Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. The Frailty Assessment
2.3. Dietary Assessment
2.4. Dietary Quality
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Assessment of Frailty
Appendix B. The Scoring Criteria of T-HEI
References
- Granic, A.; Mendonça, N.; Hill, T.R.; Jagger, C.; Stevenson, E.J.; Mathers, J.C.; Sayer, A.A. Nutrition in the Very Old. Nutrients 2018, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E. Nutrition through the Life Cycle, 7th ed.; Cengage Learning: Boston, MA, USA, 2019. [Google Scholar]
- Cesari, M.; Prince, M.; Thiyagarajan, J.A.; De Carvalho, I.A.; Bernabei, R.; Chan, P.; Robledo, L.M.G.; Michel, J.-P.; Morley, J.E.; Ong, P.; et al. Frailty: An Emerging Public Health Priority. J. Am. Med. Dir. Assoc. 2016, 17, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, M.S. From dietary guidelines to daily food guide: The Taiwanese experience. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 59–62. [Google Scholar]
- Health Promotion Administration, Ministry of Health and Welfare. Daily Food Guides Manual; Health Promotion Administration, Ministry of Health and Welfare: Taipei City, Taiwan, 2018.
- León-Muñoz, L.M.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Artalejo, F. Mediterranean Diet and Risk of Frailty in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2014, 15, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Struijk, E.A.; Hagan, K.A.; Fung, T.T.; Hu, F.B.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Diet quality and risk of frailty among older women in the Nurses’ Health Study. Am. J. Clin. Nutr. 2020, 111, 877–883. [Google Scholar] [CrossRef]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2019, 68, 770–776. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Pan, W.-H.; Lee, M.M.-S.; Yu, S.-L.; Huang, P.-C. Foods Predictive of Nutrient Intake in Chinese Diet in Taiwan: II. Vitamin A, Vitamin B1, Vitamin B2, Vitamin C and Calcium. Int. J. Epidemiol. 1992, 21, 929–934. [Google Scholar] [CrossRef]
- Lee, M.M.-S.; Pan, W.-H.; Yu, S.-L.; Huang, P.-C. Foods Predictive of Nutrient Intake in Chinese Diet in Taiwan: I. Total Calories, Protein, Fat and Fatty Acids. Int. J. Epidemiol. 1992, 21, 922–928. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lee, M.-S.; Pan, W.-H.; Wahlqvist, M.L. Validation of a simplified food frequency questionnaire as used in the Nutrition and Health Survey in Taiwan (NAHSIT) for the elderly. Asia Pac. J. Clin. Nutr. 2011, 20, 134–140. [Google Scholar]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M.; Reeve, B.B.; Basiotis, P.P. Development and Evaluation of the Healhty Eating Index-2005: Technical Report; Center for Nutrition Policy and Promotion, U.S. Department of Agriculture: Washington, DC, USA, 2007.
- Health Promotion Administration, Ministry of Health and Welfare. Daily Dietary Guidelines Manuel; Health Promotion Ad-ministration, Ministry of Health and Welfare: Taipei City, Taiwan, 2018.
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; Declerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- International Life Science Institute Taiwan. ‘Health and sarcopenia among Taiwanese Older Adults’ conference; ILSI Taiwan: Taipei City, Taiwan, 2017. [Google Scholar]
- Lo, Y.L.; Hsieh, Y.T.; Hsu, L.L.; Chuang, S.Y.; Chang, H.Y.; Hsu, C.C.; Chen, C.Y.; Pan, W.H. Dietary Pattern As-sociated with Frailty: Results from Nutrition and Health Survey in Taiwan. J. Am. Geriatr. Soc. 2017, 65, 2009–2015. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sar-copenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Yeh, N.-H.; Chang, H.-Y.; Wang, C.-F.; Hung, S.-Y.; Wu, S.-J.; Pan, W.-H. Adequate protein intake in older adults in the context of frailty: Cross-sectional results of the Nutrition and Health Survey in Taiwan 2014–2017. Am. J. Clin. Nutr. 2021, 114, 649–660. [Google Scholar] [CrossRef]
- Kobayashi, S.; The Three-Generation Study of Women on Diets and Health Study Group; Suga, H.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Group Diet with a combination of high protein and high total antioxidant capacity is strongly associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr. J. 2017, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Huang, Y.-C.; Lee, M.-S.; Chang, H.-Y.; Doong, J.-Y. Frailty Severity and Cognitive Impairment Associated with Dietary Diversity in Older Adults in Taiwan. Nutrients 2021, 13, 418. [Google Scholar] [CrossRef]
- Cuesta-Triana, F.; Verdejo-Bravo, C.; Fernández-Pérez, C.; Sánchez, F.J.M. Effect of Milk and Other Dairy Products on the Risk of Frailty, Sarcopenia, and Cognitive Performance Decline in the Elderly: A Systematic Review. Adv. Nutr. 2019, 10, S105–S119. [Google Scholar] [CrossRef]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S.; The Three-Generation Study of Women on Diets and Health Study Group. High protein intake is associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr. J. 2013, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Rahi, B.; Colombet, Z.; Harmand, M.G.-C.; Dartigues, J.-F.; Boirie, Y.; Letenneur, L.; Feart, C. Higher Protein but Not Energy Intake Is Associated With a Lower Prevalence of Frailty Among Community-Dwelling Older Adults in the French Three-City Cohort. J. Am. Med. Dir. Assoc. 2016, 17, 672.e7–672.e11. [Google Scholar] [CrossRef]
- Byrd-Bredbenner, C.; Beshgetoor, D.; Moe, G.; Berning, J. Wardlaw’s Perspective in Nutrition, 8th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Westbury, L.D.; Fuggle, N.R.; Syddall, H.E.; Duggal, N.A.; Shaw, S.C.; Maslin, K.; Dennison, E.; Lord, J.M.; Cooper, C. Relationships Between Markers of Inflammation and Muscle Mass, Strength and Function: Findings from the Hertfordshire Cohort Study. Calcif. Tissue Int. 2017, 102, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.K.; Lyass, A.; Larson, M.G.; Massaro, J.M.; Wang, N.; D’Agostino, R.B.; Benjamin, E.J.; Murabito, J.M. Biomarkers of oxidative stress are associated with frailty: The Framingham Offspring Study. AGE 2016, 38, 1. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and Immune System Alterations in Frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Zempleni, J.; Rucker, R.B.; McCormick, D.B.; Suttie, J.W. Handbook of Vitamins, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A Systemic Review of the Roles of n-3 Fatty Acids in Health and Disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M. Bioactive Foods and Ingredients for Health. Adv. Nutr. 2014, 5, 306S–311S. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Stubbs, B.; Hébert, J.R.; Cesari, M.; Schofield, P.; Soysal, P.; Maggi, S.; Veronese, N. The Relationship Between the Dietary Inflammatory Index and Incident Frailty: A Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2017, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Hung, S.Y. Evidence-based recommendations for 2011 Taiwan Food Guide. Nutr. Sci. J. 2015, 40, 1–11. [Google Scholar]
- Lee, M.S.; Huang, Y.C.; Su, H.H.; Lee, M.Z.; Wahlqvist, M.L. A simple food quality index predicts mortality in elderly Taiwanese. J. Nutr. Health Aging 2011, 15, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Fang, H.-Y. Adherence to Daily Food Guides Is Associated with Lower Risk of Metabolic Syndrome: The Nutrition and Health Survey in Taiwan. Nutrients 2020, 12, 2955. [Google Scholar] [CrossRef]
- Lee, M.-S.; Huang, L.-Y.; Chen, M.-C.; Wahlqvist, M.L. The demography of food in health security: Current experience with dairy consumption in Taiwan. Asia Pac. J. Clin. Nutr. 2009, 18, 585–589. [Google Scholar]
- Health Promotion Administration, Ministry of Health and Welfare. Nutrition and Health Survey in Taiwan. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=3998 (accessed on 2 November 2020).
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-E.; Wang, T.-D.; Ueng, K.-C.; Lin, T.-H.; Yeh, H.-I.; Chen, C.-Y.; Wu, Y.-J.; Tsai, W.-C.; Chao, T.-H.; Chen, C.-H.; et al. 2015 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hyper-tension Society for the Management of Hypertension. J. Chin. Med. Assoc. 2015, 78, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar]
- The Whoqol-Taiwan Group, Introduction to the Development of the WHOQOL-Taiwan Version. Chin. J. Public Health 2000, 19, 315–324.
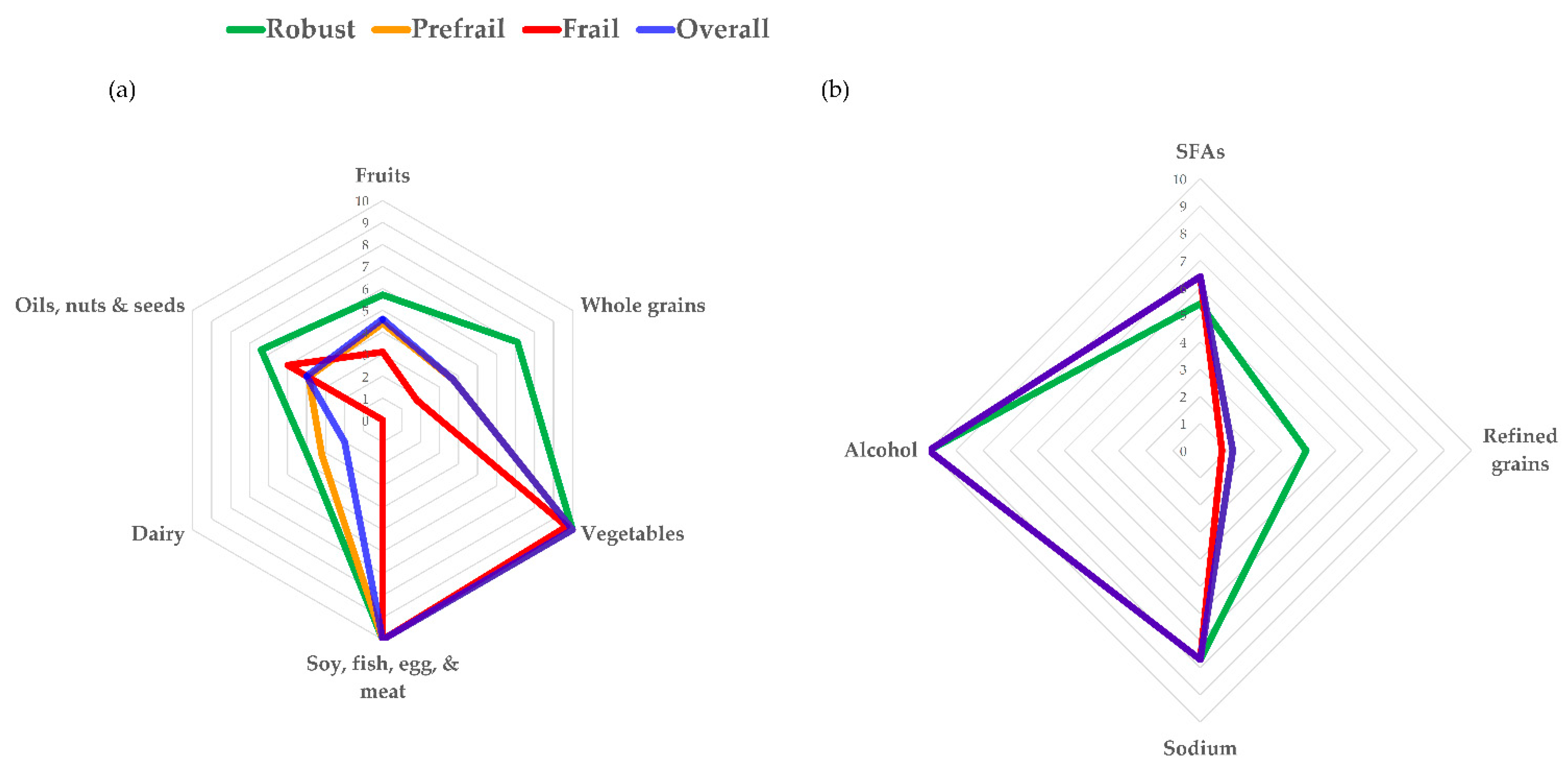
| Component | Standards for Minimum Score of Zero | Standards for Maximum Score | Maximum Score |
|---|---|---|---|
| Adequacy | |||
| Whole fruits | No whole fruits | ≥1.1 serving per 1000 kcal | 10 |
| Total vegetables | No vegetables | ≥1.7 serving per 1000 kcal | 5 |
| Dark or orange vegetables | No dark or orange vegetables | ≥0.6 serving per 1000 kcal | 5 |
| Whole grains | No whole grains | ≥2.0 serving per 1000 kcal | 10 |
| Total protein foods | No protein foods | ≥2.5 serving per 1000 kcal | 5 |
| Plant proteins & seafoods | No plant proteins & seafood | ≥0.8 serving per 1000 kcal | 5 |
| Dairy | No dairy | ≥0.6 serving per 1000 kcal | 10 |
| Fatty acids | >40% of total energy (90th percentile of distribution) | 20–30% of total energy | 5 |
| Nuts & seeds | No nuts & seeds | ≥0.4 serving per 1000 kcal | 5 |
| Moderation | |||
| Saturated fats | >10% of total energy | ≤ 6% of total energy (15th percentile of distribution) | 10 |
| Refined grains | >4.4 serving per 1000 kcal | ≤1.7 serving per 1000 kcal | 10 |
| Sodium | >1150 mg per 1000 kcal | ≤800 mg per 1000 kcal (15th percentile of distribution) | 10 |
| Alcohol | >20 g/day alcohol for man>10 g/day alcohol for woman | No alcohol | 10 |
| Overall (n = 154) | Non-Frail (n = 19) | Prefrail (n = 119) | Frail (n = 16) | p-Value 2 | |
|---|---|---|---|---|---|
| Age, years | 77.1 ± 7.4 | 72.6 ± 5.6 a | 77.6 ± 7.3 b | 78.6 ± 8.5 a,b | 0.015 |
| below 80 | 97 (63.0) | 16 (84.2) | 71 (59.7) | 10 (62.5) | |
| 80 and above | 57 (37.0) | 3 (15.8) | 48 (40.3) | 6 (37.5) | |
| Accommodation | 0.353 | ||||
| Retirement home | 58 (37.7) | 5 (26.3) | 45 (37.8) | 8 (50) | |
| Community | 96 (62.3) | 14 (73.7) | 74 (62.2) | 8 (50) | |
| Gender | 0.529 | ||||
| Male | 50 (32.5) | 5 (26.3) | 38 (31.9) | 7 (43.8) | |
| Female | 104 (67.5) | 14 (73.7) | 81 (68.1) | 9 (56.3) | |
| BMI | 24.9 ± 3.6 | 23.9 ± 2.3 | 24.9 ± 3.6 | 25.7 ± 2.9 | 0.242 |
| MNA (0–30) | 26.9 ± 2.0 | 27.6 ± 2.2 a | 26.9 ± 2.0 a,b | 26.2 ± 1.8 b | 0.015 |
| ADL (0–100) | 98.2 ± 10.6 | 98.1 ± 6.9 | 98.7 ± 10.6 | 94.7 ± 8.8 | 0.159 |
| IADL (0–24) | 20.4 ± 4.8 | 21.9 ± 3.7 a | 20.7 ± 4.0 a | 16.3 ± 6.8 b | 0.003 |
| Number of frailty criteria (0–5) | 1.5 ± 0.9 | 0 a | 1.5 ± 0.5 b | 3.2 ± 0.4 c | <0.001 |
| Exhaustion | 26 (16.9) | 0 | 15 (12.6) | 11 (68.8) | |
| Weakness | 59 (38.3) | 0 | 44 (37) | 15 (93.8) | |
| Low physical activity | 8 (5.2) | 0 | 0 | 8 (50) | |
| Shrinking | 2 (1.3) | 0 | 1 (0.8) | 1 (6.3) | |
| Slowness | 132 (85.7) | 0 | 116 (97.5) | 16 (100) |
| Overall (n = 154) | Non-Frail (n = 19) | Prefrail (n = 119) | Frail (n = 16) | ptrend 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| T-HEI | 61.0 | (55.6–71.5) | 66.5 | (55.2–77.2) | 61.3 | (55.6–71.8) | 57.6 | (51.6–62.7) | 0.025 |
| Whole fruits | 4.6 | (3.1–8.3) | 5.7 | (3.1–10.0) | 4.4 | (3.1–8.3) | 3.1 | (0.1–7.5) | 0.072 |
| Total vegetables | 5.0 | (5.0–5.0) | 5.0 | (5.0–5.0) | 5.0 | (5.0–5.0) | 5.0 | (4.7–5.0) | 0.548 |
| Dark or orange vegetables | 5.0 | (4.7–5.0) | 5.0 | (5.0–5.0) | 5.0 | (4.7–5.0) | 4.7 | (4.7–5.0) | 0.010 |
| Whole grains | 3.7 | (1.8–7.2) | 7.1 | (2.5–10.0) | 3.7 | (1.8–7.2) | 1.8 | (0.7–3.3) | 0.007 |
| Total protein foods | 5.0 | (4.8–5.0) | 5.0 | (4.1–5.0) | 5.0 | (4.8–5.0) | 5.0 | (4.9–5.0) | 0.350 |
| Plant proteins or seafoods | 5.0 | (5.0–5.0) | 5.0 | (5.0–5.0) | 5.0 | (5.0–5.0) | 5.0 | (5.0–5.0) | 0.867 |
| Dairy | 2.0 | (0–8.9) | 3.8 | (1.4–10.0) | 3.2 | (0–9.2) | 0.0 | (0–6.6) | 0.096 |
| Fatty acids | 4.0 | (1.9–5.0) | 4.7 | (1.4–5.0) | 3.9 | (2.1–5.0) | 5.0 | (0.3–5.0) | 0.772 |
| Nuts & seeds | 0.0 | (0–4.4) | 1.7 | (0–5.0) | 0.0 | (0–4.4) | 0.0 | (0–0.9) | 0.029 |
| Saturated fatty acids | 6.4 | (2.2–6.4) | 5.4 | (0–10.0) | 6.4 | (2.3–6.4) | 6.4 | (0.6–6.4) | 0.842 |
| Refined grains | 1.2 | (0–4.9) | 3.9 | (1.1–7.3) | 1.2 | (0–4.8) | 0.8 | (0–4.2) | 0.074 |
| Sodium | 7.7 | (7.6–8.7) | 7.7 | (0–10.0) | 7.7 | (7.6–8.6) | 7.7 | (7.6–9.3) | 0.958 |
| Alcohol | 10.0 | (10.0–10.0) | 10.0 | (10.0–10.0) | 10.0 | (10.0–10.0) | 10.0 | (10.0–10.0) | 0.901 |
| DASH | 24 | (22–29) | 27 | (20–30) | 22 | (22–29) | 22 | (21–26) | 0.051 |
| Fruits | 3 | (2–4) | 4 | (2–4) | 3 | (2–4) | 2 | (1–3) | 0.064 |
| Vegetables | 3 | (2–4) | 4 | (1–5) | 3 | (2–4) | 3 | (2–3) | 0.222 |
| Nuts & legumes | 3 | (2–4) | 2 | (1–4) | 3 | (2–4) | 4 | (2–4) | 0.219 |
| Whole grains | 3 | (1–5) | 5 | (2–5) | 3 | (1–5) | 1 | (1–3) | 0.003 |
| Dairy | 1 | (1–3) | 2 | (1–5) | 2 | (1–3) | 1 | (1–3) | 0.186 |
| Sodium | 3 | (3–4) | 3 | (1–5) | 3 | (3–4) | 3 | (3–4) | 0.621 |
| Red & processed meat | 3 | (2–4) | 4 | (2–4) | 3 | (2–4) | 3 | (2–4) | 0.384 |
| Sweetened beverage | 5 | (5–5) | 5 | (5–5) | 5 | (5–5) | 5 | (5–5) | 0.856 |
| MDS | 4 | (3–6) | 5 | (3–5) | 4 | (3–6) | 4 | (3–5) | 0.488 |
| Vegetables | 1 | (0–1) | 1 | (0–1) | 1 | (0–1) | 0 | (0–1) | 0.247 |
| Legumes | 1 | (0–1) | 0 | (0–1) | 1 | (0–1) | 1 | (0–1) | 0.054 |
| Fruits & nuts | 1 | (0–1) | 1 | (0–1) | 1 | (0–1) | 1 | (0–1) | 0.283 |
| Cereals/whole grains | 1 | (0–1) | 1 | (0–1) | 1 | (0–1) | 0 | (0–0) | 0.004 |
| Fish | 0 | (0–1) | 1 | (0–1) | 0 | (0–1) | 0 | (0–1) | 0.593 |
| Ethanol | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 1.000 |
| MUFA: SFA | 1 | (0–1) | 0 | (0–1) | 1 | (0–1) | 1 | (0–1) | 0.377 |
| Dairy | 0 | (0–1) | 0 | (0–1) | 0 | (0–1) | 1 | (0–1) | 0.242 |
| Meat & meat product | 0 | (0–1) | 1 | (0–1) | 0 | (0–1) | 0 | (0–1) | 0.368 |
| T-HEI | DASH | MDS | ||||
|---|---|---|---|---|---|---|
| β ± SE | p-Value | β ± SE | p-Value | β ± SE | p-Value | |
| Model 1 2 | −0.22 ± 0 | 0.006 | −0.18 ± 0.01 | 0.024 | −0.06 ± 0.02 | 0.465 |
| Model 2 3 | −0.19 ± 0 | 0.022 | −0.14 ± 0.01 | 0.094 | −0.08 ± 0.02 | 0.341 |
| Model 3 4 | −0.16 ± 0 | 0.047 | −0.10 ± 0.01 | 0.235 | −0.09 ± 0.02 | 0.269 |
| Model 4 5 | −0.14 ± 0 | 0.103 | −0.08 ± 0.01 | 0.349 | −0.08 ± 0.02 | 0.290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-Y.; Chen, I.-C.; Chan, Y.-C.; Cheong, I.-F.; Wang, Y.-Y.; Jian, Z.-R.; Lee, S.-D.; Chou, C.-C.; Yang, F.L. Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese. Nutrients 2021, 13, 4210. https://doi.org/10.3390/nu13124210
Lim K-Y, Chen I-C, Chan Y-C, Cheong I-F, Wang Y-Y, Jian Z-R, Lee S-D, Chou C-C, Yang FL. Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese. Nutrients. 2021; 13(12):4210. https://doi.org/10.3390/nu13124210
Chicago/Turabian StyleLim, Kian-Yuan, I-Chen Chen, Yun-Chun Chan, In-Fai Cheong, Yi-Yen Wang, Zi-Rong Jian, Shyh-Dye Lee, Chi-Chun Chou, and Feili Lo Yang. 2021. "Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese" Nutrients 13, no. 12: 4210. https://doi.org/10.3390/nu13124210
APA StyleLim, K.-Y., Chen, I.-C., Chan, Y.-C., Cheong, I.-F., Wang, Y.-Y., Jian, Z.-R., Lee, S.-D., Chou, C.-C., & Yang, F. L. (2021). Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese. Nutrients, 13(12), 4210. https://doi.org/10.3390/nu13124210






