In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Food Ingredient
2.3. Duodenal Bioaccessibility of Nutrients and Non-Nutrients
2.3.1. In Vitro Oral-Gastro-Intestinal Digestion
2.3.2. Glucose
2.3.3. Amino Acids and Protein
Soluble Protein Content
Gluten Content
Free Amino Acid Content
Advanced Glycation End Products (AGEs) Content
2.3.4. Phenolic Compounds
2.3.4.1. Total Polyphenolic Content
2.3.4.2. Analysis of Phenolic Compounds by HPLC-QTOF Assay
2.4. Colonic Bioaccessibility of Nutrients and Non-Nutrients
2.4.1. In Vitro Simulation of Colonic Fermentation
Microbiota Analysis
2.4.2. Sugars
2.4.3. Organic Acids
2.4.4. Short-Chain Fatty Acids
2.4.5. Phenolic Compounds
2.5. Bioactivity of Bioaccessible Compounds
2.5.1. Antioxidant Capacity
ABTS Method
ORAC Method
Intracellular Reactive Oxygen Species (ROS) Formation
2.5.2. Anti-Inflammatory Properties
2.5.3. Antidiabetic Properties
2.5.3.1. Carbohydrase Activity
2.5.3.2. Glucose Absorption
2.5.3.3. Glucose Transport Inhibition
- Determination of the kinetic mechanism of inhibition
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. Duodenal Bioaccessibility of Nutrients
3.2. Duodenal Bioaccessibility of Non-Nutrients
3.3. Colonic Bioaccessibility of Metabolites Formed by Microbial Fermentation of Nutrients: Short-Chain Fatty Acids (SCFAs)
3.4. Colonic Bioaccessibility of Non-Nutrients
3.5. Bioactivity of Bioaccessible Compounds
3.5.1. Antioxidant Capacity
3.5.2. Anti-Inflammatory Properties
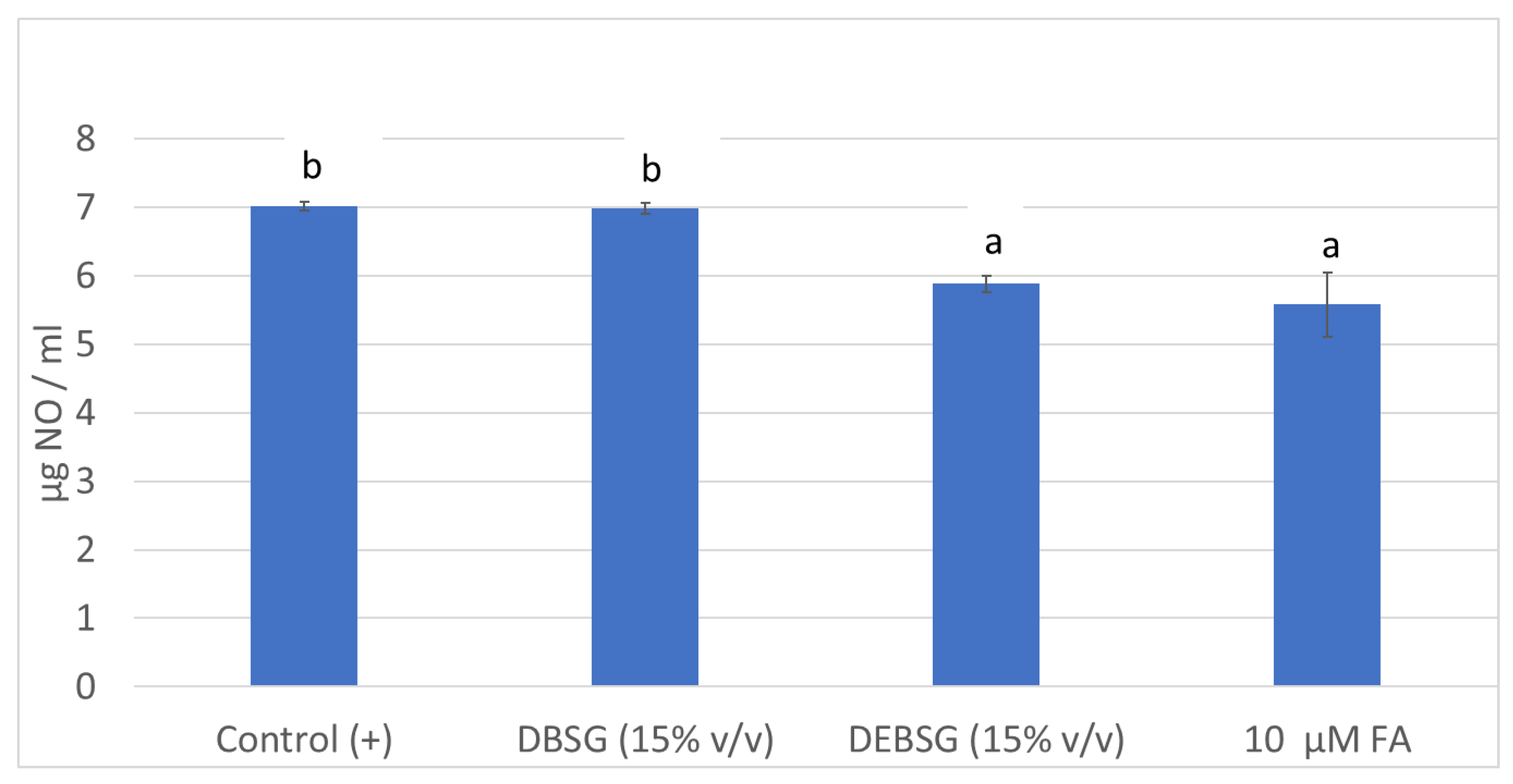
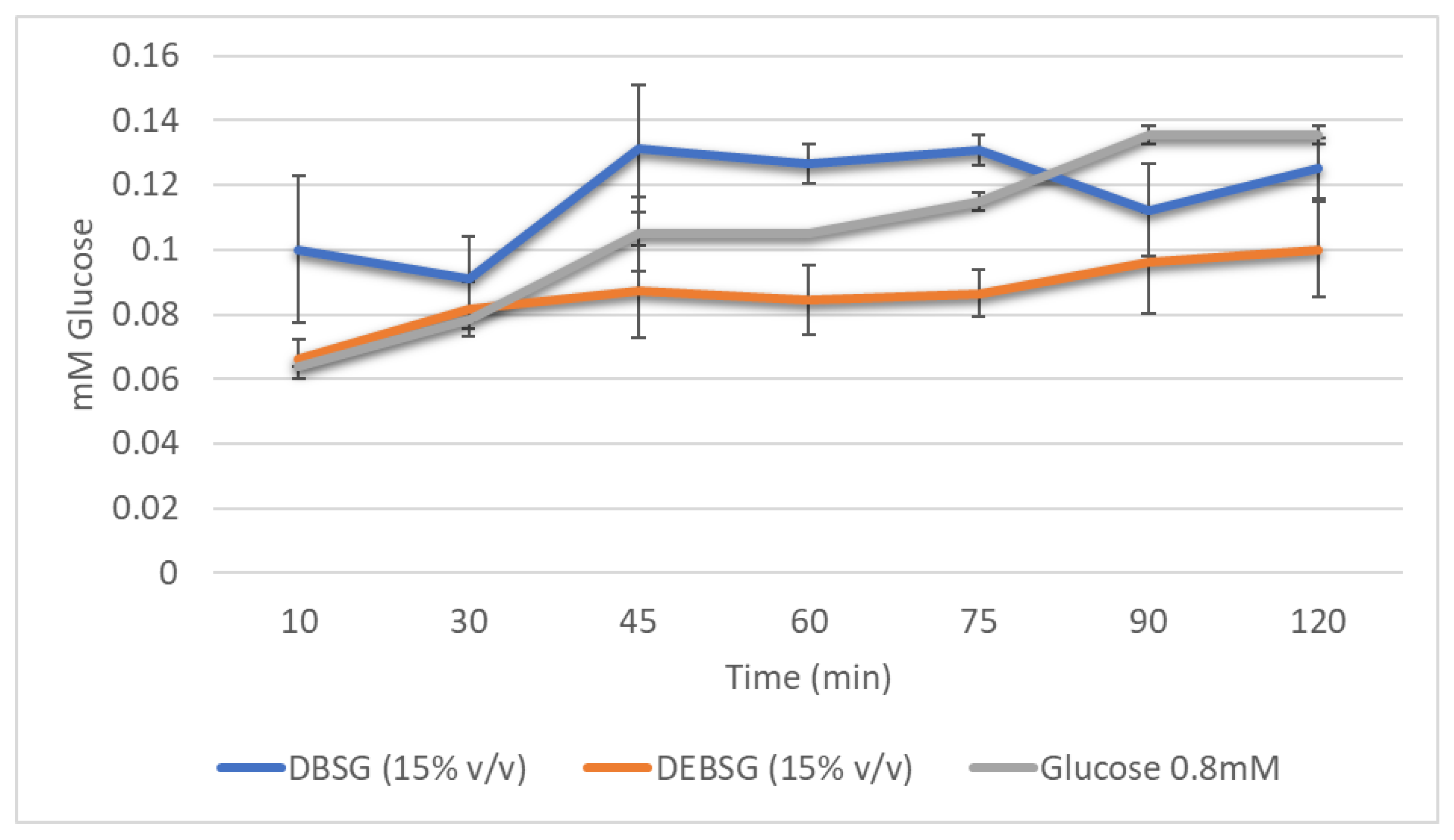
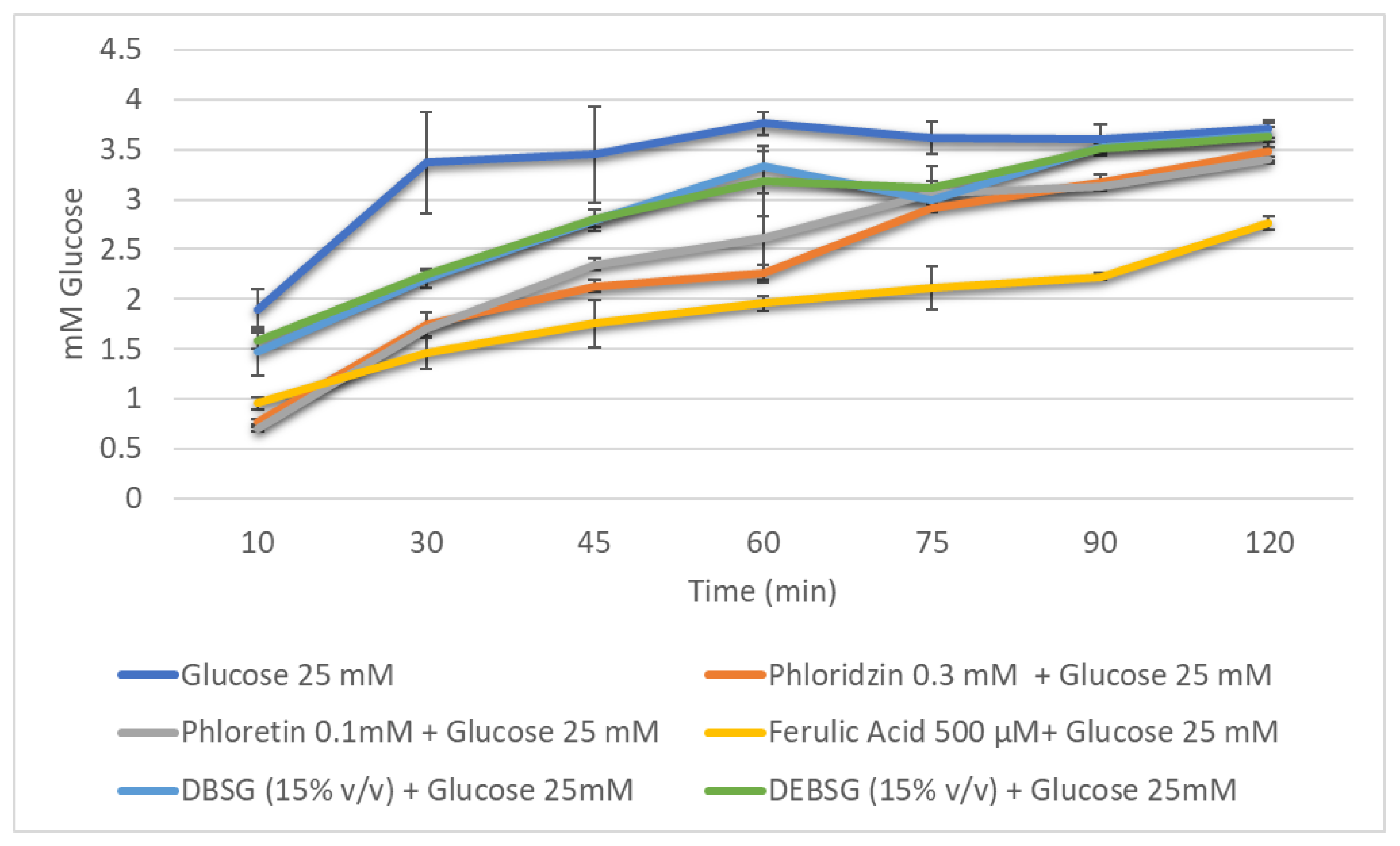
3.5.3. Antidiabetic Properties
Carbohydrase Activity
Glucose Absorption
Glucose Absorption Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN General Assembly, Transforming Our World: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 22 August 2022).
- De Foidmont-Goertz, I.; Faure, U.; Gajdzinska, M.; Haent Jens, W.; Krommer, J.; Lizaso, M.; Lutzeyer, H.-J.; Mangan, C.; Markakis, M.; Schoumacher, C.; et al. Food 2030 Pathways for Action; Fabbri, K., Ndongosi, I., Eds.; Publications Office: Luxembourg, 2020; ISBN 9789276181224. [Google Scholar]
- WHO Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Keyfacts,-andmiddle-incomecountries (accessed on 8 June 2022).
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers ’ Spent Grain: A Review with an Emphasis on Food and Health. Inst. Brew. Distill. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- ReGrained. Available online: https://www.regrained.com/pages/about-us (accessed on 29 June 2022).
- Grainstone. Available online: https://www.grainstone.com.au/ (accessed on 29 June 2022).
- Jimenez, B.; Gopi, A.; Fitting, A.; Jimenez, E. Rise. Available online: https://www.riseproducts.co/ (accessed on 29 June 2022).
- Gutiérrez-Barrutia, M.B.; del Castillo, M.D.; Arcia, P.; Cozzano, S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- AdedejAdekola, K.A. Engineering Review Food Extrusion Technology and Its Applications. J. Food Sci. Eng. 2016, 6, 149–168. [Google Scholar] [CrossRef][Green Version]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Björck, I.; Asp, N.G. The Effects of Extrusion Cooking on Nutritional Value—A Literature Review. J. Food Eng. 1983, 2, 281–308. [Google Scholar] [CrossRef]
- Obradović, V.; Babic, J.; Šubarić, D.; Ačkar, D.; Jozinović, A. Improvement of Nutritional and Functional Properties of Extruded Food Products Improvement of Nutritional and Functional Properties of Extruded Food Products. J. Food Nutr. Res. 2014, 53, 189–206. [Google Scholar]
- Grundy, M.M.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-Evaluation of the Mechanisms of Dietary Fi Bre and Implications for Macronutrient Bioaccessibility, Digestion and Postprandial Metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Francis, N.; Blanchard, C.; Santhakumar, A. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review Borkwei. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Anson, N.M.; Mateo, N.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Antioxidant and Anti-Inflammatory Capacity of Bioaccessible Compounds from Wheat Fractions after Gastrointestinal Digestion Antioxidant and Anti-Inflammatory Capacity of Bioaccessible Compounds from Wheat Fractions after Gastrointestinal Digestion. J. Cereal Sci. 2010, 51, 110–114. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao Zhang, X.Z.; Chen Yang, F.H.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flazseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.J.; Larondelle, Y.; Rogez, H. Development of a Standardised Human in Vitro Digestion Protocol Based on Macronutrient Digestion Using Response Surface Methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Hochkogler, C.M.; Somoza, V.; del Castillo, M.D. Biscuits with No Added Sugar Containing Stevia, Coffee Fibre and Fructooligosaccharides Modifies α-Glucosidase Activity and the Release of GLP-1 from HuTu-80 Cells and Serotonin from Caco-2 Cells after In Vitro Digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.K.; Olson, B.J.; Klenk, D. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ketnawa, S.; Ogawa, Y. In Vitro Protein Digestibility and Biochemical Characteristics of Soaked, Boiled and Fermented Soybeans. Sci. Rep. 2021, 11, 14257. [Google Scholar] [CrossRef]
- Mena, M.C.; Lombardía, M.; Hernando, A.; Méndez, E.; Albar, J.P. Comprehensive Analysis of Gluten in Processed Foods Using a New Extraction Method and a Competitive ELISA Based on the R5 Antibody. Talanta 2012, 91, 33–40. [Google Scholar] [CrossRef]
- Shene, C.; Asenjo, J.A.; Paredes, P.; Flores, L.; Leyton, A.; Chisti, Y. Dynamic Flux Balance Analysis of Biomass and Lipid Production by Antarctic Thraustochytrid Oblongichytrium sp. RT2316—13. Biotechnol. Bioeng. 2020, 117, 3006–3017. [Google Scholar] [CrossRef]
- Spackman, D.; Stein, W.; Moore, S. Automatic Recording Apparatus for Use in the Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Fernandez-Gomez, B.; Cai, W.; Uribarri, J.; del Castillo, M.D. In Vitro Formation of Maillard Reaction Products during Simulated Digestion of Meal-Resembling Systems. Food Res. Int. 2019, 118, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; García, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez, F.; Patricia, G.; Ignacio, M.; Andres, S.; Sanchez-Fortun, S.; Dolores, M. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.; Hinojosa-Nogueira, D. Effect of Cooking Methods on the Antioxidant Capacity of Foods of Animal Origin Submitted to In Vitro. Antioxidants 2021, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rufián-Henares, J.A.; Pastoriza, S. Towards an Improved Global Antioxidant Response Method (GAR+): Physiological-Resembling in Vitro Digestion-Fermentation Method Q. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J. Plant Extracts as Natural Modulators of Gut Microbiota Community Structure and Functionality. Heliyon 2020, 6, e05474. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-arribas, M.V. Understanding the Impact of Chia Seed Mucilage on Human Gut Microbiota by Using the Dynamic Gastrointestinal Model Simgi ®®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Hartemink, R.; Domenech, V.R.; Rombouts, F.M. Methods LAMVAB—A New Selective Medium for the Isolation of Lactobacilli from Faeces. J. Microbiol. Methods 1997, 29, 77–84. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Ramírez, B.; Velazquez Escobar, F.; del Castillo, M.D. Antioxidant Properties of High Molecular Weight Compounds from Coffee Roasting and Brewing Byproducts. Bioact. Compd. Health Dis. 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Bakondi, E.; Gonczi, M.; Szabós, É.; Bai, P.; Pacher, P.; Gergely, P.; Kovács, L.; Hunyadi, J.; Szabó, C.; Csernoch, L.; et al. Role of Intracellular Calcium Mobilization and Cell-Density-Dependent Signaling in Oxidative-Stress-Induced Cytotoxicity in HaCaT Keratinocytes. J. Investig. Dermatol. 2003, 121, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia Ficus-Indica Flowers Obtained by Different Extraction Methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-vila, A.; Stanton, C.; Ross, R.P. The a -Amylase and a -Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chuang, Y.; Hsieh, J. Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola Crenulata. Foods 2019, 8, 540. [Google Scholar] [CrossRef]
- Puthia, M.K.; Sio, S.W.S.; Lu, J.; Tan, K.S.W. Blastocystis Ratti Induces Contact-Independent Apoptosis, F-Actin Rearrangement, and Barrier Function Disruption in IEC-6 Cells. Infect. Innmunity 2006, 74, 4114–4123. [Google Scholar] [CrossRef]
- Miller, R.D.; Monsul, N.T.; Vender, J.R.; Lehmann, J.C. NMDA- and Endothelin- 1 -Induced Increases in Blood-Brain Barrier Permeability Quantitated with Lucifer Yellow. J. Neurol. Sci. 1996, 136, 37–40. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.; Leo, G.; Mabur, J.; Lenhard, J.; Hornby, P. Derived Tryptophan Indoles Increase after Gastric Bypass Surgery and Reduce Intestinal Permeability in Vitro and in Vivo. Neurogastroentology Motil. 2017, 30, e13178. [Google Scholar] [CrossRef]
- Fernández, A.; Iriondo-dehond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano, A.; del Castillo, M.D. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. [Google Scholar] [CrossRef]
- Manzano, S.; Williamson, G. Polyphenols and Phenolic Acids from Strawberry and Apple Decrease Glucose Uptake and Transport by Human Intestinal Caco-2 Cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, D.; Freitas, D.; Golding, M.; Le Feuntenun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in Vitro and in Vivo Data on Food Digestion. What Can We Predict with Static in Vitro Digestion Models? Crit. Rev. Food Sci. Nutr. 2017, 58, 2239–2261. [Google Scholar] [CrossRef]
- Becker, D.; Bakuradze, T.; Hensel, M.; Beller, S.; Corral, C.; Richling, E. Influence of Brewer’s Spent Grain Compounds on Glucose Metabolism Enzymes. Nutrients 2021, 13, 2696. [Google Scholar] [CrossRef] [PubMed]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of Glucose Absorption in the Small Intestine in Appetite Regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- ThermoFishcer Scientific Kit de Ensayo de Proteínas BCA PierceTM. Available online: https://www.thermofisher.com/order/catalog/product/23225 (accessed on 20 July 2022).
- Kapoor, K.N.; Barry, D.T.; Rees, R.C.; Dodi, I.A.; Mcardle, S.E.B.; Creaser, C.S.; Bonner, P.L.R. Estimation of Peptide Concentration by a Modified Bicinchoninic Acid Assay. Anal. Biochem. 2009, 393, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J. Protein Content and Amino Acid Composition of Commercially Available Plant—Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzynski, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. FOOD Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Gutiérrez-Uribe, J.A.; Campos-Vega, R.; Gaytán-Martínez, M. Influence of Extrusion Process on the Release of Phenolic Compounds from Mango (Mangifera indica L.) Bagasse-Added Confections and Evaluation of Their Bioaccessibility, Intestinal Permeability, and Antioxidant Capacity. Food Res. Int. 2021, 148, 110591. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11, e161086. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by- Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Bernalier-Donadille, A. Fermentative Metabolism by the Human. Gastroentérologie Clin. Biol. 2010, 34, S16–S22. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Silva, M.; Molinero, N.; Miralles, B.; Moreno-arribas, M.V. Gastrointestinal Co-Digestion of Wine Polyphenols with Glucose/Whey Proteins Affects Their Bioaccessibility and Impact on Colonic Microbiota Bego N. Food Res. Int. 2022, 155, 111010. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Abele, G.; Miggiano, D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Havensone, G.; Meija, L.; Lejnieks, A. Perspective: Physiological Benefits of Short-Chain Fatty Acids from Cereal Grain Fibre Fermentation and Metabolic Syndrome. Proc. Latv. Acad. Sci. 2020, 74, 65–67. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; De Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Bui, A.T.; Williams, B.A.; Murtaza, N.; Lisle, A.; Mikkelsen, D.; Morrison, M.; Gidley, M.J. Wheat-Based Food Form Has a Greater Effect than Amylose Content on Fermentation Outcomes and Microbial Community Shifts in an in Vitro Fermentation Model. Food Hydrocoll. 2021, 114, 106560. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Smith, C.; Haute, M.J. Van Processing Has Differential Effects on Microbiota-Accessible Carbohydrates in Whole Grains during In Vitro Fermentation. Appl. Environ. Microbiol. 2020, 86, 1705–1720. [Google Scholar] [CrossRef]
- Sajib, M.; Falck, P.; Sardari, R.R.R.; Mathew, S.; Grey, C.; Karlsson, E.N.; Adlercreutz, P. Valorization of Brewer’ s Spent Grain to Prebiotic Oligosaccharide: Production, Xylanase Catalyzed Hydrolysis, in-Vitro Evaluation with Probiotic Strains and in a Batch Human Fecal Fermentation Model. J. Biotechnol. 2018, 268, 61–70. [Google Scholar] [CrossRef]
- Reis, S.F.; Gullón, B.; Gullón, P.; Ferreira, S.; Maia, C.J.; Alonso, J.L.; Domingues, F.C.; Abu-ghannam, N. Evaluation of the Prebiotic Potential of Arabinoxylans from Brewer ’ s Spent Grain. Appl. Microb. Cell Physiol. 2014, 98, 9365–9373. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet—Microbe—Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Hole, A.S.; Kjos, N.P.; Grimmer, S.; Kohler, A.; Lea, P.; Rasmussen, B.; Lima, L.R.; Narvhus, J.; Sahlstrøm, S. Extrusion of Barley and Oat Improves the Bioaccessibility of Dietary Phenolic Acids in Growing Pigs. J. Agric. Food Chem. 2013, 61, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Chitindingu, K.; Benhura, M.A.N.; Muchuweti, M. LWT—Food Science and Technology In Vitro Bioaccessibility Assessment of Phenolic Compounds from Selected Cereal Grains: A Prediction Tool of Nutritional Ef Fi Ciency. LWT Food Sci. Technol. 2015, 63, 575–581. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil, J.; Patrick, M.; Aylin, O.R.; Paul, W.S.R.; et al. Extraction and Characterisation of Arabinoxylan from Brewers Spent Grain and Investigation of Microbiome Modulation Potential. Eur. J. Nutr. 2021, 60, 4393–4411. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ying, D.; Guo, B.; Cheng, J.; May, B. Extrusion of Apple Pomace Increases Antioxidant Activity upon in Vitro Digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; González-Aguilar, G.; Rouzaud-Sanchez, O.; Robles-Sánchez, M. Bioaccessibility of Hydroxycinnamic Acids and Antioxidant Capacity from Sorghum Bran Thermally Processed during Simulated in Vitro Gastrointestinal Digestion. J. Food Sci. Technol. 2018, 55, 2021–2030. [Google Scholar] [CrossRef]
- Vieira, E.F.; Dias, D.; Carmo, H.; Ferreira, I.M.P.L.V.O. Protective Ability against Oxidative Stress of Brewers ’ Spent Grain Protein Hydrolysates. Food Chem. 2017, 228, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Salazar Lopez, N.; Loarca-Piña, G.; Campos-Vega, R.; Martínez, M.G.; Moralez Sánchez, E.; Esquerra-Brauer, J.M.; Gonzalez-aguilar, G.A.; Robles-Sánchez, R.M. The Extrusion Process as an Alternative for Improving the Biological Potential of Sorghum Bran: Phenolic Compounds and Antiradical and Anti-Inflammatory Capacity. Evid. Based Complement. Alterantive Med. 2016, 2016, 8387975. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Alam, A. Anti-Hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Mccarthy, A.L.; Callaghan, Y.C.O.; Connolly, A.; Piggott, C.O.; Fitzgerald, R.J.; Brien, N.M.O. In Vitro Antioxidant and Anti-Inflammatory Effects of Brewers ’ Spent Grain Protein Rich Isolate and Its Associated Hydrolysates. Food Res. Int. 2013, 50, 205–212. [Google Scholar] [CrossRef]
- Cian, R.; Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Drago, S.; Sánchez de Medina, F.; Martínez-Augustin, O. Molecular Action Mechanism of Anti-Inflammatory Hydrolysates Obtained from Brewers’ Spent Grain Running. J. Sci. Food Agric. 2020, 100, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A.; Thomson, D. Separation and Characterization of Two Rat-Intestinal Amylases. Biochem. J. 1963, 89, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Connolly, A.; Piggott, C.O.; Fitzgerald, R.J. In Vitro α-Glucosidase, Angiotensin Converting Enzyme and Dipeptidyl Peptidase-IV Inhibitory Properties of Brewers’ Spent Grain Protein Hydrolysates Food Research International In Vitro α -Glucosidase, Angiotensin Converting Enzyme and Dipeptidyl Pept. Food Res. Int. 2014, 56, 100–107. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H. A Series of Cinnamic Acid Derivatives and Their Inhibitory Activity on Intestinal α-Glucosidase A Series of Cinnamic Acid Derivatives and Their Inhibitory Activity on Intestinal α-Glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 6366, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, L.; Tian, Y.; Zhang, X.; Li, B. Protein Hydrolysate from Brewer ’ s Spent Grain and Its Inhibitory Ability of α-Glucosidase. Adv. Mater. Res. 2012, 582, 138–141. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.; Sang, D.; Gao, Q.; Li, Q. The Role of Nutrition in the Prevention and Intervention of Type 2 Diabetes. Front. Bioeng. Biotechnol. 2020, 8, 575442. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Helliwell, P.A. Glucose-Induced Recruitment of GLUT2 to the Brush-Border Membrane. Biochem. J. 2000, 162, 155–162. [Google Scholar] [CrossRef]
- Helliwell, P.A.; Richardson, M.; Affleck, J.; Kellett, G.L. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000, 154, 149–154. [Google Scholar] [CrossRef]
- Helliwell, P.A.; Rumsby, M.G.; Kellett, G.L. Intestinal Sugar Absorption Is Regulated by Phosphorylation and Turnover of Protein Kinase C βII Mediated by Phosphatidylinositol 3-Kinase- and Mammalian Target of Rapamycin-Dependent Pathways *. J. Biol. Chem. 2003, 278, 28644–28650. [Google Scholar] [CrossRef]
- Nistor, L.A.; Martineau, L.C.; Benhaddou-andaloussi, A.; Arnason, J.T.; Lévy, É.; Haddad, P.S. Inhibition of Intestinal Glucose Absorption by Anti-Diabetic Medicinal Plants Derived from the James Bay Cree Traditional Pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose Transporters in the Small Intestine in Health and Disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jeffrey, S.; Duenes, B.; Sarr, M. Mechanisms of Glucose Uptake in Intestinal Cell Lines: Role of GLUT2. Natl. Institutes Health 2013, 151, 13–25. [Google Scholar] [CrossRef]
- Welsch, C.A.; Lachahce, P.A.; Wasserman, A.P. Nutrient Uptake and Transport Dietary Phenolic Compounds: Inhibition of Na- Dependent o-Glucose Uptake in Rat Intestinal Brush Border Membrane Vesicles. Am. Inst. Nutr. 1989, 119, 1698–1704. [Google Scholar] [CrossRef]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary Polyphenols Decrease Glucose Uptake by Human Intestinal Caco-2 Cells.Pdf. Fed. Eur. Soc. Bioquímicas 2005, 579, 1653–1657. [Google Scholar] [CrossRef]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of Intestinal-Glucosidase and Glucose Absorption by Feruloylated Arabinoxylan Mono- and Oligosaccharides from Corn Bran and Wheat Aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef]
- Li, M.; Koecher, K.; Hansen, L.; Ferruzzi, M.G. Phenolics from Whole Grain Oat Products as Modifiers of Starch Digestion and Intestinal Glucose Transport. J. Agric. Food Chem. 2017, 65, 6831–6839. [Google Scholar] [CrossRef]


| Glucose (mM) | Soluble Protein (mg BSA/mL) | Gluten (ppm) | Amino Acids (mM of Equivalent N-acetyl lysine) | |
|---|---|---|---|---|
| DBSG | 4.654 ± 0.347 a | 17.089 ± 0.069 a | <3 a | 55.781 ± 3.516 a |
| DEBSG | 4.788 ± 0.217 a | 18.942 ± 0.012 b | <3 a | 69.776 ± 6.389 b |
| Amino Acid (mM) | DBSG | DEBSG | |
|---|---|---|---|
| Non-essential amino acids (NEAA) | Alanine (Ala) | 5.759 ± 0.220 a,J | 6.719 ± 0.353 b,K |
| Arginine (Arg) | 3.442 ± 0.038 a,G,H | 3.363 ± 0.232 a,I | |
| Aspartic acid (Asp) | 0.235 ± 0.025 a,A | 0.235 ± 0.025 a,A | |
| Cysteine (Cys) | 0.502 ± 0.034 a,A | 0.461 ± 0.075 a,A,B | |
| Glutamic acid (Glu) | 1.309 ± 0.070 a,C,D | 1.328 ± 0.098 a,C,D,E | |
| Glycine (Gly) | 3.259 ± 0.075 a,G | 3.294 ± 0.174 a,I | |
| Proline (Pro) | 1.535 ± 0.332 a,D | 1.578 ± 0.351 a,D,E,F | |
| Serine (Ser) | 8.762 ± 0.242 a,L | 10.498 ± 0.382 b,N | |
| Tyrosine (Tyr) | 1.600 ± 0.098 a,D | 1.788 ± 0.096 b,E,F | |
| Total NEAA | 26.282 ± 0.717 a,* | 28.729 ± 1.788 b | |
| Essential amino acids (EAA) | Histidine (His) | 1.139 ± 0.034 a,B,C | 1.158± 0.455 a,C,D |
| Isoleucine (Ile) | 2.683 ± 0.092 a,F | 2.870 ± 0.133 b,H,I | |
| Leucine (Leu) | 7.360 ± 0.249 a,K | 8.729 ± 0.471 b,M | |
| Lysine (Lys) | 2.744 ± 0.060 b,F | 2.580 ± 0.154 a,H,G | |
| Methionine (Met) | 0.876 ± 0.021 a,B | 0.892 ± 0.068 a,B,C | |
| Phenylalanine (Phe) | 3.632 ± 0.189 a,H | 3.909 ± 0.295 a,J | |
| Threonine (Thr) | 1.975 ± 0.049 a,E | 2.079 ± 0.081 b,F,G | |
| Tryptophan (Trp) | n.d. | n.d. | |
| Valine (Val) | 4.541 ± 0.132 a,I | 4.824 ± 0.400 a,L | |
| Total EAA | 24.947 ± 0.661 a | 27.041 ± 1.570 b | |
| Total | 51.457 ± 1.230 a | 56.233 ± 3.074 b |
| Total Phenolic Content (mg FAeq/g of Digested Sample) | ||
|---|---|---|
| BSG | EBSG | |
| Duodenal bioaccessibility | 3.221 ± 0.116 a,A | 3.604 ± 0.111 b,A |
| Colon bioaccessibility | 4.743 ± 0.235 a,B | 5.290 ± 0.072 b,B |
| Proposed Compound | Molecular Formula | Molar Mass (g/mol) | Retention Time (min) | Duodenal Bioaccessibility | Colon Bioaccessibility | ||||
|---|---|---|---|---|---|---|---|---|---|
| DBSG 1 (%) | DEBSG 1 (%) | Variation after Extrusion (%) 2 | FBSG 1 (%) | FEBSG 1 (%) | Variation after Extrusion (%) 2 | ||||
| 2-(3-hydroxyphenyl) propionic acid | C9H10O3 | 165.1 | 16.7 | 95.0 | 94.3 | −3 | 96 | 96 | 14 |
| Ferulic acid | C15H18O8 | 193.1 | 20.0 | 0.5 | 0.5 | 3 | 0 | 0 | 0 |
| Dihydrocaffeic acid | C9H10O4 | 181.1 | 6.7 | 2.8 | 2.8 | 2 | 0 | 0 | 0 |
| Benzoic acid | C7H6O2 | 121.0 | 11.5 | 1.8 | 2.4 | 31 | 4 | 4 | 0 |
| log (CFU/mL) | Relative Percentage to Total Anaerobes | |
|---|---|---|
| Total anaerobes | 9.575 ± 0.077 d | 100.00% |
| Total aerobes | 7.504 ± 0.035 a,b | 0.842% |
| Enterobacteriaceae | 7.435 ± 0.094 a,b | 0.728% |
| Staphylococcus spp. | 7.155 ± 0.045 a | 0.377% |
| Lactic acid bacteria | 8.354 ± 0.039 b,c | 5.965% |
| Lactobacillus spp. | 7.110 ± 0.067 a | 0.342% |
| Clostridium spp. | 9.357 ± 0.157 d | 62.281% |
| Entereococcus spp. | 7.385 ± 0.027 a,b | 0.640% |
| Bifidobacteria | 9.290 ± 0.061 d,c | 51.754% |
| Short-Chain Fatty Acids (mM) | ||
|---|---|---|
| FBSG | FEBSG | |
| Acetic acid | 9.471 ± 0.139 b,D | 8.185 ± 0.674 a,C |
| Propionic acid | 5.808 ± 0.287 a,C | 5.739 ± 0.158 a,B |
| Butyric acid | 5.747 ± 0.142 a,C | 5.551 ± 0.216 a,B |
| Isobutyric acid | 0.302 ± 0.012 a,A | 0.284 ± 0.001 a,A |
| Isovaleric acid | 1.084 ± 0.048 a,B | 1.017 ± 0.090 a,A |
| Valeric acid | 1.008 ± 0.128 a,B | 0.837 ± 0.208 a,A |
| Caproic acid | 0.328 ± 0.036 a,A | 0.298 ± 0.085 a,A |
| Total | 23.748 ±0.181 b | 21.895 ± 1.035 a |
| ABTS (mM FAeq) | ORAC (mM FAeq) | |||
|---|---|---|---|---|
| Duodenal | Colonic | Duodenal | Colonic | |
| BSG | 2.899 ± 0.158 a | 1.967 ± 0.081 a | 10.809 ± 0.109 a | 1.624 ± 0.044 a |
| EBSG | 3.232 ± 0.089 b | 2.132 ± 0.088 b | 13.000 ± 0.080 b | 1.785 ± 0.053 a |
| ABTS (µmol FAeq/g of digested sample) | ORAC (µmol FAeq/g of digested sample) | |||
| BSG | EBSG | BSG | EBSG | |
| Duodenal | 19.916 ± 1.170 A | 23.900 ± 0.673 A | 12.487 ± 0.344 A | 16.950 ± 0.195 A |
| Colonic | 25.354 ± 0.920 B | 28.184 ± 1.157 B | 21.262 ± 0.262 B | 21.632 ± 0.210 B |
| IC50 Acarbose | DBSG | DEBSG | |
|---|---|---|---|
| α-amylase (µM Acarbose) | 10.522 | 0.435 ± 0.010 a,A | 0.689± 0.004 b,A |
| α-glucosidase (mM Acarbose) | 1.002 | 3.005 ± 0.123 a,B | 7.829 ± 1.560 b,B |
| Sucrase (µM Acarbose) | 15.152 | n.i.d. | n.i.d. |
| IAUC Sodium Dependent Conditions (mM × min) | IAUC Sodium Free Conditions (mM × min) | |
|---|---|---|
| Glucose 25 mM | 377.153 ± 21.049 d,A | 380.010 ± 8.862 b,A |
| Phloridzin 3 mM + Glucose 25 mM | 272.741 ± 7.324 b,A | 381.344 ± 20.569 b,B |
| Phloretin 1 mM + Glucose 25 mM | 274.627 ± 6.602 b,A | 334.700 ± 7.394 a,B |
| Ferulic acid 500µM + Glucose 25 mM | 213.868 ± 11.630 a,A | 321.482 ± 7.037 a,B |
| DBSG (15% v/v) + Glucose 25 mM | 322.757 ± 1.854 c,A | 336.658 ± 9.084 a,A |
| DEBSG (15% v/v) + Glucose 25 mM | 324.793 ± 4.320 c,A | 324.747 ± 10.224 a,A |
| Km (mM) | Vmax (mM/min) | Inhibition | |
|---|---|---|---|
| Uptake | |||
| Control (Glucose) | 19.490 ± 3.156 | 0.171 ± 0.027 | n.a. |
| DEBSG (15% v/v) | 16.273 ± 0.557 | 0.180 ± 0.029 | No |
| Ferulic acid 500 µM | 31.493 ± 3.646 * | 0.336 ± 0.056 | Competitive |
| Transport | |||
| Control (Glucose) | 128.427 ± 13.830 | 0.632 ± 0.003 | n.a. |
| DEBSG (15% v/v) | 97.726 ± 1.509 | 0.478 ± 0.015 * | Non-competitive |
| Ferulic acid 500 µM | 133.578± 19.526 | 0.546 ± 0.021 * | Non-competitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Barrutia, M.B.; Cozzano, S.; Arcia, P.; del Castillo, M.D. In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain. Nutrients 2022, 14, 3480. https://doi.org/10.3390/nu14173480
Gutierrez-Barrutia MB, Cozzano S, Arcia P, del Castillo MD. In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain. Nutrients. 2022; 14(17):3480. https://doi.org/10.3390/nu14173480
Chicago/Turabian StyleGutierrez-Barrutia, Maria Belen, Sonia Cozzano, Patricia Arcia, and Maria Dolores del Castillo. 2022. "In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain" Nutrients 14, no. 17: 3480. https://doi.org/10.3390/nu14173480
APA StyleGutierrez-Barrutia, M. B., Cozzano, S., Arcia, P., & del Castillo, M. D. (2022). In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain. Nutrients, 14(17), 3480. https://doi.org/10.3390/nu14173480







