00 |
| Score (−6)–(−1) | 4859 | 86 | 2.52 | 0.98 (0.70–1.37) | 1.02 (0.68–1.53) | 1.05 (0.70–1.57) |
| Fruits | ||||||
| Score 0 | 354 | 4 | 1.62 | 1.00 | 1.00 | 1.00 |
| Score (−6)–(−1) | 7487 | 138 | 2.62 | 1.75 (0.65–4.73) | 1.88 (0.59–6.04) | 1.95 (0.61–6.28) |
| Dairy | ||||||
| Score 0 | 25 | 1 | 5.84 | 1.00 | 1.00 | 1.00 |
| Score (−6)–(−1) | 7816 | 141 | 2.57 | 0.54 (0.07–3.82) | 0.31 (0.04–2.30) | 0.31 (0.04–2.26) |
| Soybeans | ||||||
| Score 0 | 3575 | 63 | 2.52 | 1.00 | 1.00 | 1.00 |
| Score (−6)–(−1) | 4266 | 79 | 2.62 | 1.09 (0.78–1.53) | 1.04 (0.69–1.57) | 1.03 (0.68–1.54) |
| Meats | ||||||
| Score 0 | 1544 | 23 | 2.12 | 1.00 | 1.00 | 1.00 |
| Score (−4)–(−1) | 2645 | 63 | 3.4 | 1.66 (1.03–2.68) * | 1.13 (0.67–1.90) | 1.08 (0.64–1.82) |
| Score (1)–(4) | 3652 | 56 | 2.17 | 0.95 (0.59–1.55) | 0.64 (0.37–1.12) | 0.63 (0.36–1.09) |
| Fish/shrimps | ||||||
| Score 0 | 194 | 2 | 1.47 | 1.00 | 1.00 | 1.00 |
| Score (−4)–(−1) | 7647 | 140 | 2.60 | 1.76 (0.44–7.10) | 2.02 (0.28–14.50) | 1.98 (0.28–14.20) |
| Eggs | ||||||
| Score 0 | 1042 | 17 | 2.37 | 1.00 | 1.00 | 1.00 |
| Score (−4)–(−1) | 6547 | 122 | 2.64 | 1.01 (0.61–1.68) | 0.78 (0.45–1.35) | 0.79 (0.46–1.37) |
| Score (1)–(4) | 252 | 3 | 1.69 | 0.65 (0.19–2.21) | 0.52 (0.12–2.25) | 0.50 (0.12–2.20) |
| Cooking oils | ||||||
| Score 0 | 2814 | 28 | 1.39 | 1.00 | 1.00 | 1.00 |
| Score (1)–(6) | 5027 | 114 | 3.26 | 2.60 (1.72–3.94) *** | 2.96 (1.75–5) *** | 3.00 (1.77–5.07) *** |
| Alcoholic beverages | ||||||
| Score 0 | 7353 | 130 | 2.51 | 1.00 | 1.00 | 1.00 |
| Score (1)–(6) | 488 | 12 | 3.64 | 1.62 (0.90–2.93) | 1.30 (0.60–2.80) | 1.35 (0.62–2.93) |
| Addible sugar | ||||||
| Score 0 | 7757 | 141 | 2.59 | 1.00 | 1.00 | 1.00 |
| Score (1)–(6) | 84 | 1 | 1.74 | 0.81 (0.11–5.80) | 0.84 (0.12–6.00) | 0.81 (0.11–5.81) |
| Salt | ||||||
| Score 0 | 3079 | 39 | 1.76 | 1.00 | 1.00 | 1.00 |
| Score (1)–(6) | 4762 | 103 | 3.13 | 2.04 (1.41–2.96) *** | 1.98 (1.29–3.02) ** | 2.03 (1.33–3.10) ** |
| Dietary variety | ||||||
| Score 0 | 1195 | 17 | 1.97 | 1.00 | 1.00 | 1.00 |
| Score (−12)–(−7) | 337 | 7 | 2.85 | 1.58 (0.65–3.85) | 5.24 (1.66–16.50) ** | 5.40 (1.70–17.20) ** |
| Score (−6)–(−1) | 6309 | 118 | 2.68 | 1.65 (0.99–2.76) | 1.66 (0.94–2.95) | 1.69 (0.95–3.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Su, X.; Chen, Y.; Wang, Y.; Zhou, J.; Liu, T.; Wang, N.; Fu, C. Unfavorable Dietary Quality Contributes to Elevated Risk of Ischemic Stroke among Residents in Southwest China: Based on the Chinese Diet Balance Index 2016 (DBI-16). Nutrients 2022, 14, 694. https://doi.org/10.3390/nu14030694
Wang Y, Su X, Chen Y, Wang Y, Zhou J, Liu T, Wang N, Fu C. Unfavorable Dietary Quality Contributes to Elevated Risk of Ischemic Stroke among Residents in Southwest China: Based on the Chinese Diet Balance Index 2016 (DBI-16). Nutrients. 2022; 14(3):694. https://doi.org/10.3390/nu14030694
Chicago/Turabian StyleWang, Yingying, Xu Su, Yun Chen, Yiying Wang, Jie Zhou, Tao Liu, Na Wang, and Chaowei Fu. 2022. "Unfavorable Dietary Quality Contributes to Elevated Risk of Ischemic Stroke among Residents in Southwest China: Based on the Chinese Diet Balance Index 2016 (DBI-16)" Nutrients 14, no. 3: 694. https://doi.org/10.3390/nu14030694
APA StyleWang, Y., Su, X., Chen, Y., Wang, Y., Zhou, J., Liu, T., Wang, N., & Fu, C. (2022). Unfavorable Dietary Quality Contributes to Elevated Risk of Ischemic Stroke among Residents in Southwest China: Based on the Chinese Diet Balance Index 2016 (DBI-16). Nutrients, 14(3), 694. https://doi.org/10.3390/nu14030694






