Shibi Tea (Adinandra nitida) and Camellianin A Alleviate CCl4-Induced Liver Injury in C57BL-6J Mice by Attenuation of Oxidative Stress, Inflammation, and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Establishment of Murine Liver Injury Model
2.3. Tissue Processing
2.4. Biochemical Analysis
2.5. Histological Evaluation
2.6. Western Blotting
2.7. Immunohistochemical (IHC)
2.8. Statistical Analysis
3. Results
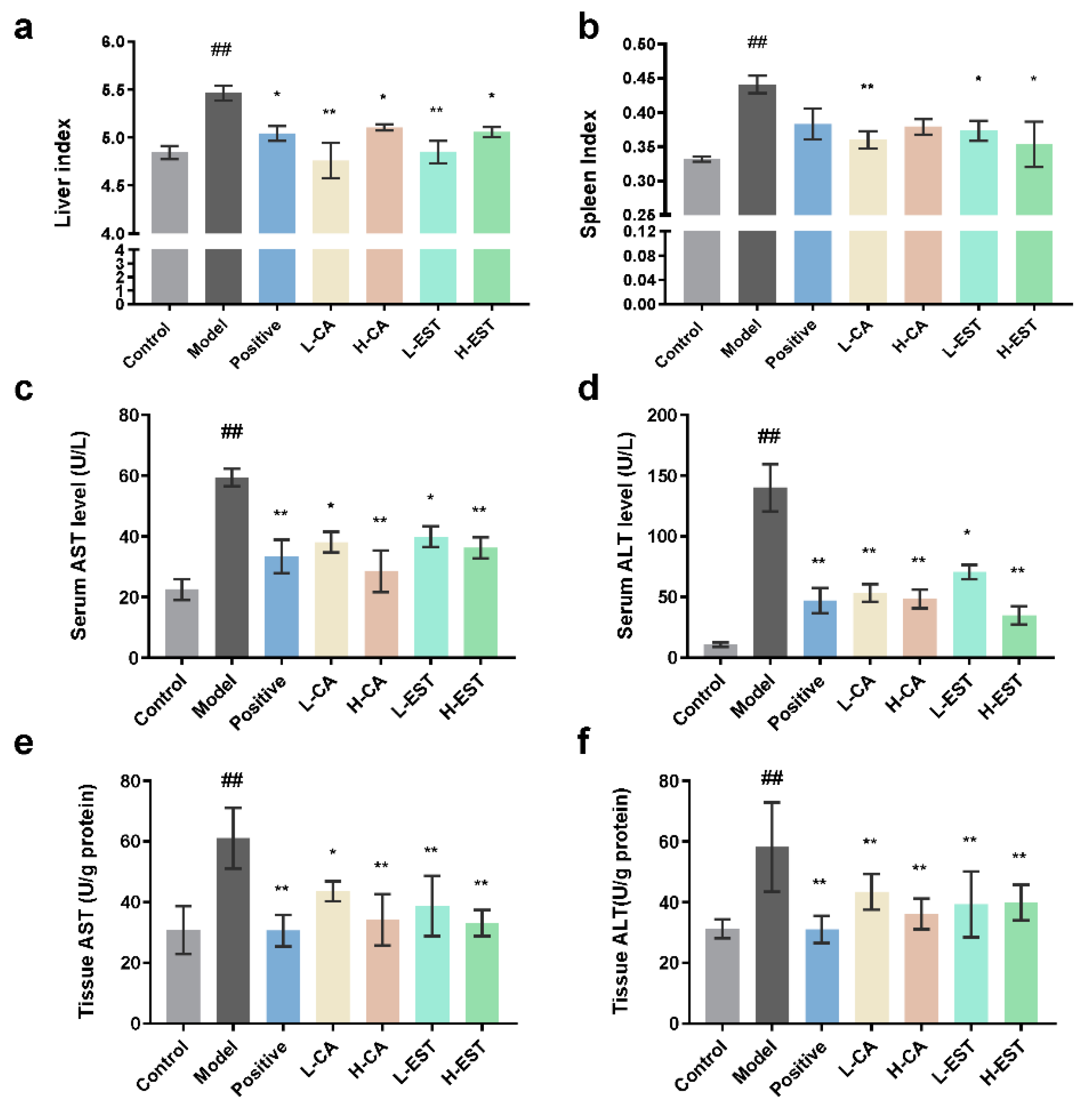
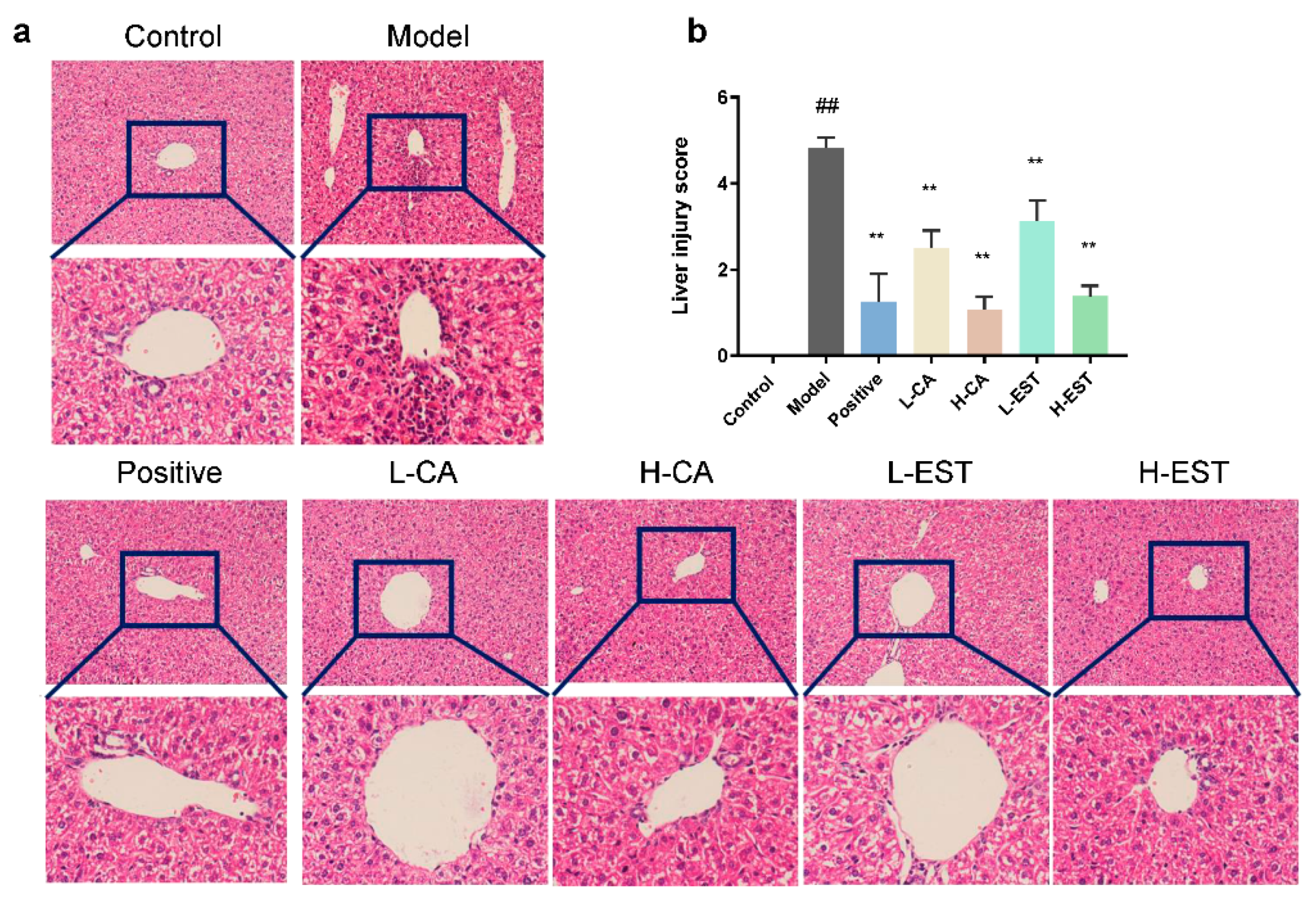
3.1. EST and CA Ameliorated the CCl4-Induced Liver Injury
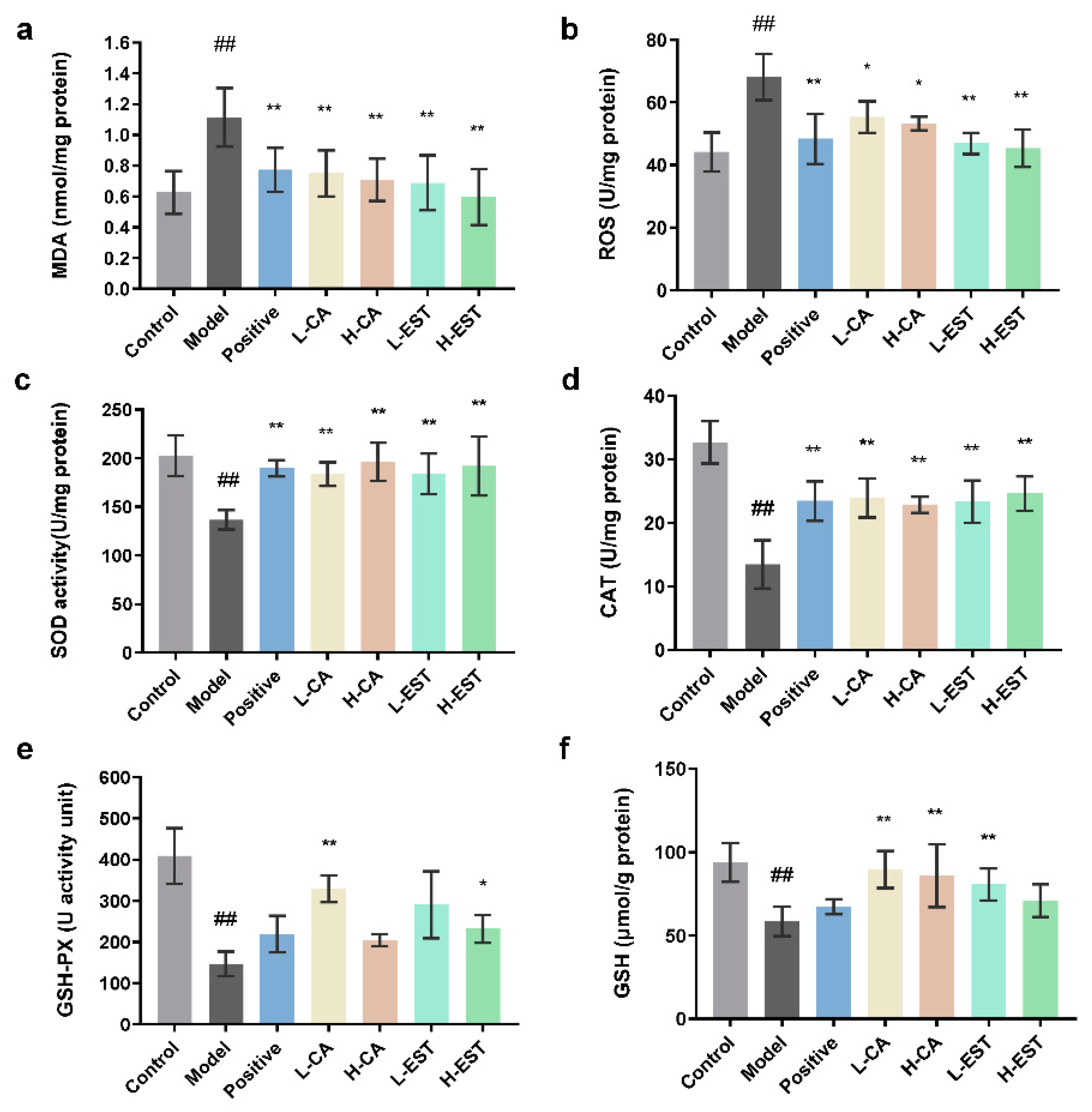
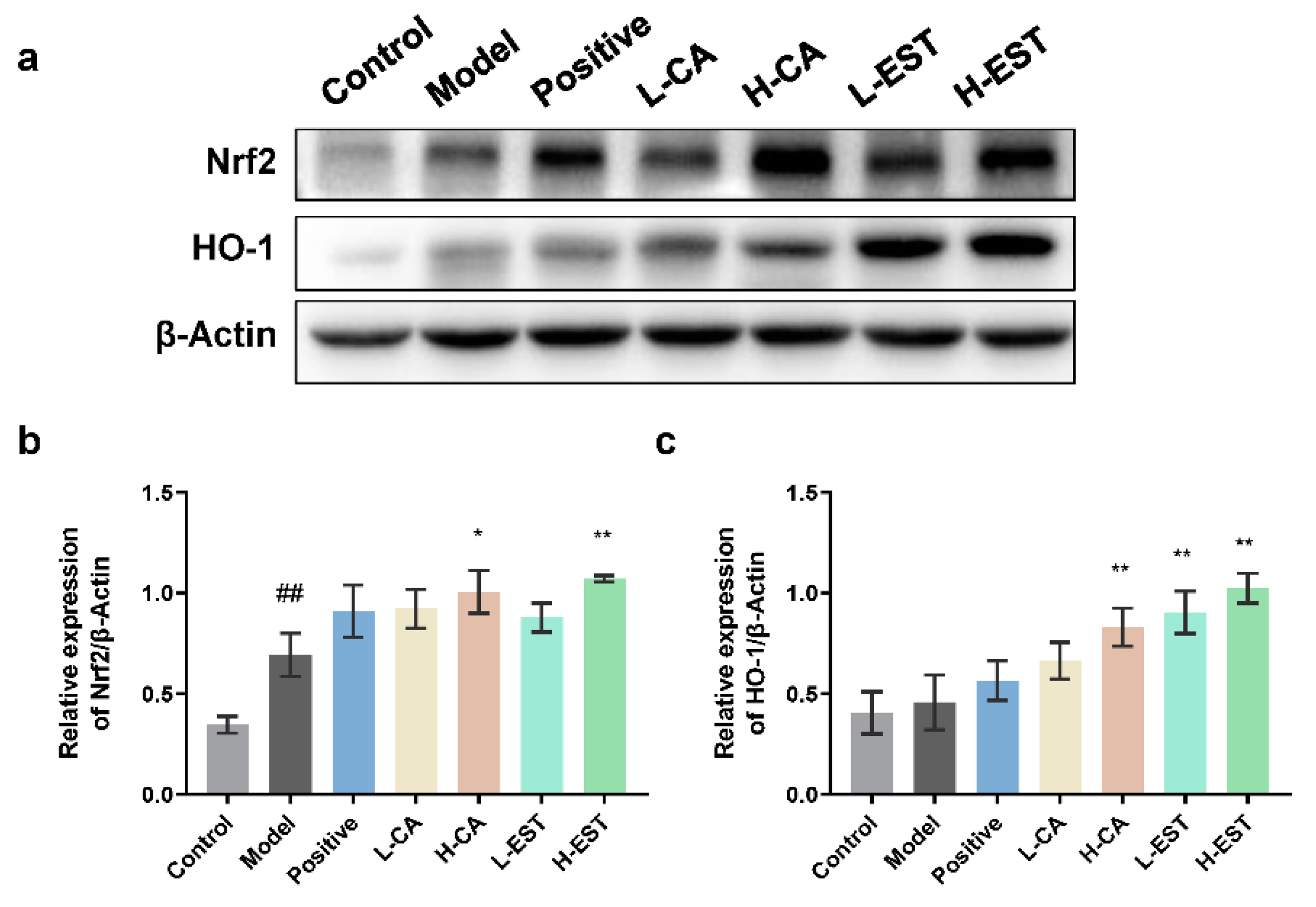
3.2. EST and CA Decrease the CCl4-Induced Oxidative Stress by Activating the Nrf2/HO-1 Pathway
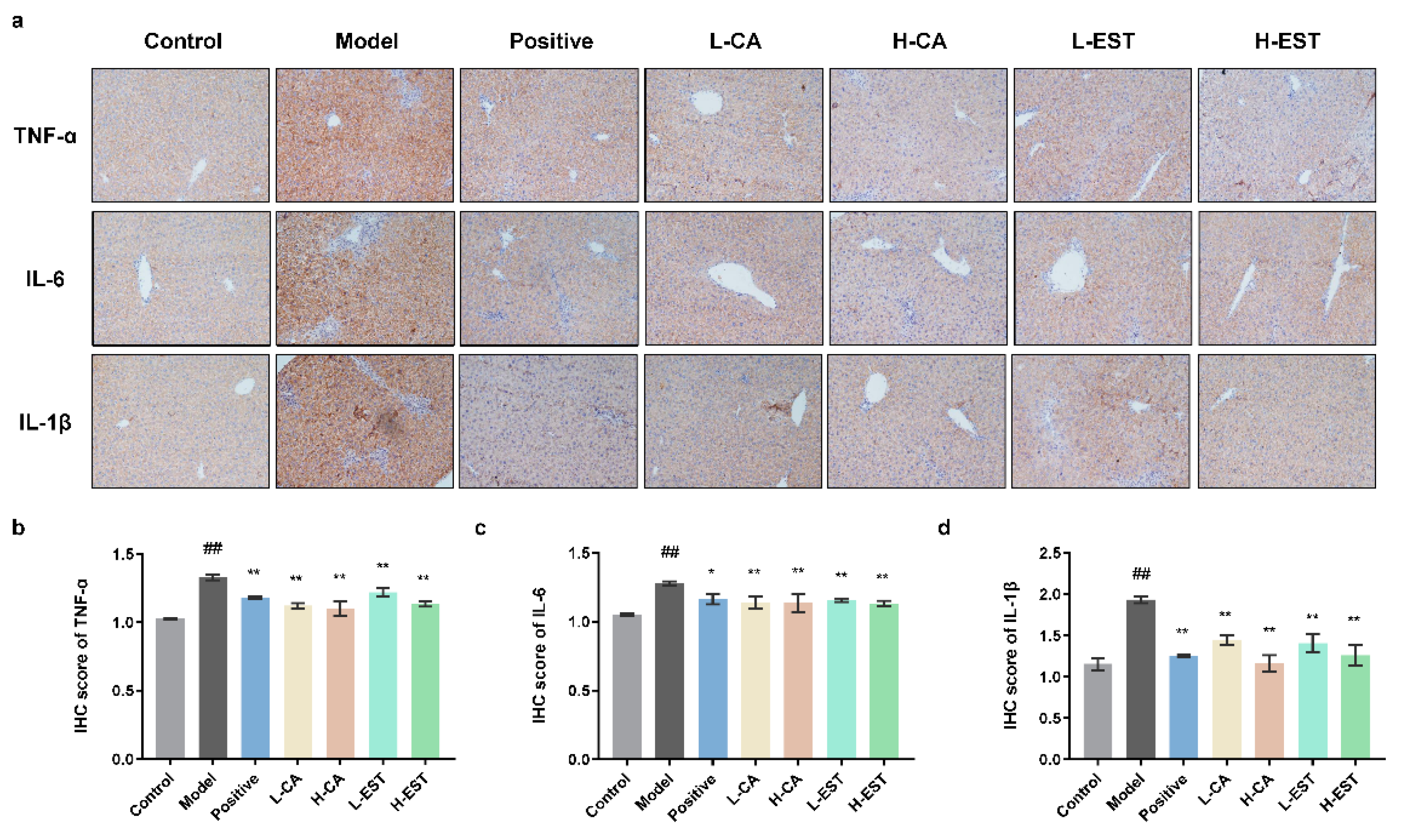
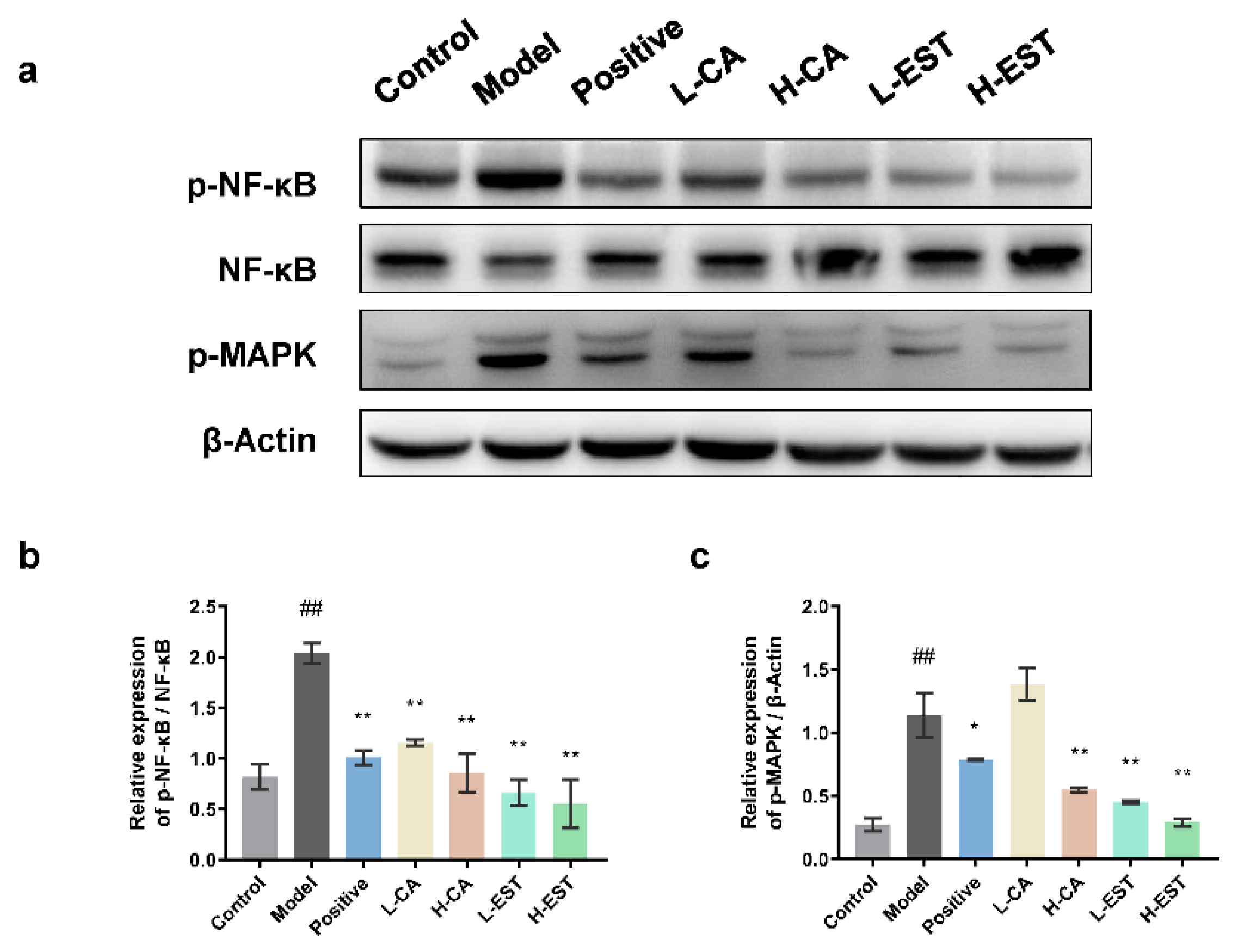
3.3. EST and CA Suppress the CCl4-Induced Pro-Inflammatory Cytokines Expression via Inhibiting the NF-κB Pathway
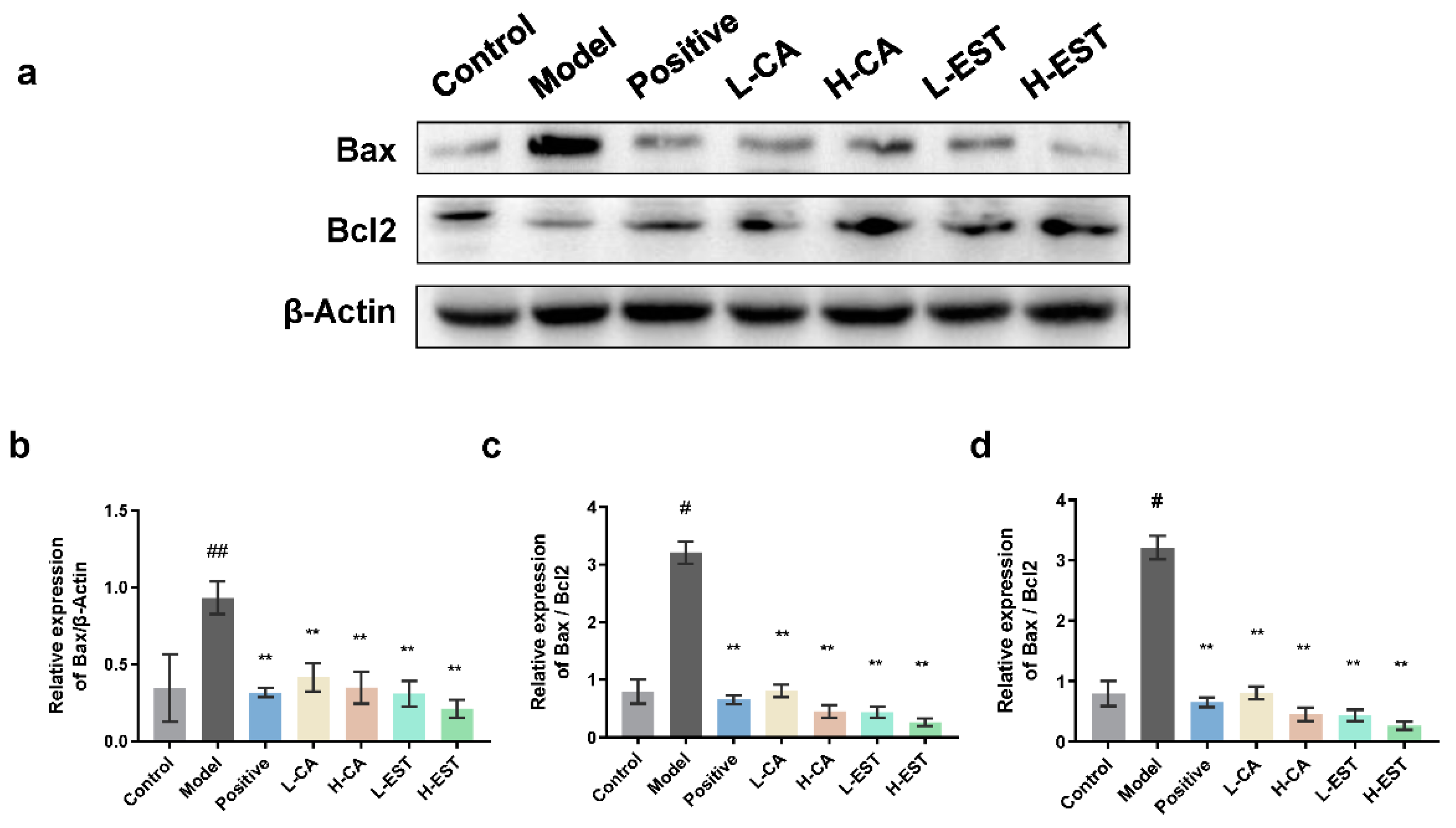
3.4. EST and CA Alleviate CCl4-Induced Hepatocyte Apoptosis by Regulating the Expression of Bax/Bcl-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. CB 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2745. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Feng, M.; Ding, J.; Wang, M.; Zhang, J.; Zhu, X.; Guan, W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int. J. Biol. Sci. 2018, 14, 1033–1040. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, P.H.; Zhang, X.X.; Dai, Y.; He, Q. Breviscapine ameliorates CCl4induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int. J. Mol. Med. 2018, 42, 755–768. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fang, S.; Liu, Q.; Gao, J.; Wang, X.; Zhu, H.; Zhu, Z.; Ji, F.; Wu, J.; Ma, Y.; et al. TGF-beta1/p65/MAT2A pathway regulates liver fibrogenesis via intracellular SAM. EBioMedicine 2019, 42, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef] [Green Version]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jie, Z.; Yang, J.; Duan, J.; Zhen, L.; Zhang, L.; Huo, Y.; Zhang, Y. Quantitative and qualitative analysis of flavonoids in leaves of Adinandra nitida by high performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Anal. Chim. Acta 2005, 532, 97–104. [Google Scholar]
- Liu, B.G.; Ma, Y.X.; Liu, Y.; Yang, Z.; Zhang, L.P. Ultrasonic-Assisted Extraction and Antioxidant Activity of Flavonoids from Adinandra nitida Leaves. Trop. J. Pharm. Res. 2013, 12, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ma, X.; Fu, X.; Yan, R. phytochemical content, cellular antioxidant activity and antiproliferative activity of adinandra nitida tea (shiyacha) infusion subjected to in vitro gastrointestinal digestion. RSC Adv. 2017, 7, 50430–50440. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lian, Y.; Li, Q.; Sun, L.; Chen, R.; Lai, X.; Lai, Z.; Yuan, E.; Sun, S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules 2020, 25, 4626. [Google Scholar] [CrossRef]
- Yuan, E.; Liu, B.; Ning, Z.; Chen, C. Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 2009, 115, 1158–1163. [Google Scholar] [CrossRef]
- Yuana, E.; Liana, Y.; Li, Q.; Lai, X.; Chen, R.; Wen, S.; Zhu, J.; Zhang, W.; Sun, S. Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice. Food Sci. Hum. Wellness 2022, 11, 1053–1063. [Google Scholar] [CrossRef]
- Wen, S.; Sun, L.; An, R.; Zhang, W.; Xiang, L.; Li, Q.; Lai, X.; Huo, M.; Li, D.; Sun, S. A combination of Citrus reticulata peel and black tea inhibits migration and invasion of liver cancer via PI3K/AKT and MMPs signaling pathway. Mol. Biol. Rep. 2020, 47, 507–519. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.W.; Gu, S.T.; Huang, X.; Deng, H.; Wang, X. Sedum sarmentosum Bunge extract ameliorates lipopolysaccharide- and D-galactosamine-induced acute liver injury by attenuating the hedgehog signaling pathway via regulation of miR-124 expression. BMC Complement. Med. 2020, 20, 88. [Google Scholar] [CrossRef] [Green Version]
- Hart, N.A.; van der Plaats, A.; Leuvenink, H.G.; Wiersema-Buist, J.; Olinga, P.; van Luyn, M.J.; Verkerke, G.J.; Rakhorst, G.; Ploeg, R.J. Initial blood washout during organ procurement determines liver injury and function after preservation and reperfusion. Am. J. Transpl. 2004, 4, 1836–1844. [Google Scholar] [CrossRef]
- Chen, R.; Lai, X.; Xiang, L.; Li, Q.; Sun, L.; Lai, Z.; Li, Z.; Zhang, W.; Wen, S.; Cao, J.; et al. Aged green tea reduces high-fat diet-induced fat accumulation and inflammation via activating the AMP-activated protein kinase signaling pathway. Food Nutr. Res. 2022, 66, 7923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Lai, X.; Peng, X.; Wen, S.; Zhang, Z.; Xie, Y.; Li, Q.; Chen, R.; Zheng, X.; et al. Gastroprotective effects of extract of Jasminum grandiflorum L. flower in HCl/EtOH-induced gastric mucosal ulceration mice. Biomed. Pharm. 2021, 144, 112268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oh, D.S.; Oh, J.Y.; Son, T.G.; Yuk, D.Y.; Jung, Y.S. Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response. Molecules 2016, 21, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, M.; Nkwocha, J.; Hawkins, E.; Pei, X.; Parker, R.E.; Kmieciak, M.; Leverson, J.D.; Sampath, D.; Ferreira-Gonzalez, A.; Grant, S. Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells. Cancer Res. 2018, 78, 3075–3086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, P.; Cui, Z.; Wang, Y.; Zhang, C.; Zhang, N.; Li, M.; Xiao, P. Commercialized non-Camellia tea: Traditional function and molecular identification. Acta Pharm. Sin. B 2014, 4, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Lv, Q.; Wan, W.; Le, L.; Xu, L.; Hu, K.; Xiao, P. Transcriptome analysis reveals the mechanism of the effect of flower tea Coreopsis tinctoria on hepatic insulin resistance. Food Funct. 2018, 9, 5607–5620. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.B.; Jie, N.I.; Yao, X.; Wen-Cai, Y.E. Chemical Studies on the Adinandra nitida. J. China Pharm. Univ. 2003, 34, 407–409. [Google Scholar]
- Liu, B.; Yang, J.; Ma, Y.; Yuan, E.; Chen, C. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of ethanol extract and pure flavonoids from Adinandra nitida leaves. Pharm. Biol. 2010, 48, 1432–1438. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, L.; Suh, J.H.; Wang, Y. Bioactivity-Guided Isolation and Identification of Antiadipogenic Compounds in Shiya Tea (Leaves of Adinandra nitida). J. Agric. Food Chem. 2019, 67, 6785–6791. [Google Scholar] [CrossRef]
- Gao, H.; Liu, B.; Liu, F.; Chen, Y. Anti-proliferative effect of camellianin A in Adinandra nitida leaves and its apoptotic induction in human Hep G2 and MCF-7 cells. Molecules 2010, 15, 3878–3886. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Animal models of drug-induced liver injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1031–1039. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Clichici, S.; Olteanu, D.; Nagy, A.L.; Oros, A.; Filip, A.; Mircea, P.A. Silymarin inhibits the progression of fibrosis in the early stages of liver injury in CCl(4)-treated rats. J. Med. Food 2015, 18, 290–298. [Google Scholar] [CrossRef]
- Kalopitas, G.; Antza, C.; Doundoulakis, I.; Siargkas, A.; Kouroumalis, E.; Germanidis, G.; Samara, M.; Chourdakis, M. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition 2021, 83, 111092. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, J.G. Protective effects of plant-derived flavonoids on hepatic injury. J. Funct. Foods 2018, 44, 283–291. [Google Scholar] [CrossRef]
- Teschke, R.; Danan, G. Molecular Research on Drug Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 216. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Xie, J.; Wang, W.; Dong, C.; Huang, L.; Wang, H.; Li, C.; Nie, S.; Xie, M. Protective effect of flavonoids from Cyclocarya paliurus leaves against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2018, 119, 392–399. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, R.; Li, J.; Xing, X.; Chen, J.; Zhou, Q.; Sun, J. Alpinetin exerts anti-inflammatory, anti-oxidative and anti-angiogenic effects through activating the Nrf2 pathway and inhibiting NLRP3 pathway in carbon tetrachloride-induced liver fibrosis. Int. Immunopharmacol. 2021, 96, 107660. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Z.; Shen, B.; Zhang, Q.; Feng, H. Protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-kappaB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2017, 45, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.C.; Fan, J.; Deng, X.W.; Liu, H.C.; Ding, H.R.; Fang, X.; Wang, J.W.; Chen, C.H.; Zhang, W.G. Dihydromyricetin ameliorates chronic liver injury by reducing pyroptosis. World J. Gastroenterol. 2020, 26, 6346–6360. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, Y.; Li, P. A potent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis 2020, 25, 412–425. [Google Scholar] [CrossRef]
- Du, X.S.; Li, H.D.; Yang, X.J.; Li, J.J.; Xu, J.J.; Chen, Y.; Xu, Q.Q.; Yang, L.; He, C.S.; Huang, C.; et al. Wogonin attenuates liver fibrosis via regulating hepatic stellate cell activation and apoptosis. Int. Immunopharmacol. 2019, 75, 105671. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Lian, Y.; Wen, S.; Li, Q.; Sun, L.; Lai, X.; Zhang, Z.; Zhu, J.; Tang, L.; Xuan, J.; et al. Shibi Tea (Adinandra nitida) and Camellianin A Alleviate CCl4-Induced Liver Injury in C57BL-6J Mice by Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Nutrients 2022, 14, 3037. https://doi.org/10.3390/nu14153037
Chen R, Lian Y, Wen S, Li Q, Sun L, Lai X, Zhang Z, Zhu J, Tang L, Xuan J, et al. Shibi Tea (Adinandra nitida) and Camellianin A Alleviate CCl4-Induced Liver Injury in C57BL-6J Mice by Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Nutrients. 2022; 14(15):3037. https://doi.org/10.3390/nu14153037
Chicago/Turabian StyleChen, Ruohong, Yingyi Lian, Shuai Wen, Qiuhua Li, Lingli Sun, Xingfei Lai, Zhenbiao Zhang, Junquan Zhu, Linsong Tang, Ji Xuan, and et al. 2022. "Shibi Tea (Adinandra nitida) and Camellianin A Alleviate CCl4-Induced Liver Injury in C57BL-6J Mice by Attenuation of Oxidative Stress, Inflammation, and Apoptosis" Nutrients 14, no. 15: 3037. https://doi.org/10.3390/nu14153037
APA StyleChen, R., Lian, Y., Wen, S., Li, Q., Sun, L., Lai, X., Zhang, Z., Zhu, J., Tang, L., Xuan, J., Yuan, E., & Sun, S. (2022). Shibi Tea (Adinandra nitida) and Camellianin A Alleviate CCl4-Induced Liver Injury in C57BL-6J Mice by Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Nutrients, 14(15), 3037. https://doi.org/10.3390/nu14153037





