Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Exercise and Dietary Interventions
2.3. Dietary Intake
2.4. Sample Collection and Preparation
2.5. Sirtuin Activity Assay
2.6. RNA Isolation and qRT-PCR
2.7. Data Analysis and Statistical Methods
3. Results
3.1. Baseline
3.2. Dietary Intake
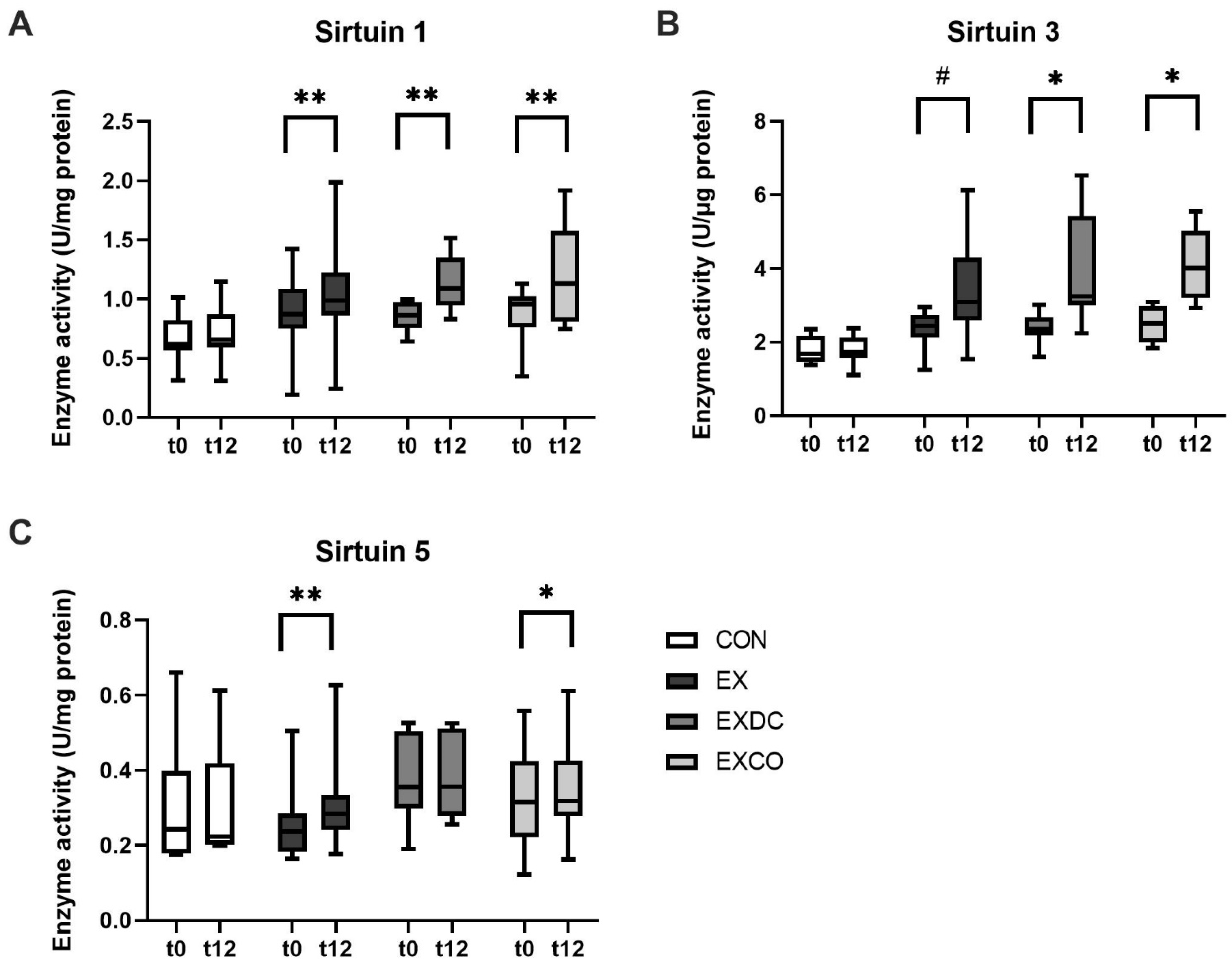
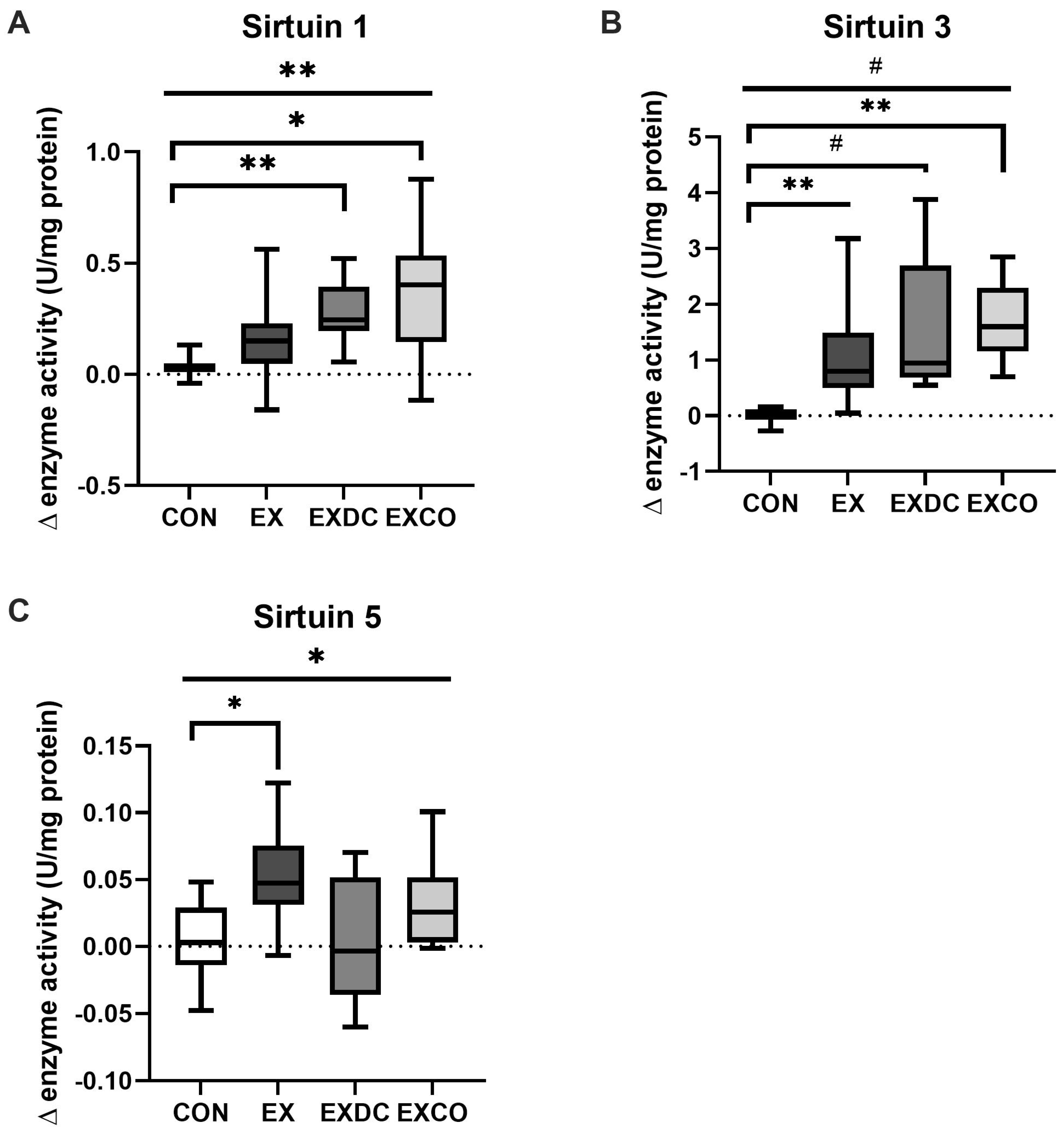
3.3. Sirtuin Activity
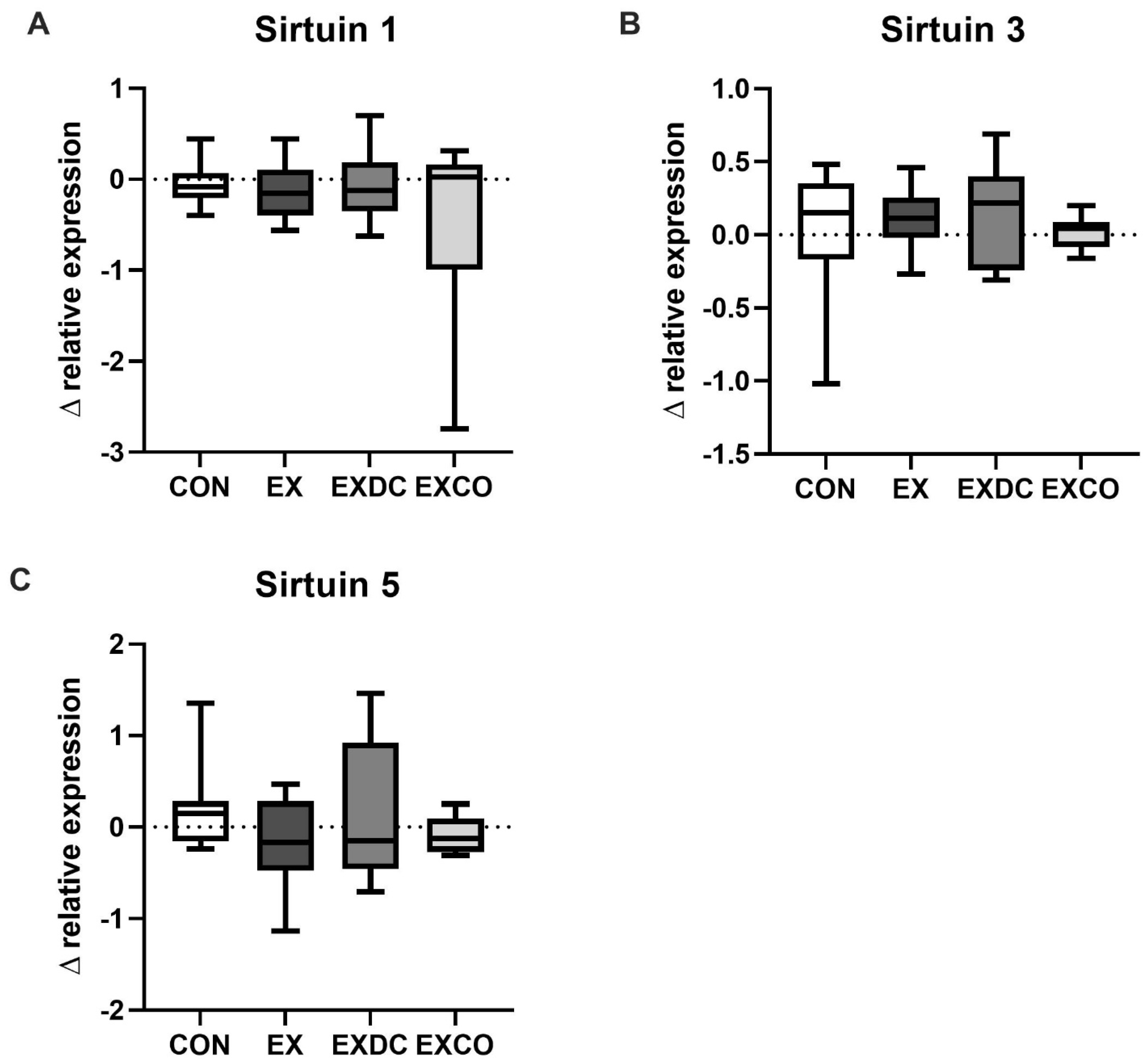
3.4. Relative Expression
4. Discussion
4.1. Exercise and Sirtuins
4.2. Diet and Sirtuins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buler, M.; Andersson, U.; Hakkola, J. Who Watches the Watchmen? Regulation of the Expression and Activity of Sirtuins. FASEB J. 2016, 30, 3942–3960. [Google Scholar] [CrossRef] [Green Version]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The Role of Sirtuins in Cellular Homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Peng, C.; Anderson, K.A.; Chhoy, P.; Xie, Z.; Dai, L.; Park, J.; Chen, Y.; Huang, H.; Zhang, Y.; et al. Lysine Glutarylation Is a Protein Posttranslational Modification Regulated by SIRT5. Cell Metab. 2014, 19, 605–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 Is a NAD-Dependent Protein Lysine Demalonylase and Desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, P.M.; Williams, S.R.; Seymour, A.-M.; Schwartz, A.; Dube, G.; Gadian, D.G.; Radda, G.K. A 31P-NMR Study of Some Metabolic and Functional Effects of the Inotropic Agents Epinephrine and Ouabain, and the Ionophore R02-2985 (X537A) in the Isolated, Perfused Rat Heart. Biochim. Biophys. Acta -Mol. Cell Res. 1982, 720, 163–171. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative Phosphorylation: Unique Regulatory Mechanism and Role in Metabolic Homeostasis. J. Appl. Physiol. 2017, 122, 611–619. [Google Scholar] [CrossRef]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef]
- Das, A.M.; Harris, D.A. Regulation of the Mitochondrial ATP Synthase in Intact Rat Cardiomyocytes. Biochem. J. 1990, 266, 355–361. [Google Scholar] [CrossRef] [Green Version]
- McCormack, J.G. Characterization of the Effects of Ca2+ on the Intramitochondrial Ca2+-Sensitive Enzymes from Rat Liver and within Intact Rat Liver Mitochondria. Biochem. J. 1985, 231, 581–595. [Google Scholar] [CrossRef] [Green Version]
- McCormack, J.G.; Denton, R.M. The Role of Intramitochondrial Ca2+ in the Regulation of Oxidative Phosphorylation in Mammalian Tissues. Biochem. Soc. Trans. 1993, 21, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. (Ed.) Sirtuin Biology in Medicine: Targeting New Avenues of Care in Development, Aging, and Disease, 1st ed.; Elsevier: Waltham, MA, USA, 2021; ISBN 978-0-12-814118-2. [Google Scholar]
- Potthast, A.B.; Nebl, J.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Hahn, A.; Das, A. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients 2020, 12, 1004. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie Restriction Promotes Mammalian Cell Survival by Inducing the SIRT1 Deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boily, G.; Seifert, E.L.; Bevilacqua, L.; He, X.H.; Sabourin, G.; Estey, C.; Moffat, C.; Crawford, S.; Saliba, S.; Jardine, K.; et al. Sirt1 Regulates Energy Metabolism and Response to Caloric Restriction in Mice. PLoS ONE 2008, 3, e1759. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Guarente, L. Calorie Restriction, SIRT1 and Metabolism: Understanding Longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef]
- Ajami, M.; Pazoki-Toroudi, H.; Amani, H.; Nabavi, S.F.; Braidy, N.; Vacca, R.A.; Atanasov, A.G.; Mocan, A.; Nabavi, S.M. Therapeutic Role of Sirtuins in Neurodegenerative Disease and Their Modulation by Polyphenols. Neurosci. Biobehav. Rev. 2017, 73, 39–47. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Khairallah, R.J.; Dabkowski, E.R. Update on Lipids and Mitochondrial Function: Impact of Dietary n-3 Polyunsaturated Fatty Acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Metabolism and Mitochondrial Bioenergetics in Older Adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [Green Version]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, E.M.; Pennington, E.R.; Sparagna, G.C.; Torres, M.J.; Neufer, P.D.; Harris, M.; Washington, J.; Anderson, E.J.; Zeczycki, T.N.; Brown, D.A.; et al. Docosahexaenoic Acid Lowers Cardiac Mitochondrial Enzyme Activity by Replacing Linoleic Acid in the Phospholipidome. J. Biol. Chem. 2018, 293, 466–483. [Google Scholar] [CrossRef] [Green Version]
- Leger, T.; Azarnoush, K.; Traoré, A.; Cassagnes, L.; Rigaudière, J.-P.; Jouve, C.; Pagès, G.; Bouvier, D.; Sapin, V.; Pereira, B.; et al. Antioxidant and Cardioprotective Effects of EPA on Early Low-Severity Sepsis through UCP3 and SIRT3 Upholding of the Mitochondrial Redox Potential. Oxidative Med. Cell. Longev. 2019, 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 Polyunsaturated Fatty Acids Suppress the Inflammatory Responses of Lipopolysaccharide-Stimulated Mouse Microglia by Activating SIRT1 Pathways. Biochim. Et Biophys. Acta -Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Z.; Wang, X.; Shi, H. Omega-3 Polyunsaturated Fatty Acids Antagonize Macrophage Inflammation via Activation of AMPK/SIRT1 Pathway. PLoS ONE 2012, 7, e45990. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-B.; Kwon, S.K.; Kwon, M.; Nagar, H.; Jeon, B.H.; Irani, K.; Yoon, S.H.; Kim, C.S. Docosahexaenoic Acid Improves Vascular Function via Up-Regulation of SIRT1 Expression in Endothelial Cells. Biochem. Biophys. Res. Commun. 2013, 437, 114–119. [Google Scholar] [CrossRef]
- Pedersen, A.M.; Vang, B.; Olsen, R.L. Oil from Calanus Finmarchicus—Composition and Possible Use: A Review. J. Aquat. Food Prod. Technol. 2014, 23, 633–646. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Nebl, J.; Schuchardt, J.P.; Müller, M.; Boßlau, T.K.; Krüger, K.; Hahn, A. Effects of Exercise Combined with a Healthy Diet or Calanus finmarchicus Oil Supplementation on Body Composition and Metabolic Markers—A Pilot Study. Nutrients 2020, 12, 2139. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung e.V. 10 Regeln Der DGE. Available online: https://www.dge.de/ernaehrungspraxis/vollwertige-ernaehrung/10-regeln-der-dge/ (accessed on 28 May 2020).
- Wasserfurth, P.; Nebl, J.; Boßlau, T.K.; Krüger, K.; Hahn, A.; Schuchardt, J.P. 12-Weeks of Calanus finmarchicus Oil Intake Improves Omega-3-Index in Healthy Older Subjects Engaging in an Exercise Program. Br. J. Nutr. 2020, 1–17. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalba, J.M.; Alcaín, F.J. Sirtuin Activators and Inhibitors. BioFactors 2012, 38, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Villanova, L.; Vernucci, E.; Pucci, B.; Pellegrini, L.; Nebbioso, M.; Mauri, C.; Marfe, G.; Spataro, A.; Fini, M.; Banfi, G.; et al. Influence of Age and Physical Exercise on Sirtuin Activity in Humans. J. Biol. Regul. Homeost. Agents 2013, 27, 497–507. [Google Scholar]
- Suwa, M.; Sakuma, K. The Potential Role of Sirtuins Regarding the Effects of Exercise on Aging- Related Diseases. Curr. Aging Sci. 2013, 6, 178–188. [Google Scholar] [CrossRef]
- Covington, J.D.; Bajpeyi, S. The Sirtuins: Markers of Metabolic Health. Mol. Nutr. Food Res. 2016, 60, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Suzuki, K.; Posa, A.; Petrovszky, Z.; Koltai, E.; Boldogh, I. The Systemic Role of SIRT1 in Exercise Mediated Adaptation. Redox Biol. 2020, 35, 101467. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, B.; Bossy-Wetzel, E. Forever Young: SIRT3 a Shield against Mitochondrial Meltdown, Aging, and Neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifici, F.; Di Cola, D.; Pastore, D.; Abete, P.; Guadagni, F.; Donadel, G.; Bellia, A.; Esposito, E.; Salimei, C.; Sinibaldi Salimei, P.; et al. Proposed Tandem Effect of Physical Activity and Sirtuin 1 and 3 Activation in Regulating Glucose Homeostasis. Int. J. Mol. Sci. 2019, 20, 4748. [Google Scholar] [CrossRef] [Green Version]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 Regulates Mitochondrial Fatty-Acid Oxidation by Reversible Enzyme Deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a New Target of PGC-1α, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Lombard, D.B. Functions of the Sirtuin Deacylase SIRT5 in Normal Physiology and Pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef]
- Braud, L.; Pini, M.; Stec, D.F.; Manin, S.; Derumeaux, G.; Stec, D.E.; Foresti, R.; Motterlini, R. Increased Sirt1 Secreted from Visceral White Adipose Tissue Is Associated with Improved Glucose Tolerance in Obese Nrf2-Deficient Mice. Redox Biol. 2021, 38, 101805. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Rinaldi, B.; Corbi, G.; Conti, V.; Stiuso, P.; Boccuti, S.; Rengo, G.; Rossi, F.; Filippelli, A. Exercise Training Promotes SIRT1 Activity in Aged Rats. Rejuvenation Res. 2008, 11, 139–150. [Google Scholar] [CrossRef]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise Training Increases Mitochondrial Biogenesis in the Brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Huffman, D.M.; Moellering, D.R.; Grizzle, W.E.; Stockard, C.R.; Johnson, M.S.; Nagy, T.R. Effect of Exercise and Calorie Restriction on Biomarkers of Aging in Mice. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2008, 294, R1618–R1627. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J.; Yoshida, Y.; McFarlan, J.T.; Holloway, G.P.; Moyes, C.D.; Heigenhauser, G.J.F.; Spriet, L.; Bonen, A. Nuclear SIRT1 Activity, but Not Protein Content, Regulates Mitochondrial Biogenesis in Rat and Human Skeletal Muscle. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2011, 301, R67–R75. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance Exercise as a Countermeasure for Aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, E.A.; Ruderman, N.B. AMPK and the Biochemistry of Exercise: Implications for Human Health and Disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.T.; Schenk, S. NAD+/NADH and Skeletal Muscle Mitochondrial Adaptations to Exercise. Am. J. Physiol. -Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.A.; Moore, J.H.; Mesquita, P.H.C.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Lopez, H.L.; Ziegenfuss, T.N.; Huggins, K.W.; et al. Resistance Training Increases Muscle NAD+ and NADH Concentrations as Well as NAMPT Protein Levels and Global Sirtuin Activity in Middle-Aged, Overweight, Untrained Individuals. Aging 2020, 12, 9447–9460. [Google Scholar] [CrossRef] [PubMed]
- de Boer, V.C.J.; de Goffau, M.C.; Arts, I.C.W.; Hollman, P.C.H.; Keijer, J. SIRT1 Stimulation by Polyphenols Is Affected by Their Stability and Metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish Oil-Derived n-3 PUFA Therapy Increases Muscle Mass and Function in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-Oil Supplementation Enhances the Effects of Strength Training in Elderly Women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Koehler, K.; Chung, S. Adaptive Thermogenesis by Dietary N-3 Polyunsaturated Fatty Acids: Emerging Evidence and Mechanisms. Biochim. Biophys. Acta -Mol. Cell Biol. Lipids 2019, 1864, 59–70. [Google Scholar] [CrossRef]
- Quesada-López, T.; Cereijo, R.; Turatsinze, J.-V.; Planavila, A.; Cairó, M.; Gavaldà-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The Lipid Sensor GPR120 Promotes Brown Fat Activation and FGF21 Release from Adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-Chain Polyunsaturated Fatty Acids Regulation of PPARs, Signaling: Relationship to Tissue Development and Aging. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic Acid Stimulates Complete Oxidation of Fatty Acids through Protein Kinase A-Dependent Activation of SIRT1-PGC1α Complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Wang, H.; Xu, Y.; Zheng, D.; Zhang, Q.; Li, W. Protective Effect of Astaxanthin against Contrast-Induced Acute Kidney Injury via SIRT1-P53 Pathway in Rats. Int. Urol. Nephrol. 2019, 51, 351–358. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin Stimulates Mitochondrial Biogenesis in Insulin Resistant Muscle via Activation of AMPK Pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eilertsen, K.-E.; Mæhre, H.K.; Jensen, I.J.; Devold, H.; Olsen, J.O.; Lie, R.K.; Brox, J.; Berg, V.; Elvevoll, E.O.; Østerud, B. A Wax Ester and Astaxanthin-Rich Extract from the Marine Copepod Calanus Finmarchicus Attenuates Atherogenesis in Female Apolipoprotein E–Deficient Mice. J. Nutr. 2012, 142, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höper, A.C.; Salma, W.; Sollie, S.J.; Hafstad, A.D.; Lund, J.; Khalid, A.M.; Raa, J.; Aasum, E.; Larsen, T.S. Wax Esters from the Marine Copepod Calanus Finmarchicus Reduce Diet-Induced Obesity and Obesity-Related Metabolic Disorders in Mice. J. Nutr. 2014, 144, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, S.; di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarnicola, A.; Genco, A.; et al. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, A.; Sultan, S. The Expression of Sirtuins, Superoxide Dismutase, and Lipid Peroxidation Status in Peripheral Blood from Patients with Diabetes and Hypothyroidism. BMC Endocr. Disord. 2019, 19, 19. [Google Scholar] [CrossRef]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.-C.; Atangan, L.; Wang, M. Resveratrol Is Not a Direct Activator of SIRT1 Enzyme Activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar] [CrossRef] [PubMed]
| CON (n = 9) | EX (n = 14) | EXDC (n = 8) | EXCO (n = 9) | p | |
|---|---|---|---|---|---|
| Sex (f/m) | 7/2 | 11/3 | 5/3 | 7/2 | 0.836 |
| Age (years) | 61 ± 5 | 60 ± 6 | 59 ± 5 | 60 ± 3 | 0.825 |
| Height (cm) | 166 ± 6 | 168 ± 7 | 170 ± 8 | 174 ± 6 | 0.112 |
| Body weight (kg) | 75.1 ± 13.6 | 78.4 ± 19.1 | 86.9 ± 20.0 | 82.3 ± 16.8 | 0.503 |
| BMI (kg/m2) | 27.2 ± 4.1 | 27.7 ± 6.0 | 30.0 ± 5.7 | 27.4 ± 6.0 | 0.701 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasserfurth, P.; Nebl, J.; Rühling, M.R.; Shammas, H.; Bednarczyk, J.; Koehler, K.; Boßlau, T.K.; Krüger, K.; Hahn, A.; Das, A.M. Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients 2021, 13, 3824. https://doi.org/10.3390/nu13113824
Wasserfurth P, Nebl J, Rühling MR, Shammas H, Bednarczyk J, Koehler K, Boßlau TK, Krüger K, Hahn A, Das AM. Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients. 2021; 13(11):3824. https://doi.org/10.3390/nu13113824
Chicago/Turabian StyleWasserfurth, Paulina, Josefine Nebl, Miriam Rebekka Rühling, Hadeel Shammas, Jolanthe Bednarczyk, Karsten Koehler, Tim Konstantin Boßlau, Karsten Krüger, Andreas Hahn, and Anibh Martin Das. 2021. "Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training" Nutrients 13, no. 11: 3824. https://doi.org/10.3390/nu13113824
APA StyleWasserfurth, P., Nebl, J., Rühling, M. R., Shammas, H., Bednarczyk, J., Koehler, K., Boßlau, T. K., Krüger, K., Hahn, A., & Das, A. M. (2021). Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients, 13(11), 3824. https://doi.org/10.3390/nu13113824








