The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Body Composition
2.3. Diet Analysis
2.4. Physical Performance
2.5. Blood Sampling
2.6. Haematological Variables
2.7. Biochemical Variables
2.8. Inflammatory Variables
2.9. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Diet Analysis
3.3. Physical Performance
3.4. Haematological Variables
3.5. Biochemical Variables
3.6. Inflammatory Variables
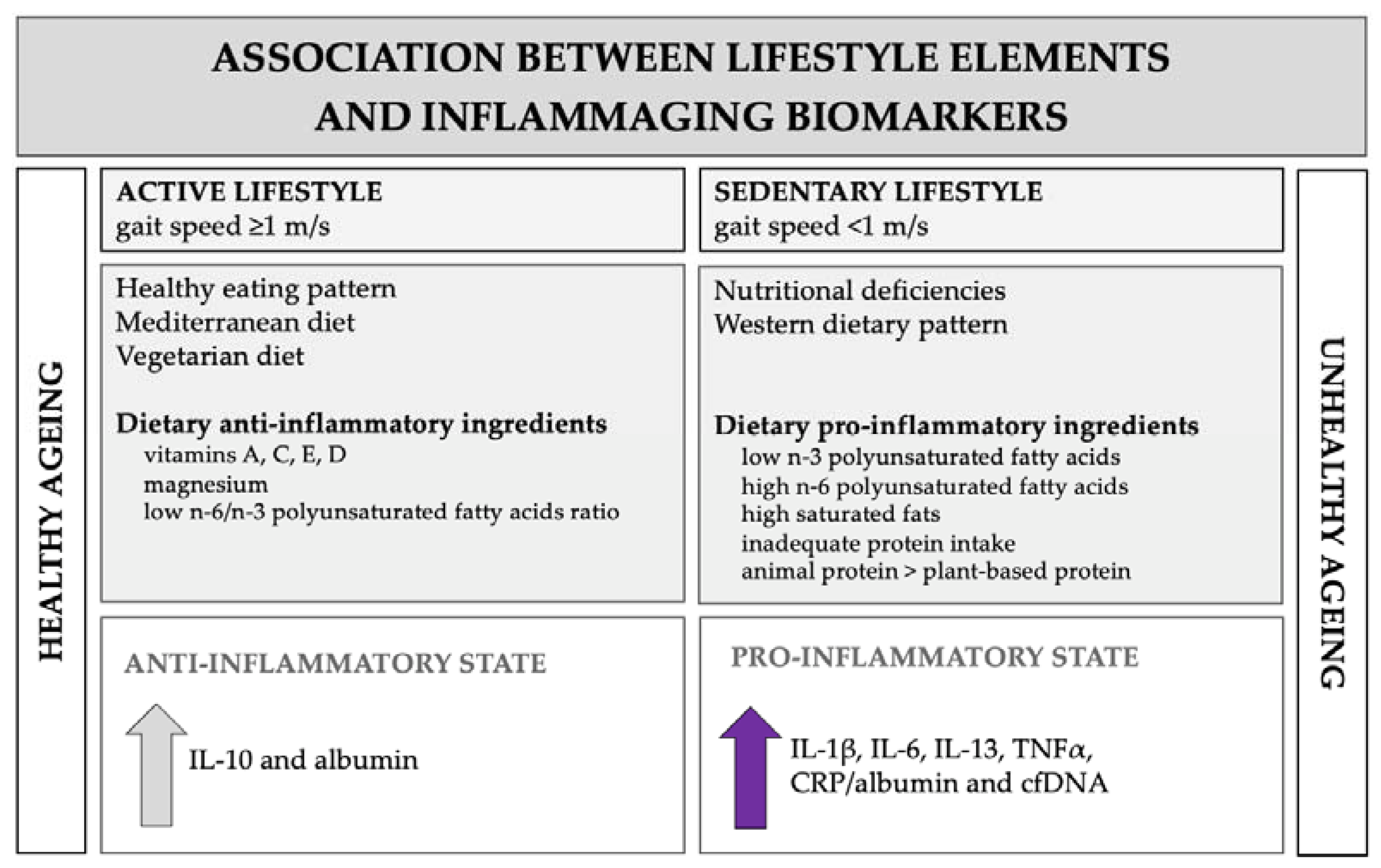
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavanagh, M.M.; Weyand, C.M.; Goronzy, J.J. Chronic Inflammation and Aging: DNA Damage Tips the Balance. Curr. Opin. Immunol. 2012, 24, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Stallone, G.; Infante, B.; Prisciandaro, C.; Grandaliano, G. mTOR and Aging: An Old Fashioned Dress. Int. J. Mol. Sci. 2019, 20, 2774. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for Evaluation of Chronic Inflammatory Status in Ageing Research: Reliability and Phenotypic Characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The Interplay between Immunosenescence and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the Aging Immune System. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can Exercise Affect Immune Function to Increase Susceptibility to Infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K. Predicting Death from Tumour Necrosis Factor-Alpha and Interleukin-6 in 80-Year-Old People. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef]
- Galland, L. Diet and Inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Bujtor, M.; Turner, A.I.; Torres, S.J.; Esteban-Gonzalo, L.; Pariante, C.M.; Borsini, A. Associations of Dietary Intake on Biological Markres of Inflammation in Children and Adolescents: A Systemic Review. Nutrients 2021, 13, 356. [Google Scholar] [CrossRef]
- Santoro, A.; Brigidi, P.; Gonos, E.S.; Bohr, V.A.; Franceschi, C. Mediterranean Diet and Inflammaging in the Elderly: The European Project NU-AGE. Preface. Mech. Ageing Dev. 2014, 136–137, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of Vegetarian Diet with Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Observational Studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary Patterns and Associations with Biomarkers of Inflammation in Adults: A Systematic Review of Observational Studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, Inflammation and Aging. Aging Dis. 2012, 3, 130–140. [Google Scholar]
- Bartlett, D.B.; Fox, O.; McNulty, C.L.; Greenwood, H.L.; Murphy, L.; Sapey, E.; Goodman, M.; Crabtree, N.; Thøgersen-Ntoumani, C.; Fisher, J.P.; et al. Habitual Physical Activity Is Associated with the Maintenance of Neutrophil Migratory Dynamics in Healthy Older Adults. Brain Behav. Immun. 2016, 56, 12–20. [Google Scholar] [CrossRef]
- Woods, J.A.; Davis, J.M.; Smith, J.A.; Nieman, D.C. Exercise and Cellular Innate Immune Function. Med. Sci. Sports Exerc. 1999, 31, 57–66. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Narang, V.; Lu, Y.; Camous, X.; Nyunt, M.S.Z.; Carre, C.; Tan, C.; Xian, C.H.; Chong, J.; Chua, M.; et al. Hallmarks of Improved Immunological Responses in the Vaccination of More Physically Active Elderly Females. Exerc. Immunol. Rev. 2019, 25, 20–33. [Google Scholar]
- Tylutka, A.; Morawin, B.; Gramacki, A.; Zembron-Lacny, A. Lifestyle Exercise Attenuates Immunosenescence; Flow Cytometry Analysis. BMC Geriatr. 2021, 21, 200. [Google Scholar] [CrossRef]
- World Health Organization. Men Ageing and Health: Achieving Health across the Life Span. 1999, WHO/NMH/NPH/01.2. Available online: https://apps.who.int/iris/bitstream/handle/10665/66941/WHO_NMH_NPH_01.2.pdf (accessed on 9 October 2021).
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Zielenkiewicz, M.; Paczek, L. Inflammatory Markers Change with Age, but Do Not Fall Beyond Reported Normal Ranges. Arch. Immunol. Ther. Exp. 2016, 64, 249–254. [Google Scholar] [CrossRef]
- Morawin, B.; Tylutka, A.; Chmielowiec, J.; Zembron-Lacny, A. Circulating Mediators of Apoptosis and Inflammation in Aging; Physical Exercise Intervention. Int. J. Environ. Res. Public Health 2021, 18, 3165. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Zywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego: Warsaw, Poland, 2020; ISBN 978-83-65870-28-5. [Google Scholar]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation, Geneva, 28 January–1 February 2002. Technical Report Series 916. 2003. Available online: https://apps.who.int/iris/handle/10665/42665 (accessed on 9 October 2021).
- Różańska, D.; Kawicka, A.; Konikowska, K.; Salomon, A.; Zatońska, K.; Szuba, A.; Regulska-Ilow, B. Assessment of Glycemic Load and Intake of Carbohydrates in the Diet of Wroclaw Medical University Students (Poland). Rocz. Panstw. Zakl. Hig. 2016, 67, 301–308. [Google Scholar]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An Official European Respiratory Society/American Thoracic Society Technical Standard: Field Walking Tests in Chronic Respiratory Disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking Speed: The Functional Vital Sign. J. Aging Phys. Act 2015, 23, 314–322. [Google Scholar] [CrossRef]
- Zhou, T.; Zhan, J.; Hong, S.; Hu, Z.; Fang, W.; Qin, T.; Ma, Y.; Yang, Y.; He, X.; Zhao, Y.; et al. Ratio of C-Reactive Protein/Albumin Is an Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-Cell Lung Cancer. Sci. Rep. 2015, 5, 10481. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 9 October 2021).
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.S.; Dong, M.H.; Bettencourt, R.; Barrett-Connor, E.; Loomba, R. Association of Serum Bilirubin with Aging and Mortality. J. Clin. Exp. Hepatol. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum Albumin and Health in Older People: Review and Meta Analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Liperoti, R.; Russo, A.; Carter, C.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F. Interleukin-6, C-Reactive Protein, and Tumor Necrosis Factor-Alpha as Predictors of Mortality in Frail, Community-Living Elderly Individuals. J. Am. Geriatr. Soc. 2011, 59, 1679–1685. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health Relevance of the Modification of Low Grade Inflammation in Ageing (Inflammageing) and the Role of Nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Martínez-Gayo, A.; Cobo, M.J.; Pérez-Chávez, A.; Ibáñez-Santos, J.; Palacios Samper, N.; Goikoetxea Galarza, I.; Cuervo, M.; García-Unciti, M.; González-Muniesa, P.; et al. Effects of DHA-Rich n-3 Fatty Acid Supplementation and/or Resistance Training on Body Composition and Cardiometabolic Biomarkers in Overweight and Obese Post-Menopausal Women. Nutrients 2021, 13, 2465. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D Deficiency Is Associated with Inflammation in Older Irish Adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef]
- Dasinger, J.H.; Fehrenbach, D.J.; Abais-Battad, J.M. Dietary Protein: Mechanisms Influencing Hypertension and Renal Disease. Curr. Hypertens. Rep. 2020, 22, 13. [Google Scholar] [CrossRef]
- Lopez-Legarrea, P.; de la Iglesia, R.; Abete, I.; Navas-Carretero, S.; Martinez, J.A.; Zulet, M.A. The Protein Type within a Hypocaloric Diet Affects Obesity-Related Inflammation: The RESMENA Project. Nutrition 2014, 30, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, H.; Masetto, A.C.; Mesquita, E.T. C-Reactive Protein: An Inflammatory Marker with Prognostic Value in Patients with Decompensated Heart Failure. Arq. Bras. Cardiol. 2007, 88, 585–589. [Google Scholar] [CrossRef]
- Devran, O.; Karakurt, Z.; Adıgüzel, N.; Güngör, G.; Moçin, O.Y.; Balcı, M.K.; Celik, E.; Saltürk, C.; Takır, H.B.; Kargın, F.; et al. C-Reactive Protein as a Predictor of Mortality in Patients Affected with Severe Sepsis in Intensive Care Unit. Multidiscip. Respir. Med. 2012, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.M.A.; Lobo, F.R.M.; Bota, D.P.; Lopes-Ferreira, F.; Soliman, H.M.; Mélot, C.; Vincent, J.-L. C-Reactive Protein Levels Correlate with Mortality and Organ Failure in Critically Ill Patients. Chest 2003, 123, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Can Dogan, A.; Demirci, G.; Riza Demir, A.; Yilmaz, E.; Zencirkiran Agus, H.; Kemal Kalkan, A.; Uzun, F.; Erturk, M. The Prognostic Value of C-Reactive Protein to Albumin Ratio in Patients with Isolated Degenerative Aortic Valve Stenosis Undergoing Surgical Aortic Valve Replacement. Braz. J. Cardiovasc. Surg. 2020, 35, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging Markers Characteristic of Advanced Age Show Similar Levels with Frailty and Dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef]
- Onem, Y.; Terekeci, H.; Kucukardali, Y.; Sahan, B.; Solmazgül, E.; Senol, M.G.; Nalbant, S.; Sayan, O.; Top, C.; Oktenli, C. Albumin, hemoglobin, body mass index, cognitive and functional performance in elderly persons living in nursing homes. Arch. Gerontol. Geriatr. 2010, 50, 56–59. [Google Scholar] [CrossRef]
- Sun, P.; Chen, C.; Xia, Y.; Bi, X.; Liu, P.; Zhang, F.; Yang, H.; An, X.; Jiang, W.; Wang, F. The Ratio of C-Reactive Protein/Albumin Is a Novel Inflammatory Predictor of Overall Survival in Cisplatin-Based Treated Patients with Metastatic Nasopharyngeal Carcinoma. Dis. Markers 2017, 2017, 6570808. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Palmeri, M.; Misiano, G.; Malaguarnera, M.; Forte, G.I.; Vaccarino, L.; Milano, S.; Scola, L.; Caruso, C.; Motta, M.; Maugeri, D.; et al. Cytokine Serum Profile in a Group of Sicilian Nonagenarians. J. Immunoass. Immunochem. 2012, 33, 82–90. [Google Scholar] [CrossRef][Green Version]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The Origins of Age-Related Proinflammatory State. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The Immune System in Extreme Longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Ma, L.; Sha, G.; Zhang, Y.; Li, Y. Elevated Serum IL-6 and Adiponectin Levels Are Associated with Frailty and Physical Function in Chinese Older Adults. Clin. Interv. Aging 2018, 13, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory Biomarkers of Frailty. Exp. Gerontol. 2020, 133, 110858. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Jylhävä, J.; Nevalainen, T.; Marttila, S.; Jylhä, M.; Hervonen, A.; Hurme, M. Characterization of the Role of Distinct Plasma Cell-Free DNA Species in Age-Associated Inflammation and Frailty. Aging Cell 2013, 12, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.V.; Capri, M.; Morsiani, C.; Pizza, G.; Faria, A.M.C.; Franceschi, C.; Neretti, N. Cell-Free DNA as a Biomarker of Aging. Aging Cell 2019, 18, e12890. [Google Scholar] [CrossRef]
- Crigna, A.T.; Samec, M.; Koklesova, L.; Liskova, A.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Cell-Free Nucleic Acid Patterns in Disease Prediction and Monitoring-Hype or Hope? EPMA J. 2020, 11, 1–25. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Kroeze, A.; Hoogendijk, A.J.; Soussan, A.A.; Ellen van der Schoot, C.; Wuillemin, W.A.; Voermans, C.; van der Poll, T.; Zeerleder, S. Systemic Inflammation Induces Release of Cell-Free DNA from Hematopoietic and Parenchymal Cells in Mice and Humans. Blood Adv. 2019, 3, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Jylhä, M.; Lehtimäki, T.; Hervonen, A.; Hurme, M. Circulating Cell-Free DNA Is Associated with Mortality and Inflammatory Markers in Nonagenarians: The Vitality 90+ Study. Exp. Gerontol. 2012, 47, 372–378. [Google Scholar] [CrossRef]

| Females n = 28 | Males n = 32 | Females vs. Males p Level | |
|---|---|---|---|
| Age [year] | 79.8 ± 8.4 | 80.7 ± 8.0 | 0.752 |
| Weight [kg] | 67.6 ± 9.7 | 78.1 ± 14.2 | <0.05 |
| Height [cm] | 155.5 ± 6.2 | 166.2 ± 8.2 | <0.001 |
| BMI [kg/m2] | 27.4 ± 4.2 | 28.4 ± 4.7 | 0.506 |
| FM [kg] | 22.6 ± 7.4 | 19.7 ± 7.8 | <0.05 |
| FM% | 31.1 ± 9.0 | 23.9 ± 6.6 | <0.05 |
| FFM [kg] | 44.2 ± 5.3 | 59.1 ± 8.1 | <0.001 |
| SBP [mmHg] | 140 ± 23 | 147 ± 20 | 0.383 |
| DBP [mmHg] | 72 ± 10 | 80 ± 14 | 0.056 |
| 6MWT [m] | 279 ± 121 | 368 ± 104 | <0.05 |
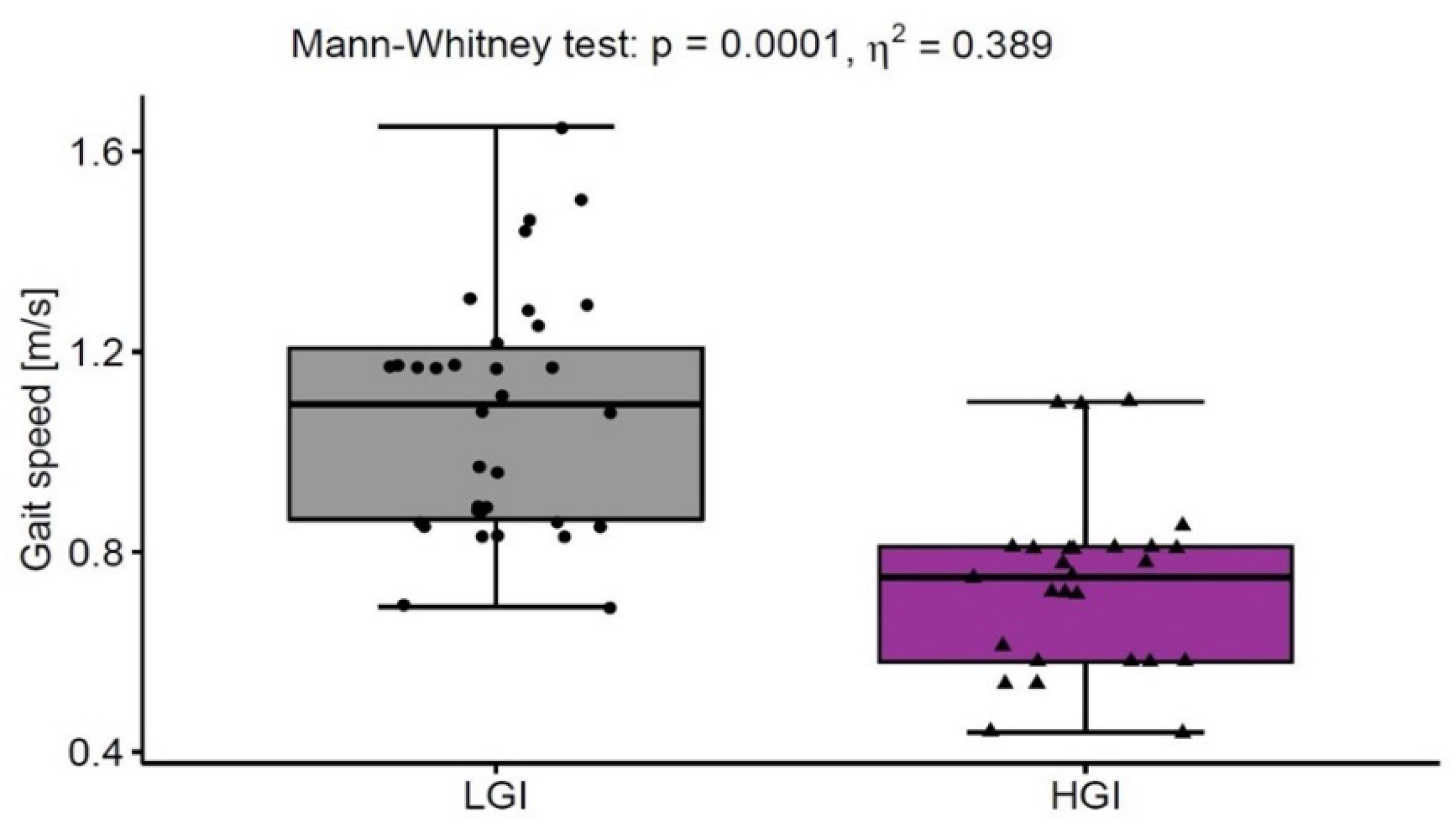
| Gait speed [m/s] | 0.78 ± 0.34 | 1.02 ± 0.29 | <0.05 |
| LGI n = 33 | HGI n = 27 | LGI vs. HGI p Level | |
|---|---|---|---|
| Age [year] | 80.9 ± 7.4 | 79.3 ± 8.3 | 0.458 |
| Weight [kg] | 69.4 ± 11.4 | 80.3 ± 13.2 | p < 0.05 |
| Height [cm] | 159.8 ± 8.2 | 164.3 ± 9.4 | 0.146 |
| BMI [kg/m2] | 26.8 ± 4.4 | 29.9 ± 3.7 | p < 0.05 |
| FM [kg] | 19.6 ± 7.5 | 23.3 ± 7.1 | 0.096 |
| FM% | 26.3 ± 8.8 | 28.6 ± 7.3 | 0.416 |
| FFM [kg] | 49.4 ± 8.5 | 57.7 ± 10.4 | 0.277 |
| Recommended Intake [23] | LGI n = 26 | HGI n = 15 | LGI vs. HGI p Level | |
|---|---|---|---|---|
| Total fat [% of energy] | 20–35 | 34.95 ± 9.24 | 32.56 ± 7.07 | 0.393 |
| SFA [% of energy] | <6 | 14.99 ± 5.83 | 13.49 ± 4.91 | 0.405 |
| MUFA [% of energy] | <20 | 11.49 ± 4.41 | 11.64 ± 3.22 | 0.908 |
| PUFA [% of energy] | 6–10 | 5.17 ± 2.56 | 4.63 ± 1.55 | 0.466 |
| Linoleic acid (C18:2) [mg] | n/a | 7950 ± 4560 | 9030 ± 4060 | <0.05 |
| Arachidonic acid (C20:4) [mg] | n/a | 70 ± 50 | 130 ± 140 | 0.527 |
| α-Linoleic acid (C18:3) [mg] | n/a | 1300 ± 940 | 1740 ± 1190 | 0.111 |
| EPA and DHA [mg] | 250 | 260 ± 560 | 90 ± 120 | 0.487 |
| n-6 PUFA [g] | n/a | 8.03 ± 4.56 | 9.17 ± 4.06 | 0.428 |
| n-3 PUFA [g] | n/a | 2.09 ± 1.08 | 1.61 ± 0.92 | 0.075 |
| n-6/n-3 PUFA [g] | <5:1 | 4.80 ± 4.22 | 6.42 ± 2.44 | <0.05 |
| Total protein [% of energy] | 15–20 | 14.01 ± 4.19 | 17.09 ± 3.72 | < 0.05 |
| Total carbohydrates [% of energy] | 45–65 | 50.05 ± 7.74 | 48.89 ± 8.03 | 0.653 |
| Dietary fibre [g] | 18–38 | 18.35 ± 8.07 | 21.69 ± 7.85 | 0.206 |
| Glycaemic index | low < 55 medium 55–70 high > 70 | 56.21 ± 6.38 | 59.91 ± 5.87 | 0.073 |
| Glycaemic load [g] | low ≤ 80 medium 81–120 high ≥ 120 | 119 ± 56 | 142 ± 49 | 0.188 |
| Vitamin D [μg] | 15 | 4.77 ± 7.43 | 2.41 ± 1.60 | 0.234 |
| Vitamin C [mg] | F 75 M 90 | 94.63 ± 61.02 | 97.15 ± 54.22 | 0.895 |
| Vitamin E [mg] | F 8 M 10 | 8.24 ± 4.80 | 9.59 ± 5.02 | 0.396 |
| Vitamin A [μg RAE] | F 700 M 900 | 1100 ± 951 | 1241 ± 1031 | 0.660 |
| β-carotene [μg] | n/a | 3098 ± 4494 | 3107 ± 3970 | 0.995 |
| Magnesium [mg] | F 320 M 420 | 235 ± 87 | 293 ± 119 | 0.082 |
| Reference Values | LGI n = 33 | HGI n = 27 | LGI vs. HGI p Level | η2 | |
|---|---|---|---|---|---|
| Leucocytes [103/µL] | 5.0–11.6 | 6.19 ± 1.65 | 7.83 ± 2.54 | <0.001 | 0.219 |
| Lymphocytes [103/µL] | 1.3–4.0 | 1.64 ± 0.66 | 1.52 ± 0.71 | 0.883 | 0.001 |
| Granulocytes [103/µL] | 2.4–7.6 | 4.26 ± 1.27 | 5.88 ± 2.10 | <0.001 | 0.371 |
| LYM% | 19.1–48.5 | 28.46 ± 11.46 | 20.08 ± 8.05 | <0.05 | 0.150 |
| GRA% | 43.6–73.4 | 67.98 ± 8.53 | 72.42 ± 10.33 | <0.001 | 0.200 |
| RBC [103/µL] | F 4.0–5.5 M 4.5–6.6 | 4.19 ± 0.67 | 4.31 ± 0.98 | 0.841 | 0.001 |
| HB [g/dL] | F 12.5–16.0 M 13.5–18.0 | 12.13 ± 1.65 | 12.60 ± 1.96 | 0.474 | 0.010 |
| HCT% | F 37–47 M 40.0–51.0 | 33.99 ± 4.19 | 35.99 ± 5.69 | 0.572 | 0.006 |
| PLT [103/µL] | 150–400 | 244 ± 74 | 230 ± 87 | 0.456 | 0.011 |
| Reference Values | LGI n = 33 | HGI n = 27 | LGI vs. HGI p Level | η2 | |
|---|---|---|---|---|---|
| TG [mg/dL] | <150 | 116 ± 63 | 134 ± 70 | 0.165 | 0.033 |
| TC [mg/dL] | <200 | 185 ± 48 | 184 ± 49 | 0.735 | 0.002 |
| LDL [mg/dL] | <130 | 105 ± 44 | 106 ± 41 | 0.940 | 0.001 |
| HDL [mg/dL] | desirable > 60 | 57.26 ± 14.95 | 54.00 ± 13.22 | 0.094 | 0.048 |
| non-HDL [mg/dL] | <130 | 128 ± 50 | 133 ± 41 | 0.211 | 0.027 |
| oxLDL [mg/dL] | - | 199.74 ± 65.65 | 173.43 ± 50.55 | 0.821 | 0.003 |
| Glucose [mg/dL] | 60–115 | 94.50 ± 16.45 | 98.91 ± 13.55 | 0.205 | 0.028 |
| Lactate [mmol/L] | <2.2 | 2.75 ± 0.78 | 2.96 ± 0.72 | 0.066 | 0.056 |
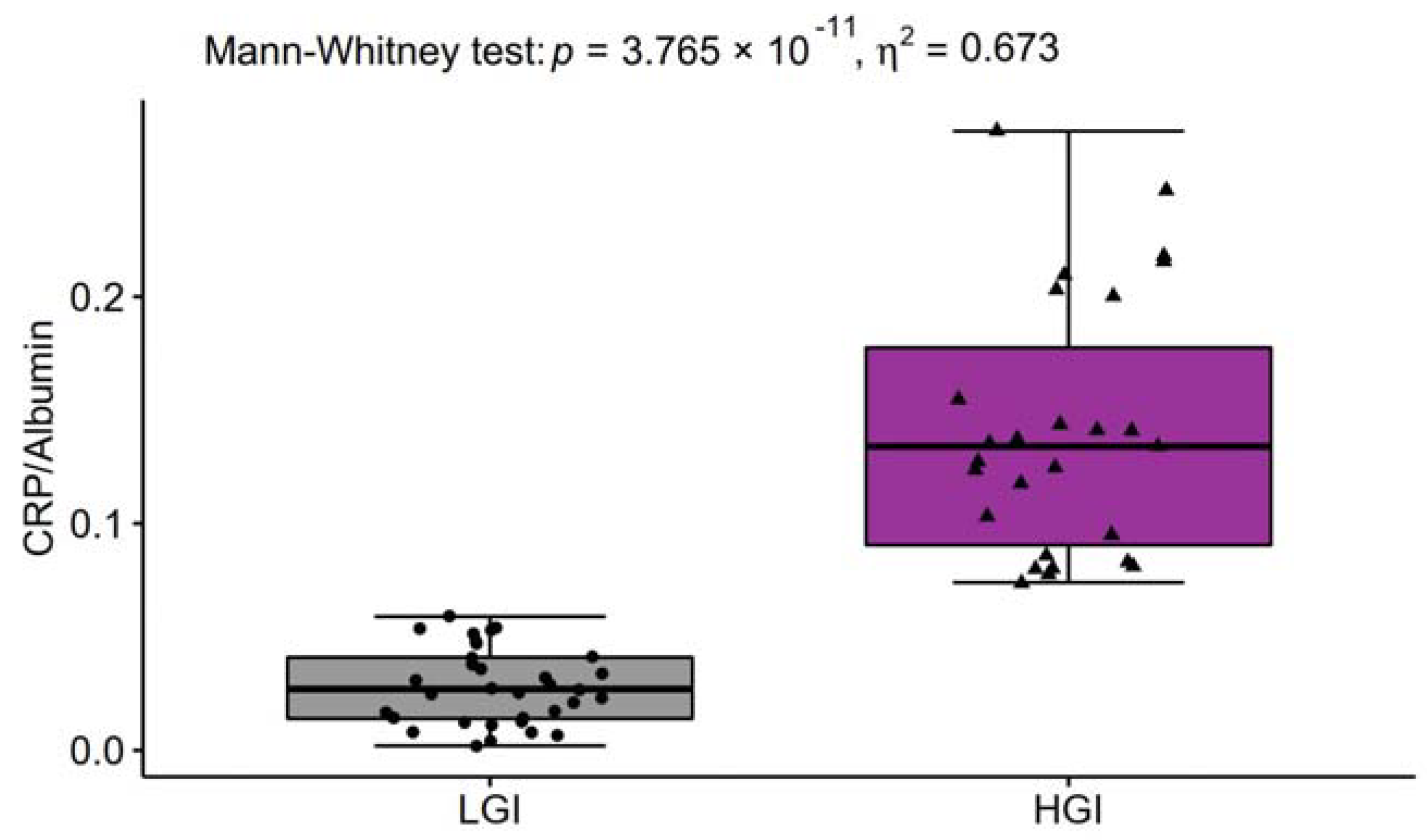
| Bilirubin [mg/dL] | <1.0 | 0.31 ± 0.11 | 0.41 ± 0.16 | <0.05 | 0.125 |
| Albumin [g/L] | F 37–53 M 42–55 | 45.90 ± 3.55 | 43.81 ± 3.49 | <0.05 | 0.152 |
| LGI n = 33 | HGI n = 27 | LGI vs. HGI p Level | η2 | |
|---|---|---|---|---|
| CRP [mg/L] | 1.28 ± 0.75 | 6.07 ± 2.25 | <0.0001 | 0.695 |
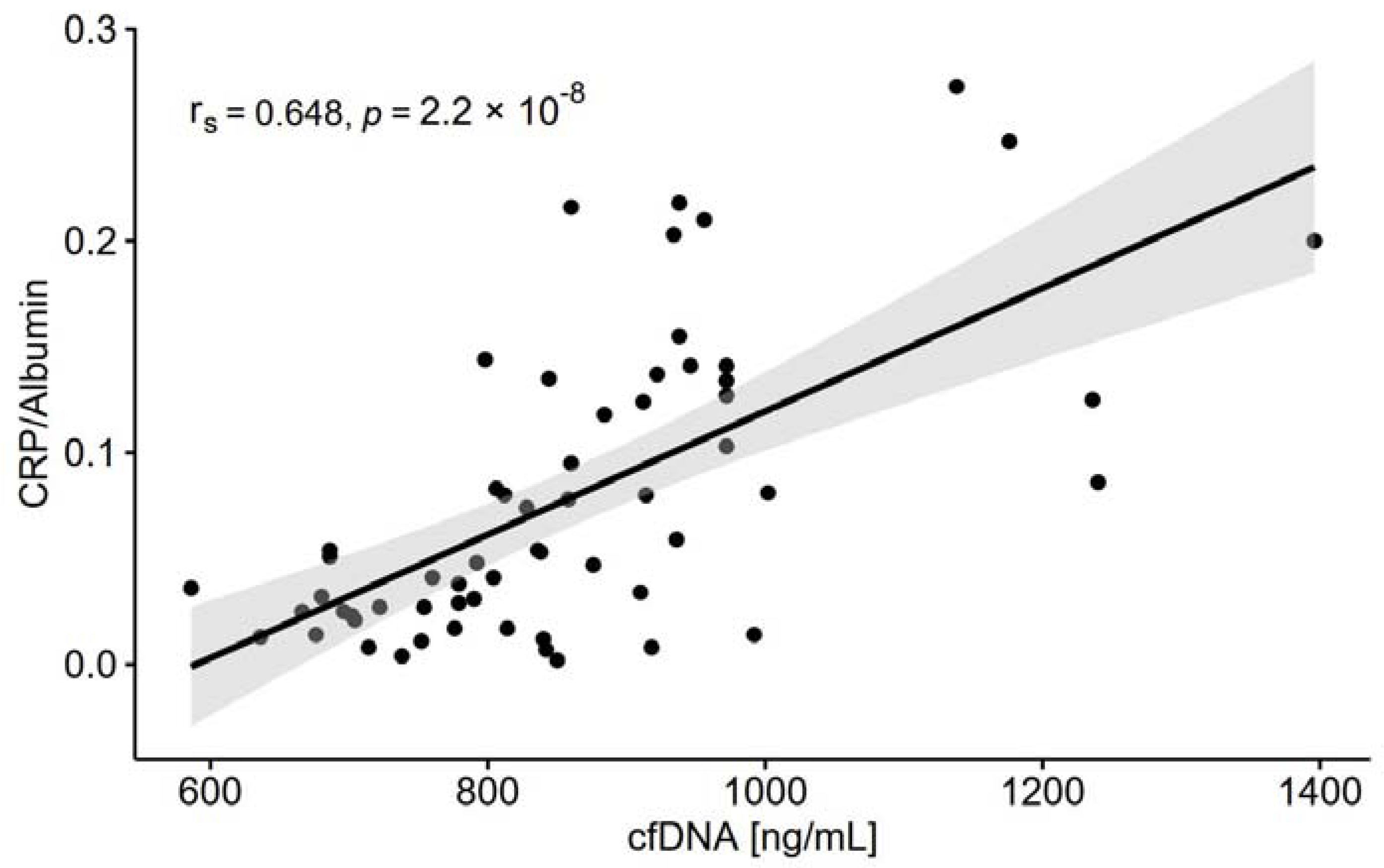
| cfDNA [ng/mL] | 747 ± 93 | 966 ± 148 | <0.001 | 0.381 |
| IL-1β [pg/mL] | 1215 ± 493 | 1459 ± 562 | 0.068 | 0.018 |
| IL-6 [pg/mL] | 48.15 ± 15.32 | 62.12 ± 22.82 | <0.05 | 0.178 |
| IL-8 [ng/mL] | 8.69 ± 4.68 | 8.35 ± 3.57 | 0.486 | 0.007 |
| IL-10 [pg/mL] | 51.42 ± 2.81 | 48.75 ± 2.95 | <0.001 | 0.281 |
| IL-13 [pg/mL] | 2.39 ± 1.44 | 3.21 ± 1.34 | <0.05 | 0.211 |
| TNFα [pg/mL] | 63.97 ± 27.54 | 82.27 ± 29.83 | <0.05 | 0.075 |
| CRP [mg/L] | IL-1β [pg/mL] | IL-6 [pg/mL] | IL-8 [ng/mL] | IL-10 [pg/mL] | IL-13 [pg/mL] | TNFα [pg/mL] | Bilirubin [mg/dL] | Albumin [g/L] | |
|---|---|---|---|---|---|---|---|---|---|
| cfDNA [ng/mL] | rs = 0.646 p < 0.0001 | rs = 0.326 p < 0.05 | rs = 0.172 p = 0.188 | rs = 0.020 p = 0.882 | rs= −0.421 p < 0.0001 | rs = 0.141 p = 0.282 | rs = 0.341 p < 0.01 | rs = 0.361 p < 0.01 | rs= −0.351 p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak-Gramacka, E.; Hertmanowska, N.; Tylutka, A.; Morawin, B.; Wacka, E.; Gutowicz, M.; Zembron-Lacny, A. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients 2021, 13, 3696. https://doi.org/10.3390/nu13113696
Wawrzyniak-Gramacka E, Hertmanowska N, Tylutka A, Morawin B, Wacka E, Gutowicz M, Zembron-Lacny A. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients. 2021; 13(11):3696. https://doi.org/10.3390/nu13113696
Chicago/Turabian StyleWawrzyniak-Gramacka, Edyta, Natalia Hertmanowska, Anna Tylutka, Barbara Morawin, Eryk Wacka, Marzena Gutowicz, and Agnieszka Zembron-Lacny. 2021. "The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging" Nutrients 13, no. 11: 3696. https://doi.org/10.3390/nu13113696
APA StyleWawrzyniak-Gramacka, E., Hertmanowska, N., Tylutka, A., Morawin, B., Wacka, E., Gutowicz, M., & Zembron-Lacny, A. (2021). The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients, 13(11), 3696. https://doi.org/10.3390/nu13113696






