Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Classification of Tumors
2.3. Variables and Data Collection
2.3.1. Assessment of Nutrient Intake
2.3.2. Analysis of PLP Intake
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.A.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 6868, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (concord-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Available online: https://www.wcrf.org/sites/default/files/Summary-of-Third-Expert-Report-2018.pdf (accessed on 10 June 2020).
- Corso, G.; Roncalli, F.; Marrelli, D.; Carneiro, F.; Roviello, F. History, pathogenesis, and management of familial gastric cancer: Original study of John XXIII’s family. BioMed. Res. Int. 2013, 2013, 385132. [Google Scholar] [CrossRef] [PubMed]
- Tessmer, M.S.; Flaherty, K.T. AACR Cancer Progress Report 2017: Harnessing Research Discoveries to Save Lives. Clin. Cancer Res. 2017, 2323, 5325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 2424, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 22, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Vitelli-Storelli, F.; Zamora-Ros, R.; Molina, A.J.; Fernández-Villa, T.; Castelló, A.; Barrio, J.P.; Amiano, P.; Ardanaz, E.; Obón-Santacana, M.; Gómez-Acebo, I.; et al. Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status. Nutrients 2020, 1212, 994. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 88, 515. [Google Scholar] [CrossRef]
- Vitelli, S.F.; Molina, A.J.; Zamora-Ros, R. Flavonoids and the Risk of Gastric Cancer: An Exploratory Case-Control Study in the MCC-Spain Study. Nutrients 2019, 1111, 967. [Google Scholar] [CrossRef] [Green Version]
- Arts, I.C.; Hollman, P.C. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [Green Version]
- Lunet, N.; Valbuena, C.; Vieira, A.L.; Fernández-Villa, T.; Roussou, V.; Romaguera, D.; Aragonés, N.; Obón-Santacana, M.; Guevara, M.; Gómez-Acebo, I.; et al. Fruit and Vegetable Consumption and Gastric Cancer by Location and Histological Type: Case-Control and Meta-Analysis. Eur. J. Cancer Prev. 2007, 1616, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Rusiecki, J.A.; Zhu, K.; Potter, J.; Devesa, S.S. Stomach Carcinoma Incidence Patterns in the United States by Histologic Type and Anatomic Site. Cancer Epidemiol. Biomark. Prev. 2009, 1818, 1945–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano-Vinyals, G.; Aragones, N.; Perez-Gomez, B.; Martín, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; de Sanjosé, S.; Jiménez-Moleón, J.J.; et al. Population-based multicase control study in common tumors in spain (MCC-Spain): Rationale and study design. Gac. Sanit. 2015, 2929, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley Orgánica. 15/1999, de 13 de diciembre, de Protección de Datos de Carácter Personal. Available online: https://www.boe.es/eli/es/lo/1999/12/13/15/con (accessed on 1 June 2020).
- Garcia-Closas, R.; Garcia-Closas, M.; Kogevinas, M.; Malats, N.; Silverman, D.; Serra, C.; Tardón, A.; Carrato, A.; Castaño-Vinyals, G.; Dosemeci, M.; et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J. Cancer 2007, 4343, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartaker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 109109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010. [Google Scholar] [CrossRef]
- Balentine, D.A.; Dwyer, J.T.; Erdman, J.W.; Ferruzzi, M.G.; Courtney, G.; Harnly, J.M.; Kwik-Uribe, C.L. Recommendations on reporting requirements for flavonoids in research. Am. J. Clin. Nutr. 2015, 101101, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Zumel, N.M. Practical Data Science with R; Manning Publications, Co.: Shelter Island, NY, USA, 2014. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Python Software Foundation. Python Language Reference. 3.6.5; Python Software Foundation: Wilmington, DE, USA, 2018. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 3.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Hernández-Ramírez, R.U.; Galván-Portillo, M.V.; Ward, M.H.; Agudo, A.; Oñate-Ocaña, L.F.; Herrera-Goepfert, R.; Palma-Coca, O.; López-Carrillo, L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer 2009, 125125, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wolk, A.; Håkansson, N.; Lagergren, J.; Lu, Y. Dietary intake of lignans and risk of esophageal and gastric adenocarcinoma: A cohort study in Sweden. Cancer Epidemiol. Biomark. Prev. 2013, 2222, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. Dietary intake of lignans and risk of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control 2012, 2323, 837–844. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 5858, 1428–1447. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, A.; Qi, B.; Ma, Z.; Xiong, Y.; Dou, J.; Wang, J. Resveratrol Protects against Helicobacter pylori-Associated Gastritis by Combating Oxidative Stress. Int. J. Mol. Sci. 2015, 1616, 27757–27769. [Google Scholar] [CrossRef] [Green Version]
- Zulueta, A.; Caretti, A.; Signorelli, P.; Ghidoni, R. Resveratrol: A potential challenger against gastric cancer. World. J. Gastroenterol. 2015, 2121, 10636–10643. [Google Scholar] [CrossRef]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food. Chem. 2007, 5555, 7777–7785. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Xu, L.; Ma, S.; Di, S.; Gao, Y.; Li, X.; Yan, X.; Zhang, H. Emerging Actions of Pterostilebene on Cancer Research. Zhongguo Fei Ai Za Zhi. 2018, 2121, 931–936. [Google Scholar] [CrossRef]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Arctigenin induces cell cycle arrest by blocking the phosphorylation of Rb via the modulation of cell cycle regulatory proteins in human gastric cancer cells. Int. Immunopharmacol. 2011, 1111, 1573–1577. [Google Scholar] [CrossRef]
- Liu, X.N.; Zhang, C.Y.; Jin, X.D.; Li, Y.Z.; Zheng, X.Z.; Li, L. Inhibitory effect of schisandrin B on gastric cancer cells in vitro. World J. Gastroenterol. 2007, 1313, 6506–6511. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Hu, Y.; Su, F.; Gao, Z.; Hu, T.; Yang, Y.; Cao, X.; Qian, F. Schisantherin A induces cell apoptosis through ROS/JNK signaling pathway in human gastric cancer cells. Biochem. Pharmacol. 2020, 173, 113673. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.; Karran, P. Vanillins-A novel family of DNA-PK inhibitors. Nucleic Acids Res. 2003, 3131, 5501–5512. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, X.; Zhang, H.; He, Z.; Zhi, W.; Liu, F.; Niu, X. Gastroprotective effect of esculin on ethanol-induced gastric lesion in mice. Fundam. Clin. Pharmacol. 2017, 3131, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.R.; Rocha, N.F.; Venâncio, E.T.; Moura, B.; Feitosa, M.; Carqueira, G.; Soares, P.M.; Woods, D.J.; de Sousa, F.C.; Leal, L.K.; et al. Mechanisms involved in the gastroprotective activity of esculin on acute gastric lesions in mice. Chem. Biol. Interact. 2010, 188188, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, M.; Yao, Y.; Wang, J.; Li, J. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 814, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, B.H.; Lv, W. Esculetin induces apoptosis in human gastric cancer cells through a cyclophilin D-mediated mitochondrial permeability transition pore associated with ROS. Chem. Biol. Interact. 2015, 242, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Ogawa, K.; Sugiura, M.; Tano, M.; Yoshizawa, Y. The antiproliferative effect of coumarins on several cancer cell lines. Anticancer Res. 2001, 21, 917–923. [Google Scholar]
- Weber, U.S.; Steffen, B.; Siegers, C.P. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol Pharmacol. 1998, 9999, 193–206. [Google Scholar]
- Alirezaei, M.; Dezfoulian, O.; Neamati, S.; Rashidipour, M.; Tanideh, N.; Kheradmand, A. Oleuropein prevents ethanol-induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J. Physiol. Biochem. 2012, 6868, 583–592. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Mohd, A.; Mohd, A. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. Bioallied. Sci. 2011, 33, 361–367. [Google Scholar] [CrossRef]
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern Med. Rev. 2007, 1212, 331–342. [Google Scholar]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedore, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 1717, 76–84. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136136, 487–490. [Google Scholar] [CrossRef]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, 5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacera, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Xiao, F.T.; Gong, B.C.; Liu, F.N. Alcohol drinking and gastric cancer risk: A meta-analysis of observational studies. Oncotarget 2017, 88, 99013–99023. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic Drinks and the Risk of Cancer. Available online: dietandcancerreport.org (accessed on 7 July 2020).
- Amor, S.; Châlons, P.; Aires, V.; Delmans, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 66, 106. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Ha, S.K.; Cho, E.; Choi, I. Resveratrol as a Bioenhancer to Improve Anti-Inflammatory Activities of Apigenin. Nutrients 2015, 77, 9650–9661. [Google Scholar] [CrossRef] [Green Version]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menéndez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 1111, 2039. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panangiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 99, 1063. [Google Scholar] [CrossRef]
- Du, S.; Li, Y.; Su, Z.; Shi, X.; Johnson, N.L.; Li, P.; Zhang, Y.; Zhang, Q.; Wen, L.; Li, K.; et al. Index-based dietary patterns in relation to gastric cancer risk: A systematic review and meta-analysis. Br. J. Nutr. 2020, 123123, 964–974. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Cayssials, V.; Jenab, M.; Rothwell, J.A.; Fedirko, V.; Aleksandrova, K.; Tjonneland, A.; Kyro, C.; Overvad, K.; Boutron-Rauault, M.C.; et al. Dietary intake of total polyphenol and polyphenol classes and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur. J. Epidemiol. 2018, 3333, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 7575, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Interindividual differences in response to plant-based diets: Implications for cancer risk. Am. J. Clin. Nutr. 2009, 8989, 1553S–1557S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 88, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébert, J.R. Social Desirability Trait: Biaser or Driver of Self-Reported Dietary Intake? J. Acad. Nutr. Diet. 2016, 116116, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 1818, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- de Kok, T.M.; van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef]
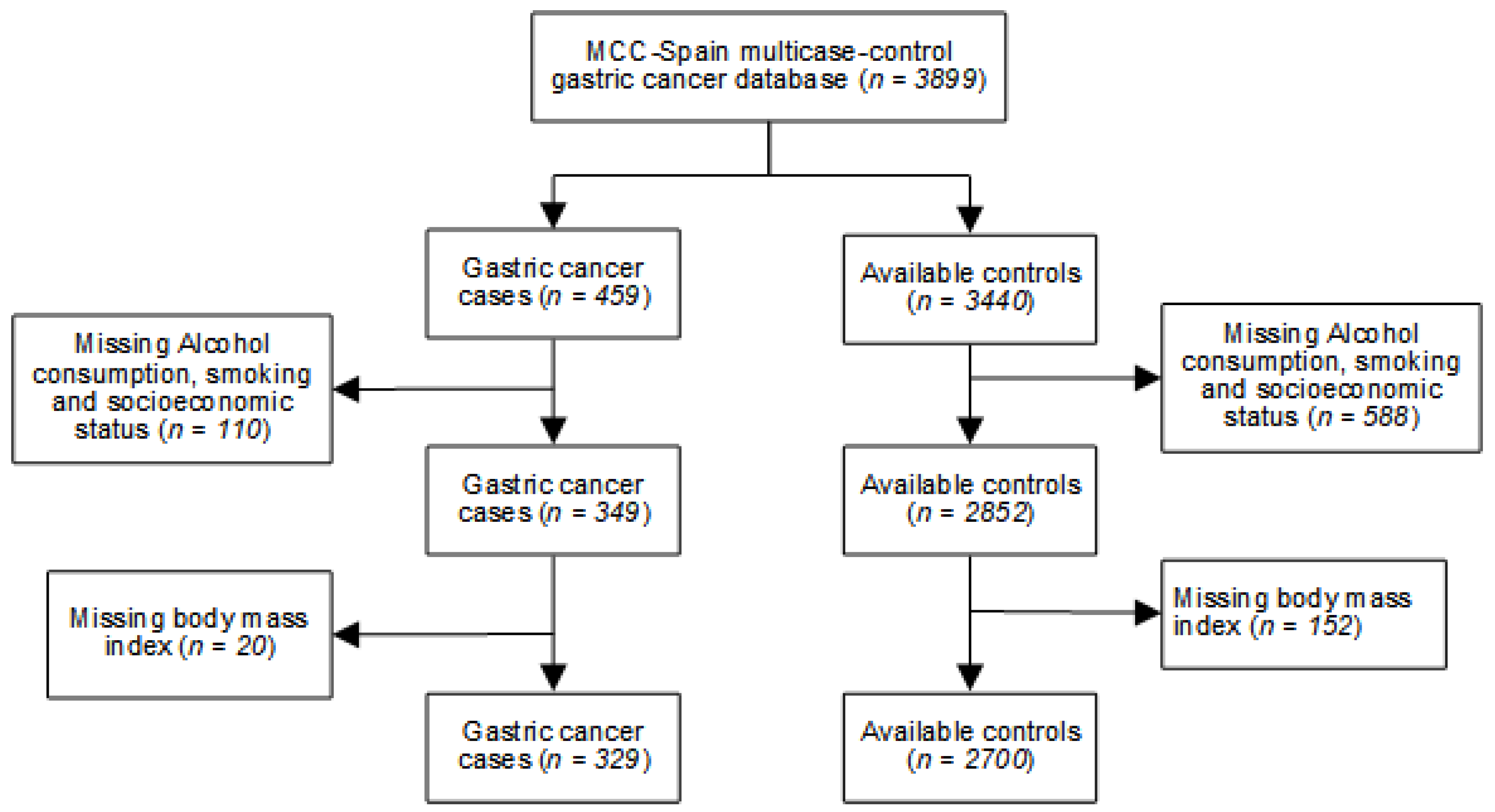
| Variables | Controls | Cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | By Anatomical Subtypes | By Histological Subtypes | ||||||||
| (n = 2700) | (n = 329) | p-Value 1 | Cardia (n = 84) | Non-Cardia (n = 238) | p-Value 2 | Intestinal (n = 122) | Diffuse (n = 75) | p-Value 3 | ||
| Age (year) mean (SE) | 63.5 (0.2) | 65.4 (0.7) | 0.001 | 63.4 (1.3) | 66.1 (0.8) | 0.036 | 69.5 (1.0) | 61.8 (1.6) | 0.000 | |
| Sex (men, %) | 1522 (56.4) | 239 (72.6) | 0.000 | 77 (91.7) | 156 (65.6) | 0.000 | 87 (70.7) | 45 (60.0) | 0.120 | |
| Socioeconomic status | High (%) | 448 (16.6) | 27 (8.2) | 0.000 | 9 (10.7) | 18 (7.6) | 0.679 | 8 (6.6) | 8 (10.7) | 0.372 |
| Medium (%) | 1361 (50.4) | 146 (44.4) | 38 (45.2) | 103 (43.3) | 49 (40.2) | 34 (45.3) | ||||
| Low (%) | 891 (33.0) | 156 (47.4) | 37 (44.1) | 117(49.1) | 65 (53.3) | 33 (44.0) | ||||
| Smoking status (%) | yes | 1531 (56.7) | 201 (61.1) | 0.138 | 65 (77.4) | 130 (54.6) | 0.000 | 60 (49.2) | 45 (60.0) | 0.229 |
| no | 1169 (43.3) | 128 (38.9) | 19 (22.6) | 108 (45.4) | 62 (50.8) | 30 (40.0) | ||||
| GC family history (%) | yes | 170 (6.3) | 53 (16.1) | 0.000 | 11 (13.0) | 40 (16.8) | 0.423 | 27 (22.1) | 13 (17.3) | 0.432 |
| no | 2530 (93.7) | 276 (83.9) | 73 (87.0) | 198 (83.2) | 95 (77.9) | 62 (82.7) | ||||
| Physical activity (MET-h/week) | <8 | 1374 (50.9) | 201 (61.1) | 0.000 | 50 (59.5) | 147 (61.8) | 0.717 | 66 (54.1) | 48 (64.0) | 0.187 |
| ≥8 | 1326 (49.1) | 128 (38.9) | 34 (40.5) | 91 (38.2) | 56 (45.9) | 27 (36.0) | ||||
| Body mass index (kg/m2) | ≤25 | 1026 (38.0) | 103 (31.3) | 0.057 | 21 (25.0) | 80 (33.6) | 0.135 | 41 (33.6) | 32 (42.7) | 0.415 |
| >25–30 | 1129 (41.8) | 150 (45.6) | 37 (44.0) | 110 (46.2) | 59 (48.4) | 31 (41.3) | ||||
| ≥30 | 545 (20.2) | 76 (23.1) | 26 (31.0) | 48 (20.2) | 22 (18.0) | 12 (16.0) | ||||
| Alcohol consumption (g/day) | 0 | 418 (15.5) | 47 (14.3) | 0.000 | 8 (9.5) | 39 (16.4) | 0.002 | 22 (18.0) | 13 (17.3) | 0.592 |
| <12 | 1179 (43.7) | 103 (31.3) | 16 (19.0) | 84 (35.3) | 40 (32.8) | 27 (36.0) | ||||
| 12–47 | 787 (29.1) | 101 (30.7) | 34 (40.5) | 66 (27.7) | 30 (24.6) | 22 (29.4) | ||||
| >47 | 316 (11.7) | 78 (23.7) | 26 (31.0) | 49 (20.6) | 30 (24.6) | 13 (17.3) | ||||
| Vegetables total intake (g/d), mean (SE) | 191.3 (2.4) | 180.8 (7.0) | 0.112 | 184.8 (18.3) | 177.9 (7.0) | 0.821 | 189.3 (170.1) | 185.6 (13.9) | 0.626 | |
| Red meat intake (g/d), mean (SE) | 64.0 (0.8) | 84.4 (2.9) | 0.000 | 97.50 (6.5) | 80.10 (3.3) | 0.006 | 84.6 (4.6) | 73.1 (5.2) | 0.143 | |
| Sodium intake (mg/d), mean (SE) | 3008.6 (24.0) | 3529.3 (86.3) | 0.000 | 3758.6 (200.7) | 3443.9 (94.9) | 0.175 | 3403.2 (144.6) | 3821.4 (187.7) | 0.044 | |
| Total phenolic acid intake (mg/d), mean (SE) | 166.5 (2.0) | 170.7 (5.4) | 0.233 | 191.0 (12.2) | 161.1 (5.8) | 0.023 | 178.4 (10.3) | 164.5 (10.6) | 0.667 | |
| Total stilbene intake (mg/d), mean (SE) | 1.9 (0.1) | 1.67 (0.2) | 0.022 | 2.4 (0.4) | 1.4 (0.2) | 0.061 | 1.6 (0.3) | 1.3 (0.3) | 0.719 | |
| Total lignan intake (mg/d), mean (SD) | 2.7 (1.7) | 2.5 (1.4) | 0.085 | 2.5 (1.3) | 2.5 (1.4) | 0.712 | 2.7 (1.5) | 2.4 (1.3) | 0.260 | |
| Total other polyphenol intake (mg/d), mean (SE) | 16.4 (0.3) | 16.3 (0.9) | 0.844 | 16.1 (1.1) | 15.3 (1.3) | 0.395 | 15.3 (1.3) | 18.8 (2.6) | 0.305 | |
| PLP Classes | Total | Anatomical | Histological | PLP Intake mg/day ± SD | Foods with Highest Contribution in All Cases and Controls | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cardia | Non-cardia | Intestinal | Diffuse | First (%) | Second (%) | Third (%) | ||||
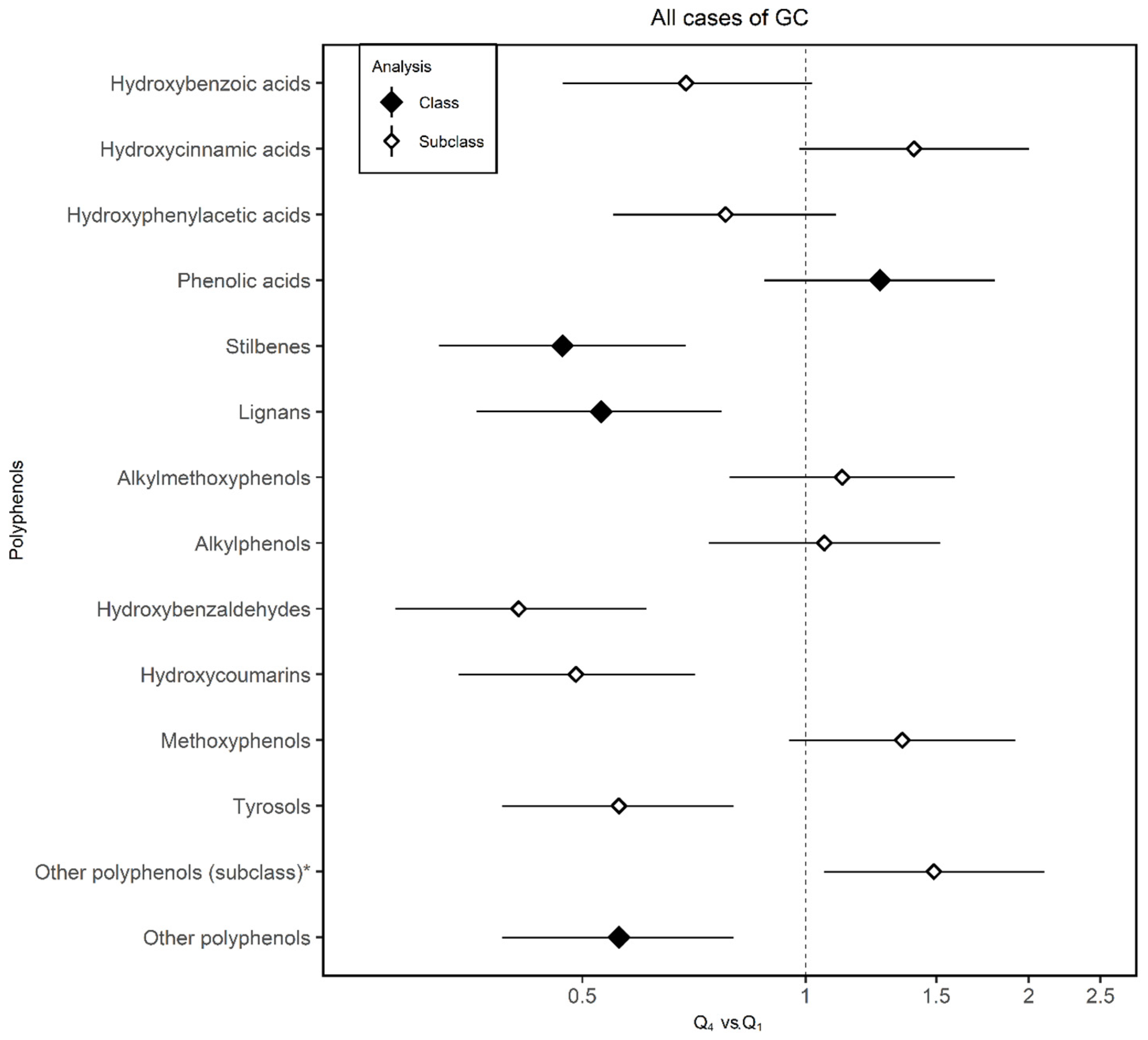
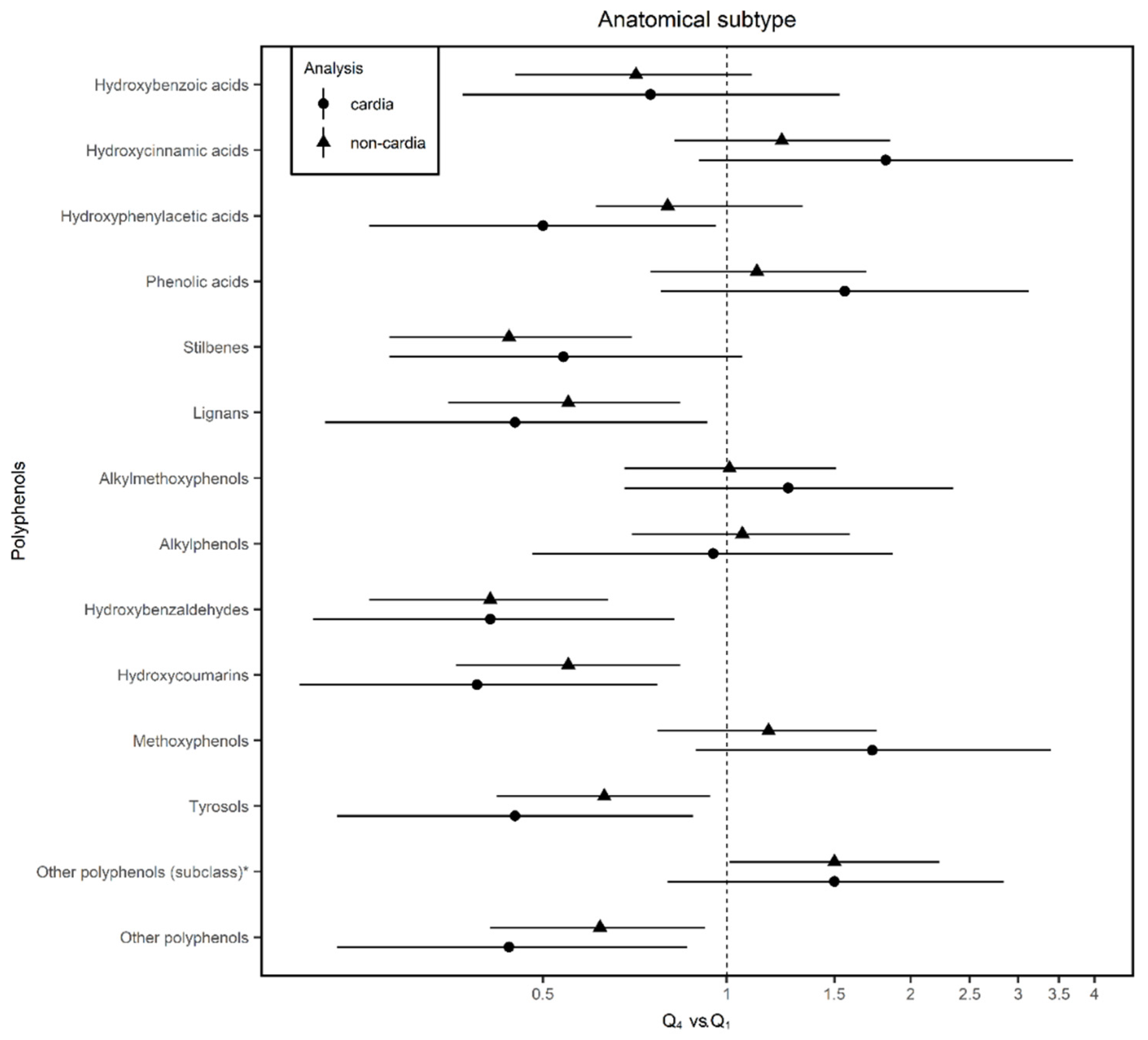
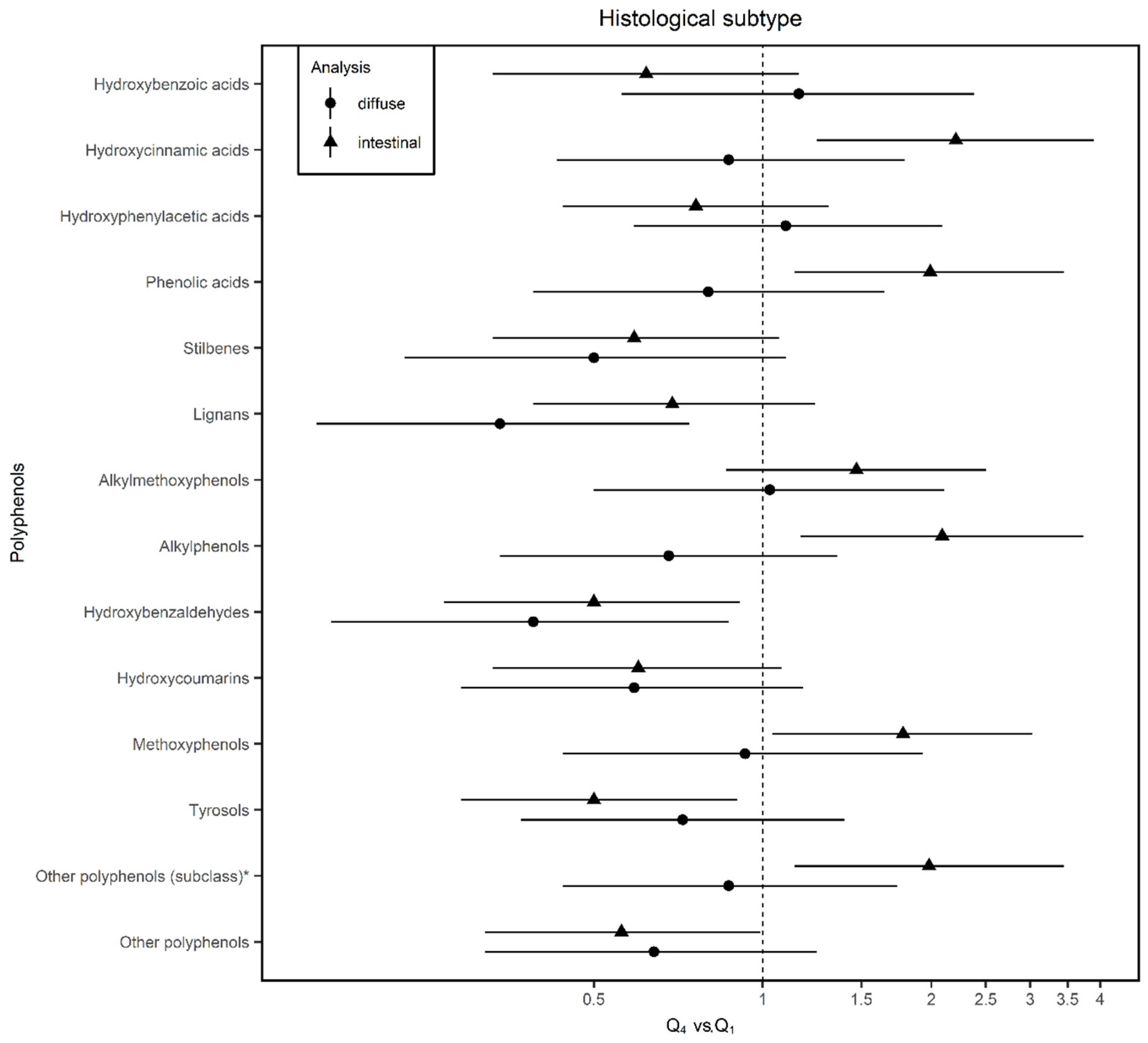
| Phenolic acids | Hydroxybenzoic acids | ↓ | ↓ | ↓ | ↓ | ↑ | 15.73 ± 11.72 | Swiss chard (24.4) | Wine (Red) (22.3) | Nuts (14.2) |
| Hydroxycinnamic acids | ↑ | ↑ | ↑ | ↑ * | ↓ | 150.51 ± 99.41 | Coffee (45) | Coffee (decaffeinated) (25) | Apple (5.3) | |
| Hydroxyphenylacetic acids | ↓ | ↓ * | ↓ | ↓ | ↑ | 0.71 ± 1.13 | Olives (83.5) | Wine (Red) (11.12) | Beer (Ale/Regular) (3.8) | |
| Phenolic acids (class) | ↑ | ↑ | ↑ | ↑ * | ↓ | |||||
| Stilbenes | Stilbenes | ↓ * | ↓ | ↓ * | ↓ | ↓ | 1.86 ± 3.06 | Wine (Red) (92.2) | Wine (Rosé) (3.8) | Grape (2) |
| Lignans | Lignans | ↓ * | ↓ * | ↓ * | ↓ | ↓ * | 2.71 ± 1.70 | Brassica oleracea (22.4) | Green bean (17.4) | Orange tangerine (11.9) |
| Other polyphenols | Alkylmethoxyphenols | ↑ | ↑ | ↑ | ↑ | ↑ | 0.74 ± 0.92 | Coffee (70.4) | Coffee (decaffeinated (22.3) | Beer (Ale/Regular) (7.3) |
| Alkylphenols | ↑ | ↓ | ↑ | ↑ * | ↓ | 0.09 ± 0.09 | Coffee (98) | Beer (1.8) | Cocoa powder (0.2) | |
| Hydroxybenzaldehydes | ↓ * | ↓ * | ↓ * | ↓ * | ↓ * | 0.38 ± 0.63 | Wine (Red) (92.7) | Wine (Rosé) (2.8) | Beer (Ale/Regular) (18) | |
| Hydroxycoumarins | ↓ * | ↓ * | ↓ * | ↓ | ↓ | 0.07 ± 0.14 | Beer (Ale/Regular) (55.2) | Wine (Rosé) (44) | Sherry (0.7) | |
| Methoxyphenol | ↑ | ↑ | ↑ | ↑ * | ↓ | 0.10 ± 0.13 | Coffee (100) | |||
| Tyrosols | ↓ * | ↓ * | ↓ * | ↓ * | ↓ | 14.06 ± 16.18 | Olives (58.2) | Olive oil (26.4) | Wine (Red) (12.8) | |
| Other polyphenols (subclass) a | ↑ * | ↑ | ↑ * | ↑* | ↓ | 0.89 ± 0.90 | Coffee (36.9) | Orange juice (23.2) | Other juice (13.7) | |
| Other polyphenols (class) | ↑ | ↑ | ↑ | ↑* | ↓ | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubín-García, M.; Vitelli-Storelli, F.; Molina, A.J.; Zamora-Ros, R.; Aragonés, N.; Adarnaz, E.; Castaño-Vinyals, G.; Obón-Santacana, M.; Gómez-Acebo, I.; Molina-Barceló, A.; et al. Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain. Nutrients 2020, 12, 3281. https://doi.org/10.3390/nu12113281
Rubín-García M, Vitelli-Storelli F, Molina AJ, Zamora-Ros R, Aragonés N, Adarnaz E, Castaño-Vinyals G, Obón-Santacana M, Gómez-Acebo I, Molina-Barceló A, et al. Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain. Nutrients. 2020; 12(11):3281. https://doi.org/10.3390/nu12113281
Chicago/Turabian StyleRubín-García, María, Facundo Vitelli-Storelli, Antonio José Molina, Raúl Zamora-Ros, Nuria Aragonés, Eva Adarnaz, Gemma Castaño-Vinyals, Mireia Obón-Santacana, Inés Gómez-Acebo, Ana Molina-Barceló, and et al. 2020. "Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain" Nutrients 12, no. 11: 3281. https://doi.org/10.3390/nu12113281
APA StyleRubín-García, M., Vitelli-Storelli, F., Molina, A. J., Zamora-Ros, R., Aragonés, N., Adarnaz, E., Castaño-Vinyals, G., Obón-Santacana, M., Gómez-Acebo, I., Molina-Barceló, A., Fernández-Tardón, G., Jiménez-Moleón, J. J., Alguacil, J., Chirlaque, M. D., Toledo, E., Pérez-Gómez, B., Pollán, M., Kogevinas, M., & Martín, V. (2020). Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain. Nutrients, 12(11), 3281. https://doi.org/10.3390/nu12113281







