Beneficial Activities of Alisma orientale Extract in a Western Diet-Induced Murine Non-Alcoholic Steatohepatitis and Related Fibrosis Model via Regulation of the Hepatic Adiponectin and Farnesoid X Receptor Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AO Extract
2.3. UHPLC-PDA-ESI-MS Analysis of AO Extract
2.4. Animals and Treatment
2.5. Glucose Tolerance Test
2.6. Liver Tissue Preparation
2.7. Serum Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction
2.9. Western Blot Analysis
2.10. Histopathologic Staining and NAFLD Activity Scores
2.11. Immunohistochemistry
2.12. Enzyme-Linked Immunosorbent Assays
2.13. Statistical Analysis
3. Results
3.1. Identification of Phytochemicals in AO Extract
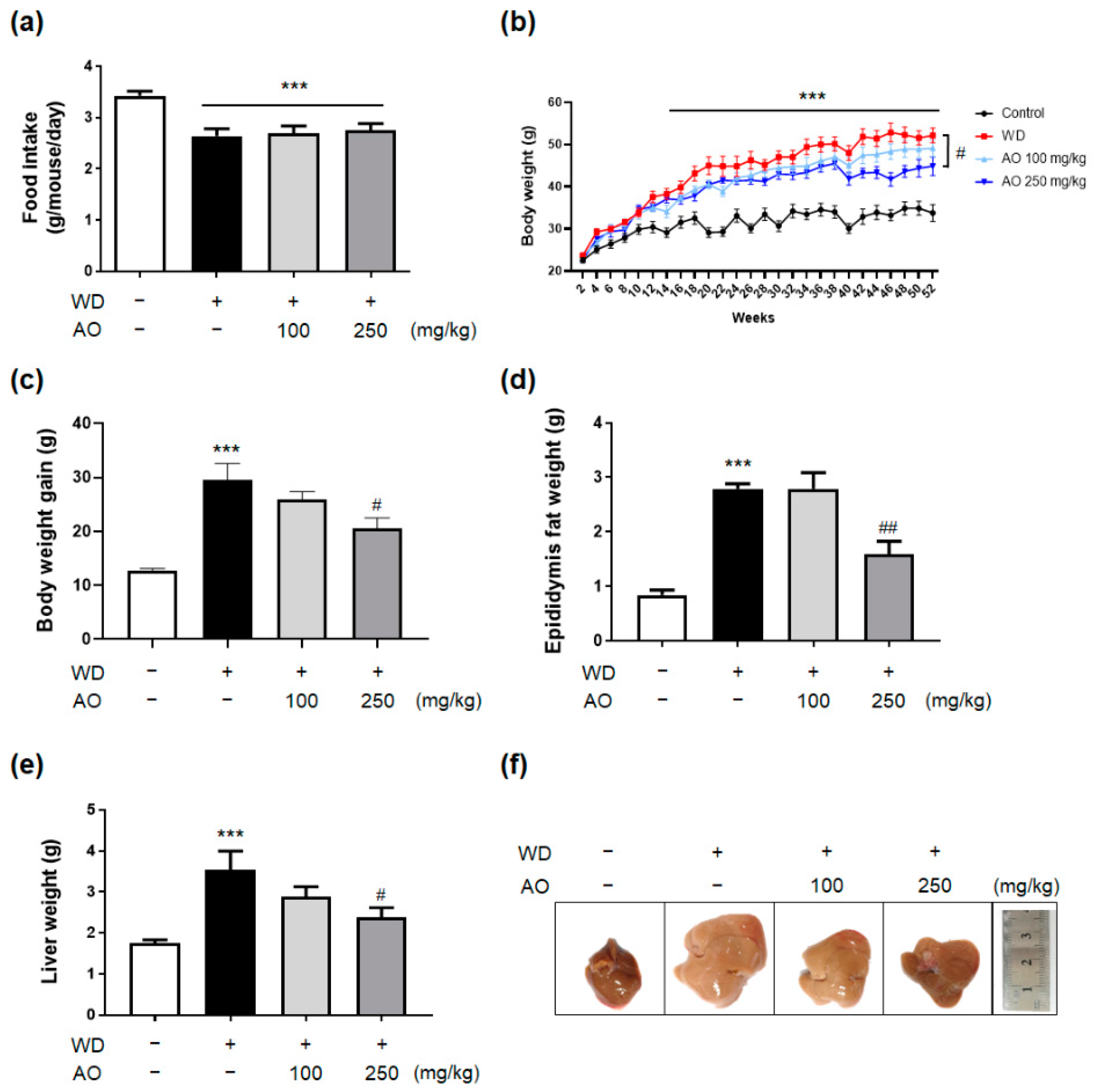
3.2. Administration of AO Extract Decreases WD-Induced Body and Liver Weight Gain and Prevented Hepatomegaly
3.3. Administration of AO Extract Improves Serum Liver Function, Lipid Profile and Glucose Level
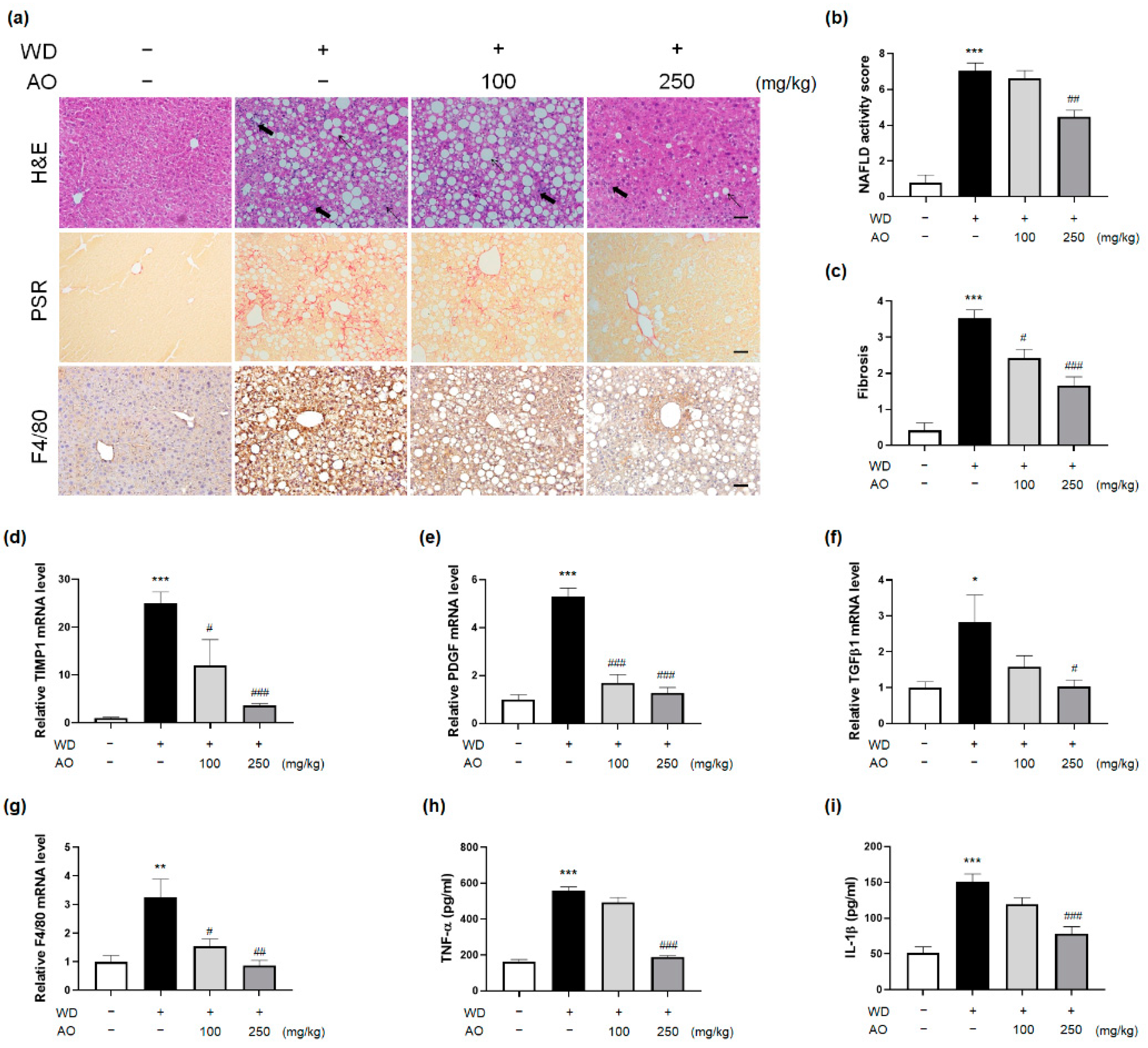
3.4. AO Extract Intervention Resolves Histological Injury of NASH with Fibrosis
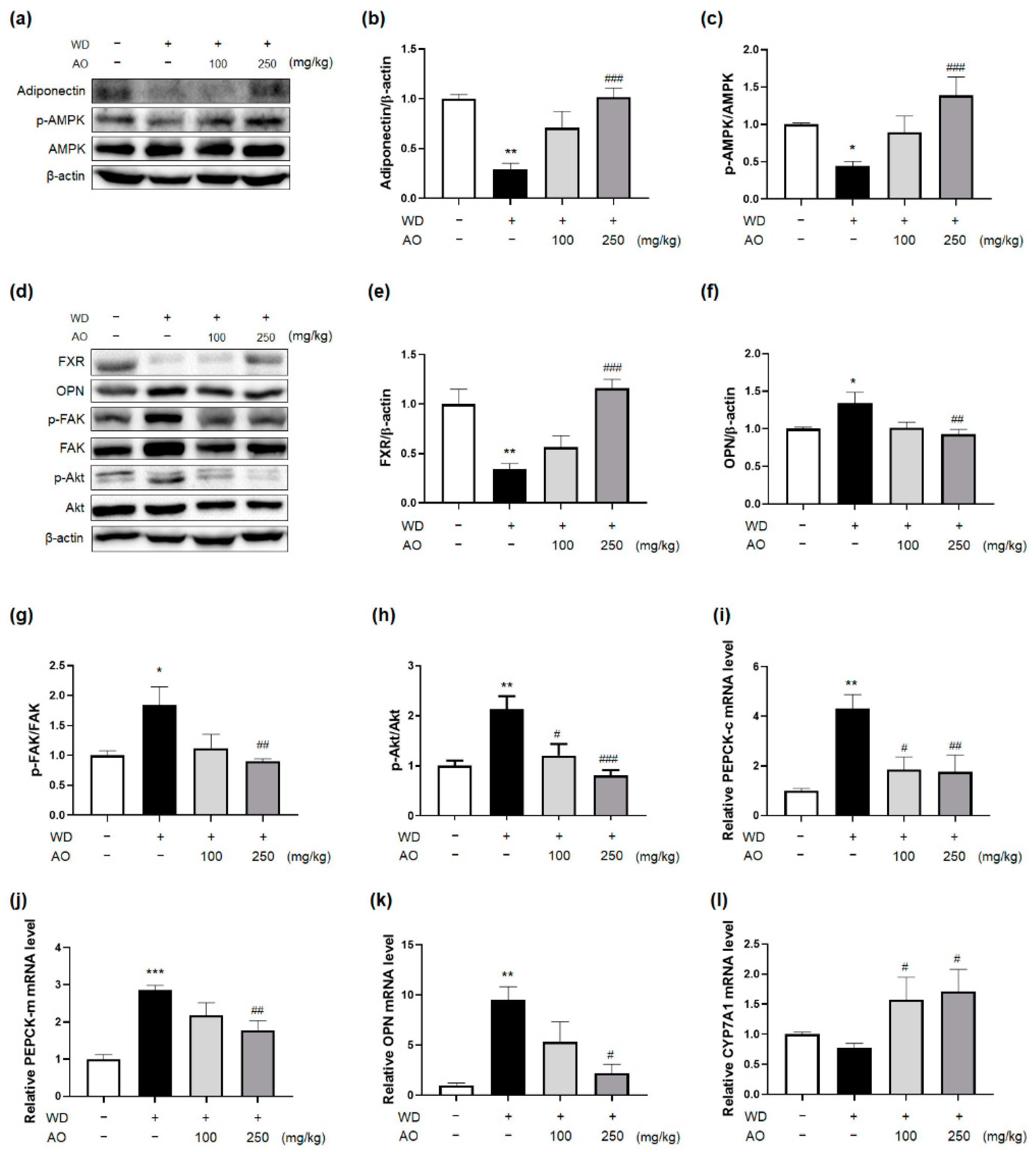
3.5. Administration of AO Extract Affects Adiponectin and FXR Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drenth, J.P.; Schattenberg, J.M. The nonalcoholic steatohepatitis (NASH) drug development graveyard: Established hurdles and planning for future success. Expert Opin. Investig. Drugs 2020, 29, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Johnson, A.L.; Horsfall, L.U.; Moser, C.; Valery, P.C.; Powell, E.E. Detecting non-alcoholic fatty liver disease and risk factors in health databases: Accuracy and limitations of the ICD-10-AM. BMJ Open Gastroenterol. 2021, 8, e000572. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Premkumar, M.; Kulkarni, A.V.; Kumar, P.; Reddy, D.N.; Rao, N.P. Drugs for Non-alcoholic Steatohepatitis (NASH): Quest for the Holy Grail. J. Clin. Transl. Hepatol. 2021, 9, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Celsa, C.; Spatola, F.; Dallio, M.; Federico, A.; Petta, S. Pharmacological therapy of non-alcoholic fatty liver disease: What drugs are available now and future perspectives. Int. J. Environ. Res. Public Health 2019, 16, 4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Finan, B.; Parlee, S.D.; Yang, B. Nuclear hormone and peptide hormone therapeutics for NAFLD and NASH. Mol. Metab. 2021, 46, 101153. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, W.; Zhang, C.; Chen, F.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Herbal Medicine in the Treatment of Non-Alcoholic Fatty Liver Diseases-Efficacy, Action Mechanism, and Clinical Application. Front. Pharmacol. 2020, 11, 601. [Google Scholar] [CrossRef]
- Choi, E.; Jang, E.; Lee, J.-H. Pharmacological activities of Alisma orientale against nonalcoholic fatty liver disease and metabolic syndrome: Literature review. Evid. Based Complement. Alternat. Med. 2019, 2019, 2943162. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.S. Efficacy of Alismatis Orientale Rhizoma on obesity induced by high fat diet. Korean J. Herbol. 2013, 28, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Huo, X.-K.; Liu, J.; Yu, Z.-L.; Wang, Y.-F.; Wang, C.; Tian, X.-G.; Ning, J.; Feng, L.; Sun, C.-P.; Zhang, B.-J.; et al. Alisma orientale extract exerts the reversing cholestasis effect by activation of farnesoid X receptor. Phytomedicine 2018, 42, 34–42. [Google Scholar] [CrossRef]
- Lee, J.K.; Jin, H.K.; Endo, S.; Schuchman, E.H.; Carter, J.E.; Bae, J.S. Intracerebral Transplantation of Bone Marrow-Derived Mesenchymal Stem Cells Reduces Amyloid-Beta Deposition and Rescues Memory Deficits in Alzheimer’s Disease Mice by Modulation of Immune Responses. Stem Cells 2009, 28, 329–343. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, N.; Ju, Y.-J.; Gee, M.S.; Lee, D.; Lee, J.K. Phytohormone Abscisic Acid Improves Memory Impairment and Reduces Neuroinflammation in 5xFAD Mice by Upregulation of LanC-Like Protein 2. Int. J. Mol. Sci. 2020, 21, 8425. [Google Scholar] [CrossRef]
- Juluri, R.; Vuppalanchi, R.; Olson, J.; Ünalp, A.; Van Natta, M.L.; Cummings, O.W.; Tonascia, J.; Chalasani, N. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2011, 45, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kotiaho, T.; Eberlin, M.N.; Vainiotalo, P.; Kostiainen, R. Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J. Am. Soc. Mass Spectrom. 2000, 11, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, D.; Zhao, Y.; Gao, H.; Hu, Y.-H.; Hu, J.-F. Fructose-derived carbohydrates from Alisma orientalis. Nat. Prod. Res. 2009, 23, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Pu, J.; Chen, L.; Zhang, Y.; Rahman, K.; Qin, L.; Zheng, C. Alisma orientale: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Am. J. Clin. Med. 2016, 44, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Huang, L.; Huang, X.; Huang, R.; Peng, M.; Le, Z.; Song, Y.; Yu, S.; Fang, N. Characterization of Protostane Triterpenoids in Dried Tuber of Alisma orientalis by Q-TOF Mass Spectrometry in Both Positive and Negative Modes. Asian J. Chem. 2013, 25, 548–557. [Google Scholar] [CrossRef]
- Peng, C.; Stewart, A.G.; Woodman, O.L.; Ritchie, R.H.; Qin, C.X. Non-alcoholic steatohepatitis: A review of its mechanism, models and medical treatments. Front. Pharmacol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Sanyal, A.J.; Neuschwander-Tetri, B.A.; Network, N.S.C.R. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: Results from the nonalcoholic steatohepatitis clinical research network treatment trials. Hepatology 2019, 70, 522–531. [Google Scholar]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, P. AMP activated protein kinase: A next generation target for total metabolic control: Oncologic, Endocrine & Metabolic. Expert Opin. Ther. Targets 2008, 12, 91–100. [Google Scholar] [PubMed]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Migliorati, M.; Cipriani, S.; Distrutti, E.; Santucci, L.; Fiorucci, S. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J. Immunol. 2009, 183, 6657–6666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P. Modeling nafld disease burden in china, france, germany, italy, japan, spain, united kingdom, and united states for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Non-alcoholic fatty liver disease: Growing burden, adverse outcomes and associations. J. Clin. Transl. Hepatol. 2020, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Sanches, S.C.L.; Ramalho, L.N.Z.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic steatohepatitis: A search for factual animal models. BioMed Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.W.; Jeong, H.; Shin, D.G.; Seo, J.H.; Kim, J.H.; Lim, C.W.; Han, K.M.; Kim, B. Fermented ginseng, GBCK25, ameliorates steatosis and inflammation in nonalcoholic steatohepatitis model. J. Ginseng Res. 2019, 43, 196–208. [Google Scholar] [CrossRef]
- Kamboj, P.; Sarkar, S.; Gupta, S.K.; Bisht, N.; Kumari, D.; Alam, M.J.; Barge, S.; Kashyap, B.; Deka, B.; Bharadwaj, S.; et al. Methanolic Extract of Lysimachia Candida Lindl. Prevents High-Fat High-Fructose-Induced Fatty Liver in Rats: Understanding the Molecular Mechanism Through Untargeted Metabolomics Study. Front. Pharmacol. 2021, 12, 653872. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Lee, J.-H. Promising Anticancer Activities of Alismatis rhizome and Its Triterpenes via p38 and PI3K/Akt/mTOR Signaling Pathways. Nutrients 2021, 13, 2455. [Google Scholar] [CrossRef] [PubMed]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9. [Google Scholar]
- Sinn, D.H.; Kang, D.; Cho, S.J.; Paik, S.W.; Guallar, E.; Cho, J.; Gwak, G.-Y. Lean non-alcoholic fatty liver disease and development of diabetes: A cohort study. Eur. J. Endocrinol. 2019, 181, 185–192. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. New perspective on type 2 diabetes, dyslipidemia and non-alcoholic fatty liver disease. J. Diabetes Investig. 2020, 11, 532–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshraghian, A. Current and emerging pharmacological therapy for non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 7495. [Google Scholar] [CrossRef] [PubMed]
- Lien, F.; Berthier, A.; Bouchaert, E.; Gheeraert, C.; Alexandre, J.; Porez, G.; Prawitt, J.; Dehondt, H.; Ploton, M.; Colin, S. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J. Clin. Investig. 2014, 124, 1037–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimbeni, F.; Pellegrini, E.; Lugari, S.; Mondelli, A.; Bursi, S.; Onfiani, G.; Carubbi, F.; Lonardo, A. Statins and nonalcoholic fatty liver disease in the era of precision medicine: More friends than foes. Atherosclerosis 2019, 284, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franckhauser, S.; Muñoz, S.; Pujol, A.; Casellas, A.; Riu, E.; Otaegui, P.; Su, B.; Bosch, F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 2002, 51, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Gomez, V.; Lu, L.; Yang, X.; Wu, X.; Xiao, S.Y. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2009, 24, 233–237. [Google Scholar] [CrossRef]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.; Patsch, J.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawad, A.S.; Levy, C. FXR agonists: From bench to bedside, a guide for clinicians. Dig. Dis. Sci. 2016, 61, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
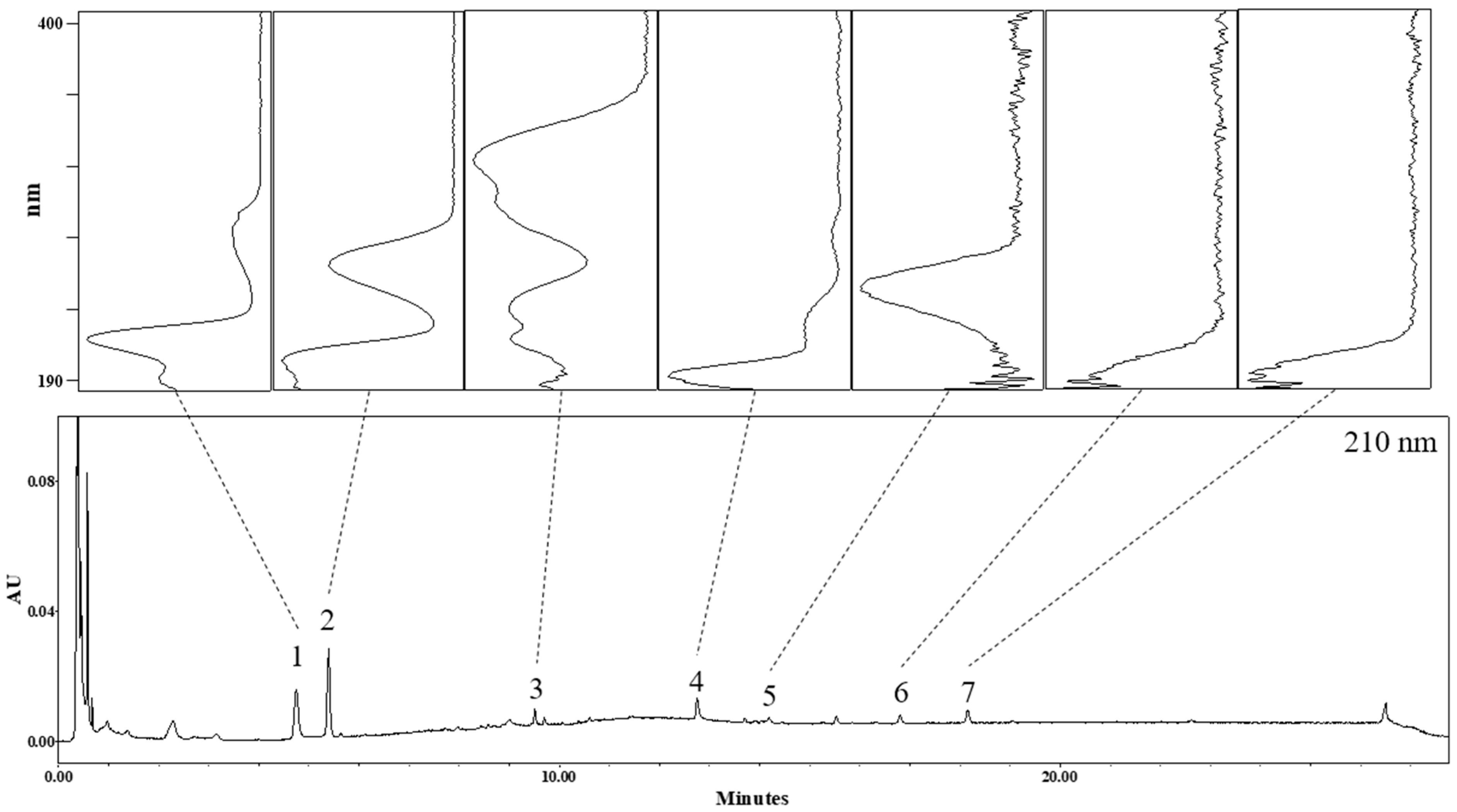

| No | Rt (min) | Identification | Molecular Formula | Ionization Mode | Observed Mass (m/z) | Mass Difference (mmu) |
|---|---|---|---|---|---|---|
| 1 | 4.7 | Tryptophan | C11H12N2O2 | Positive | 159.08491 [M-H2O-CO+H]+ 188.07128 [M-NH3+H]+ # 205.09845 [M+H]+ | −7.31 0.13 0.74 |
| Negative | 203.07321 [M-H]− 225.05624 [M+Na-2H]− | −8.84 −7.75 | ||||
| 2 | 5.4 | Adenosine | C10H13N5O4 | Positive | # 268.10469 [M+H]+ 290.08099 [M+Na]+ 309.12944 [M+CH3CN+H]+ 331.11222 [M+CH3CN+Na]+ | 0.11 −5.53 −1.69 −0.85 |
| Negative | 266.08804 [M-H]− 380.09201 [M+CF3COOH-H]− | −0.89 10.22 | ||||
| 3 | 9.5 | Unknown | C26H30O8 | Positive | 471.20419 [M+H]+ 493.19201 [M+Na]+ | 2.29 8.18 |
| Negative | 469.17523 [M-H]− | −11.02 | ||||
| 4 | 12.8 | Unknown | C30H46O5 | Positive | # 487.34498 [M+H]+ # 509.32263 [M+Na]+ 550.34522 [M+CH3CN+Na]+ | 2.64 −1.66 −5.62 |
| Negative | 467.32944 [M-H2O-H]− 485.33938 [M-H]− 531.34098 [M+HCOOH-H]− | 13.30 12.68 8.80 | ||||
| 5 | 14.2 | Alisol C 23-acetate | C32H48O6 | Positive | # 529.34650 [M+H]+ 551.33350 [M+Na]+ 592.35418 [M+CH3CN+Na]+ | −6.41 −1.35 −7.23 |
| Negative | ND | |||||
| 6 | 16.8 | * Alisol B | Positive | 490.38761 [M+NH4]+ 495.34093 [M+Na]+ 536.36193 [M+CH3CN+Na]+ | −2.02 −4.10 −9.65 | |
| 7 | 18.2 | * Alisol B acetate | Positive | 515.36592 [M+H]+ 537.34832 [M+Na]+ 578.38175 [M+CH3CN+Na]+ | −7.73 −7.27 −0.39 |
| Test | Control Group | WD Group | AO (100 mg/kg) | AO (250 mg/kg) |
|---|---|---|---|---|
| Steatosis (0–3) | 0.27 ± 0.19 | 2.59 ± 0.21 *** | 2.47 ± 0.27 | 1.53 ± 0.23 # |
| Lobular inflammation (0–3) | 0.13 ± 0.08 | 2.54 ± 0.21 *** | 2.33 ± 0.32 | 1.40 ± 0.36 |
| Ballooning (0–2) | 0.40 ± 0.19 | 1.92 ± 0.08 *** | 1.83 ± 0.11 | 1.61 ± 0.23 |
| NAS (0–8) | 0.80 ± 0.43 | 7.05 ± 0.44 *** | 6.64 ± 0.41 | 4.47 ± 0.37 ## |
| Fibrosis stage (0–4) | 0.42 ± 0.21 | 3.53 ± 0.23 *** | 2.56 ± 0.29 # | 1.89 ± 0.11 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.H.; Jang, E.; Park, G.; Lee, Y.; Jang, Y.P.; Lee, K.-T.; Inn, K.-S.; Lee, J.K.; Lee, J.-H. Beneficial Activities of Alisma orientale Extract in a Western Diet-Induced Murine Non-Alcoholic Steatohepatitis and Related Fibrosis Model via Regulation of the Hepatic Adiponectin and Farnesoid X Receptor Pathways. Nutrients 2022, 14, 695. https://doi.org/10.3390/nu14030695
Jeon SH, Jang E, Park G, Lee Y, Jang YP, Lee K-T, Inn K-S, Lee JK, Lee J-H. Beneficial Activities of Alisma orientale Extract in a Western Diet-Induced Murine Non-Alcoholic Steatohepatitis and Related Fibrosis Model via Regulation of the Hepatic Adiponectin and Farnesoid X Receptor Pathways. Nutrients. 2022; 14(3):695. https://doi.org/10.3390/nu14030695
Chicago/Turabian StyleJeon, Seung Ho, Eungyeong Jang, Geonha Park, Yeongae Lee, Young Pyo Jang, Kyung-Tae Lee, Kyung-Soo Inn, Jong Kil Lee, and Jang-Hoon Lee. 2022. "Beneficial Activities of Alisma orientale Extract in a Western Diet-Induced Murine Non-Alcoholic Steatohepatitis and Related Fibrosis Model via Regulation of the Hepatic Adiponectin and Farnesoid X Receptor Pathways" Nutrients 14, no. 3: 695. https://doi.org/10.3390/nu14030695
APA StyleJeon, S. H., Jang, E., Park, G., Lee, Y., Jang, Y. P., Lee, K.-T., Inn, K.-S., Lee, J. K., & Lee, J.-H. (2022). Beneficial Activities of Alisma orientale Extract in a Western Diet-Induced Murine Non-Alcoholic Steatohepatitis and Related Fibrosis Model via Regulation of the Hepatic Adiponectin and Farnesoid X Receptor Pathways. Nutrients, 14(3), 695. https://doi.org/10.3390/nu14030695







