Investigation of Nicotianamine and 2′ Deoxymugineic Acid as Enhancers of Iron Bioavailability in Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Metabolite and Fe solutions
2.3. Caco-2 Assays
2.4. Graphical Representation and Statistical Analysis
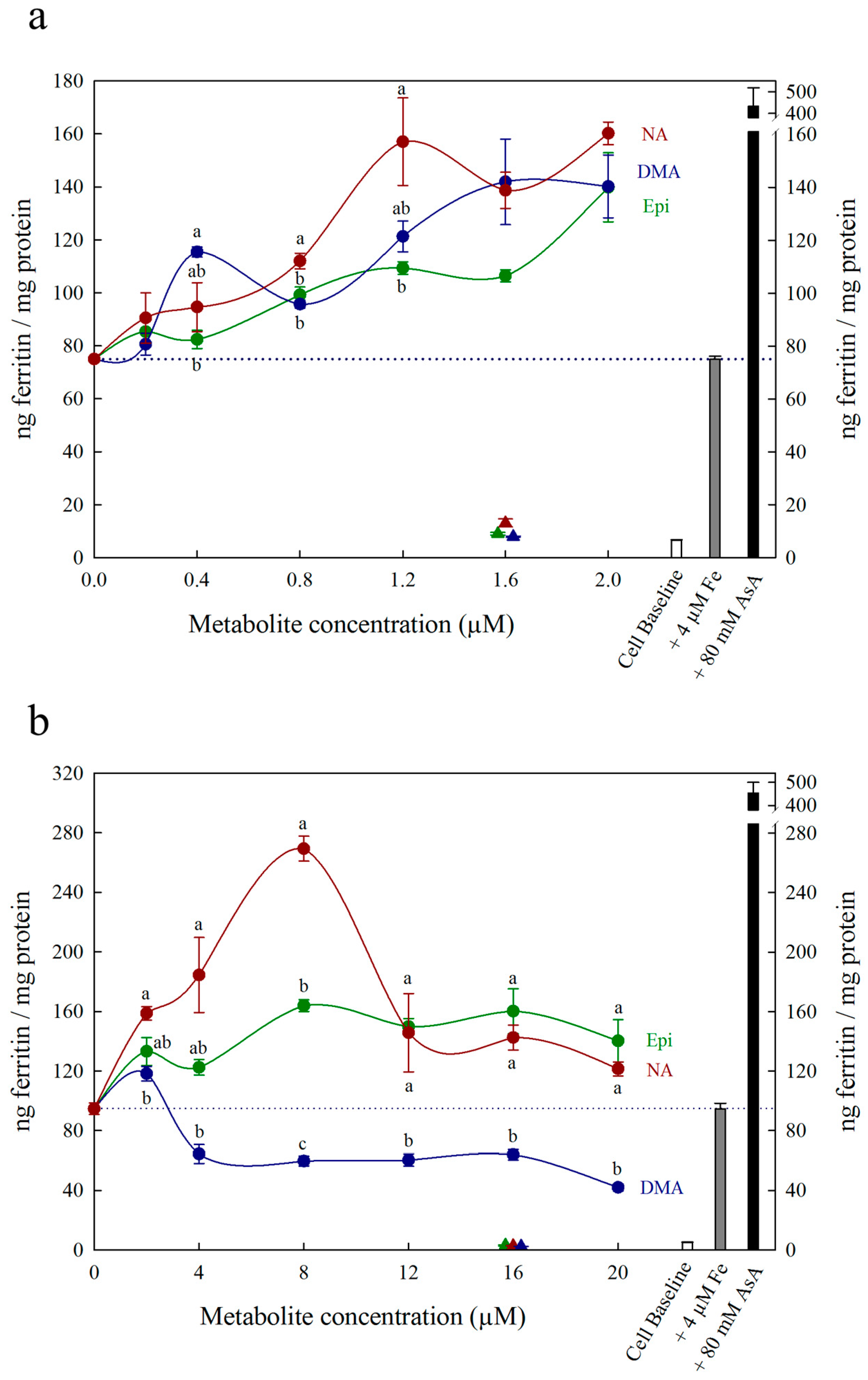
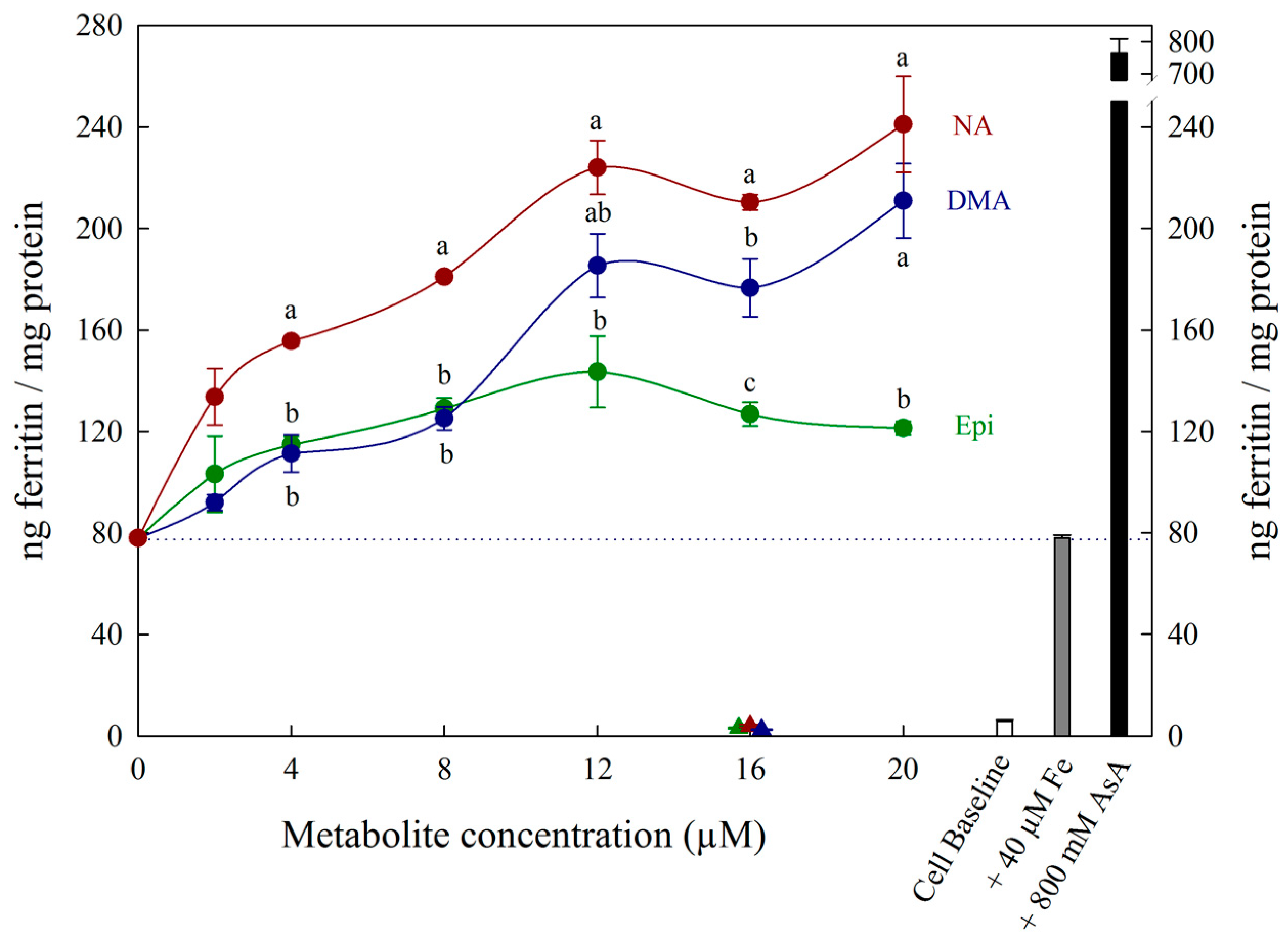
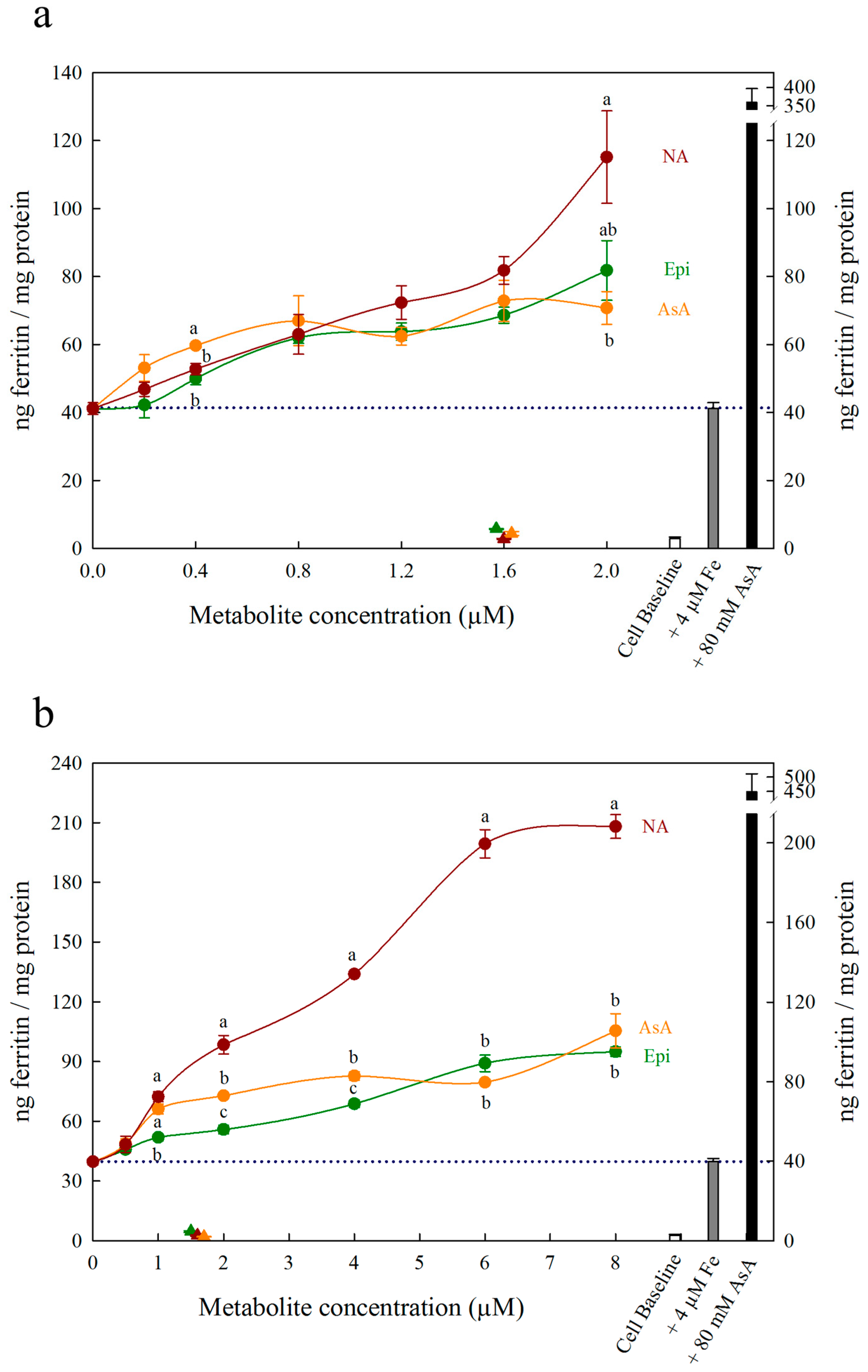
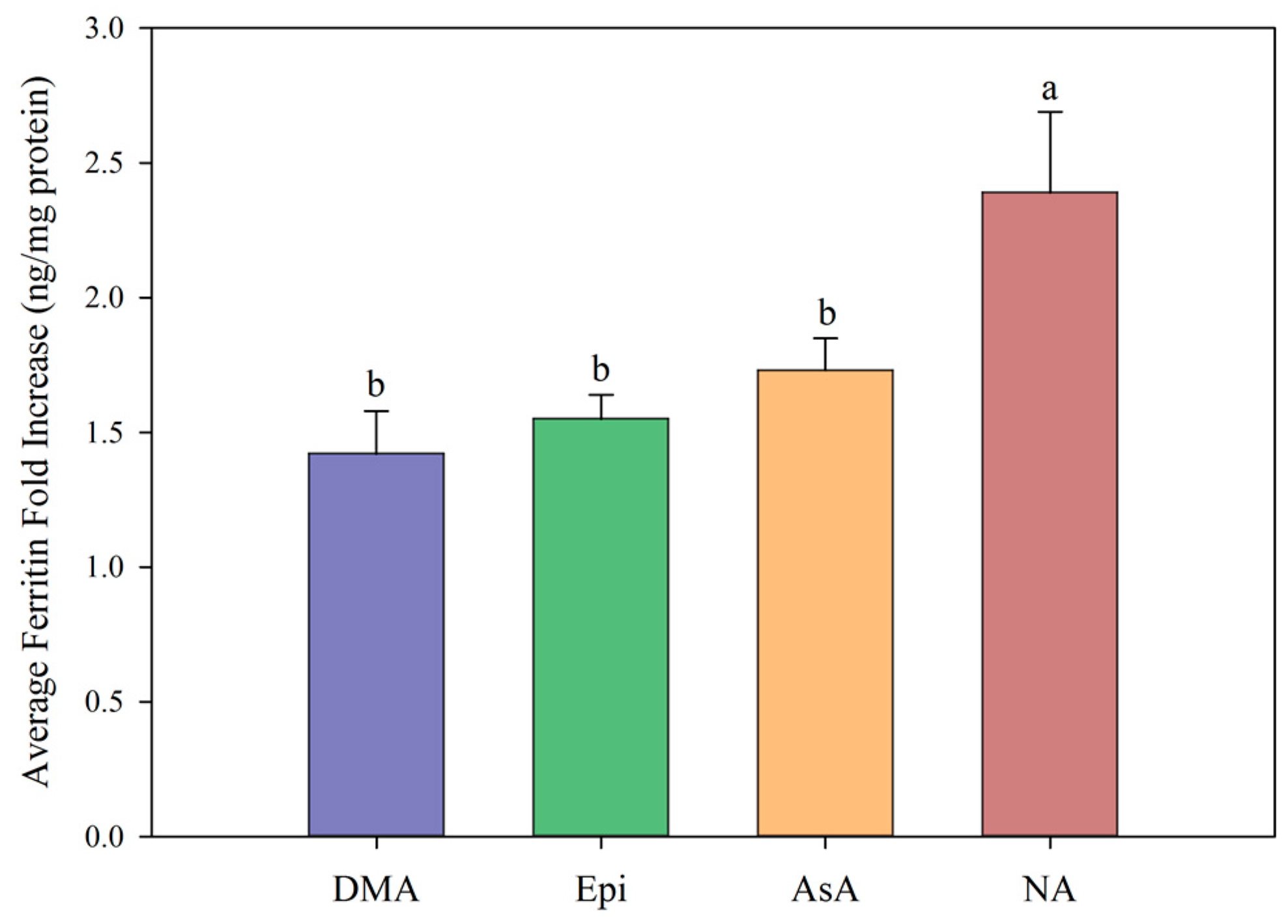
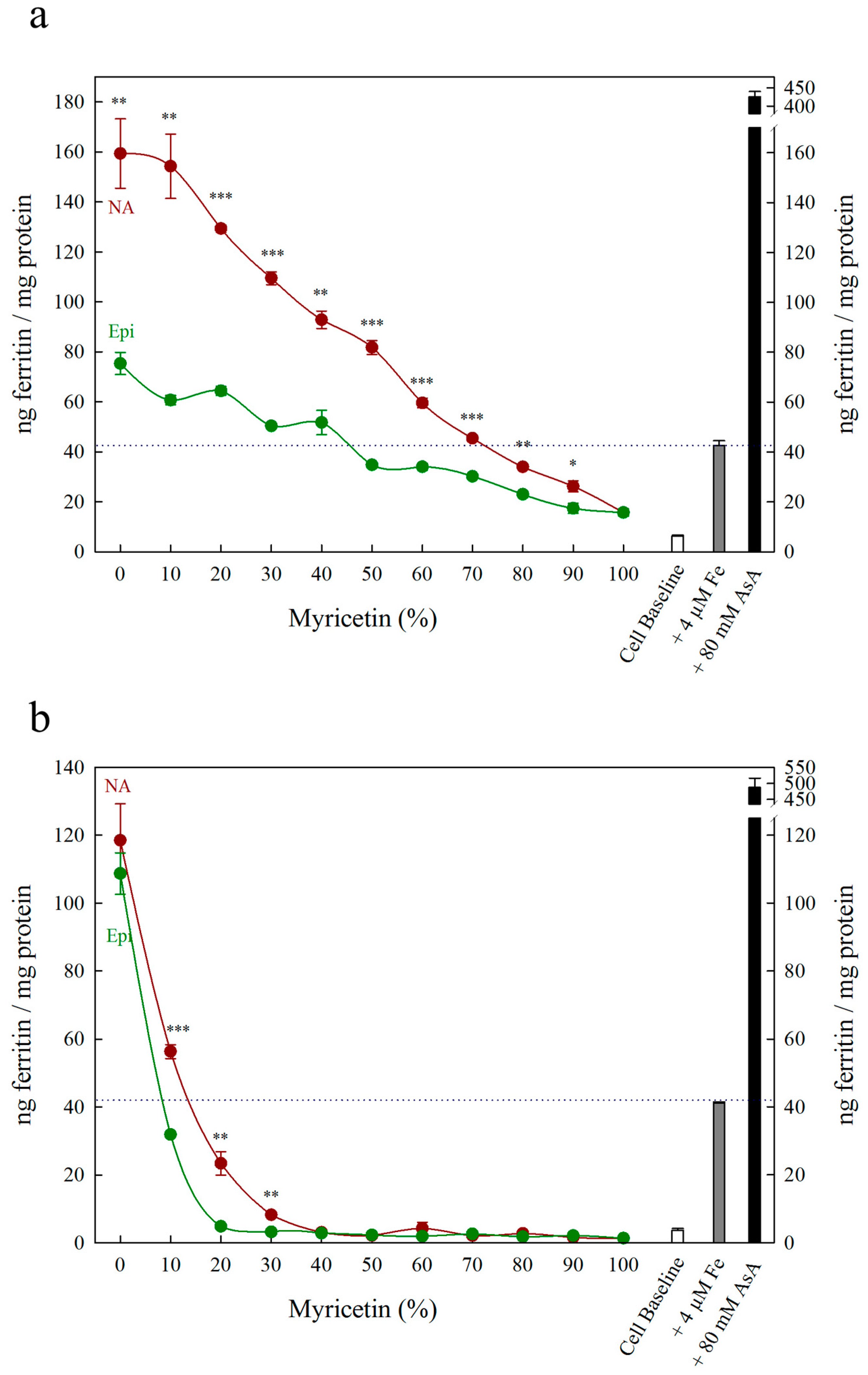
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Briat, J.F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2018, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants-a brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.T.; Bonneau, J.P.; Johnson, A.A.T. Characterisation of the nicotianamine aminotransferase and deoxymugineic acid synthase genes essential to Strategy II iron uptake in bread wheat (Triticum aestivum L.). PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Dawidziak, M. Plant ferritin—A source of iron to prevent its deficiency. Nutrients 2015, 7, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Cvitanich, C.; Przybyłowicz, W.J.; Urbanski, D.F.; Jurkiewicz, A.M.; Mesjasz-Przybyłowicz, J.; Blair, M.W.; Astudillo, C.; Jensen, E.Ø.; Stougaard, J. Iron and ferritin accumulate in separate cellular locations in Phaseolus seeds. BMC Plant Biol. 2010, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of iron bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the Caco-2 cell in vitro model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar] [CrossRef]
- Hoppler, M.; Schönbächler, A.; Meile, L.; Hurrell, R.F.; Walczyk, T. Ferritin-Iron Is Released during Boiling and In Vitro Gastric Digestion. J. Nutr. 2008, 138, 878–884. [Google Scholar] [CrossRef]
- Gillooly, M.; Charlton, R.W.; Mills, W.; MacPhail, A.P.; Mayet, F.; Bezwoda, W.R.; Bothwell, T.H.; Torrance, J.D.; Derman, D.P. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331. [Google Scholar] [CrossRef]
- Blair, M.W.; Izquierdo, P.; Astudillo, C.; Grusak, M.A. A legume biofortification quandary: variability and genetic control of seed coat micronutrient accumulation in common beans. Front. Plant Sci. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of Iron in Arabidopsis Seed Requires the Vacuolar Membrane Transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Nozoye, T.; Kim, S.; Kakei, Y.; Takahashi, M.; Nakanishi, H.; Nishizawa, N.K. Enhanced levels of nicotianamine promote iron accumulation and tolerance to calcareous soil in soybean. Biosci. Biotechnol. Biochem. 2014, 78, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Z.H.; Das Bhowmik, S.S.; Hoang, T.M.L.; Karbaschi, M.R.; Long, H.; Cheng, A.; Bonneau, J.P.; Beasley, J.T.; Johnson, A.A.T.; Williams, B.; et al. Investigation of Baseline Iron Levels in Australian Chickpea and Evaluation of a Transgenic Biofortification Approach. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Connorton, J.M.; Wan, Y.; Lovegrove, A.; Moore, K.L.; Uauy, C.; Sharp, P.A.; Shewry, P.R. Improving wheat as a source of iron and zinc for global nutrition. Nutr. Bull. 2019, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- De Brier, N.; Gomand, S.V.; Donner, E.; Paterson, D.; Smolders, E.; Delcour, J.A.; Lombi, E. Element distribution and iron speciation in mature wheat grains (Triticum aestivum L.) using synchrotron X-ray fluorescence microscopy mapping and X-ray absorption near-edge structure (XANES) imaging. Plant. Cell Environ. 2016, 39, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.T.; Bonneau, J.P.; Sánchez-Palacios, J.T.; Moreno-Moyano, L.T.; Callahan, D.L.; Tako, E.; Glahn, R.P.; Lombi, E.; Johnson, A.A.T. Metabolic engineering of bread wheat improves grain iron concentration and bioavailability. Plant Biotechnol. J. 2019, 1–13. [Google Scholar] [CrossRef]
- Eagling, T.; Wawer, A.A.; Shewry, P.R.; Zhao, F.; Fairweather-Tait, S.J. Iron Bioavailability in Two Commercial Cultivars of Wheat: Comparison between Wholegrain and White Flour and the Effects of Nicotianamine and 2′-Deoxymugineic Acid on Iron Uptake into Caco-2 Cells. J. Agric. Food Chem. 2014, 62, 10320–10325. [Google Scholar] [CrossRef]
- Kyriacou, B.; Moore, K.L.; Paterson, D.; de Jonge, M.D.; Howard, D.L.; Stangoulis, J.; Tester, M.; Lombi, E.; Johnson, A.A.T. Localization of iron in rice grain using synchrotron X-ray fluorescence microscopy and high resolution secondary ion mass spectrometry. J. Cereal Sci. 2014, 59, 173–180. [Google Scholar] [CrossRef]
- Johnson, A.A.T.; Kyriacou, B.; Callahan, D.L.; Carruthers, L.; Stangoulis, J.; Lombi, E.; Tester, M. Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 2011, 6, e24476. [Google Scholar] [CrossRef]
- Hart, J.J.; Tako, E.; Glahn, R.P. Characterization of Polyphenol Effects on Inhibition and Promotion of Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, 330–375. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Sungur, Ş.; Uzar, A. Investigation of complexes tannic acid and myricetin with Fe(III). Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2008, 69, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Asenstorfer, R.E.; Wang, Y.; Mares, D.J. Chemical structure of flavonoid compounds in wheat (Triticum aestivum L.) flour that contribute to the yellow colour of Asian alkaline noodles. J. Cereal Sci. 2006, 43, 108–119. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Tako, E.; Glahn, R.P.; Miller, D.D. Isolated Glycosaminoglycans from Cooked Haddock Enhance Nonheme Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 10346–10351. [Google Scholar] [CrossRef]
- Huh, E.C.; Hotchkiss, A.; Brouillette, J.; Glahn, R.P. Carbohydrate Fractions from Cooked Fish Promote Iron Uptake by Caco-2 Cells. J. Nutr. 2004, 134, 1681–1689. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.S.; Jeon, U.S.; Kim, Y.K.; Schjoerring, J.K.; An, G. Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol. Cells 2012, 33, 269–275. [Google Scholar] [CrossRef]
- Trijatmiko, K.R.; Duenãs, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cheng, Z.; Ai, C.; Jiang, X.; Bei, X.; Zheng, Y.; Glahn, R.P.; Welch, R.M.; Miller, D.D.; Lei, X.G.; et al. Nicotianamine, a Novel Enhancer of Rice Iron Bioavailability to Humans. PLoS ONE 2010, 5, e10190. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.; Tako, E.; Hart, J.; Haas, J.; Lung’aho, M.; Beebe, S. Iron Bioavailability Studies of the First Generation of Iron-Biofortified Beans Released in Rwanda. Nutrients 2017, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, Z.; Heller, L.I.; Krasnoff, S.B.; Glahn, R.P.; Welch, R.M. Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human Caco-2 cell model. J. Agric. Food Chem. 2006, 54, 9254–9261. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of Black Bean (Phaseolus vulgaris L.) Polyphenols That Inhibit and Promote Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Bar, H.; Glahn, R.P. The combined application of the Caco-2 cell bioassay coupled with in vivo (Gallus gallus) feeding trial represents an effective approach to predicting Fe bioavailability in humans. Nutrients 2016, 8, 732. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Heal. Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood J. 2014, 123, 615–625. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Bechoff, A.; Dhuique-Mayer, C. Factors influencing micronutrient bioavailability in biofortified crops. Ann. N. Y. Acad. Sci. 2017, 1390, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef]
- Singh, S.P.; Keller, B.; Gruissem, W.; Bhullar, N.K. Rice NICOTIANAMINE SYNTHASE 2 expression improves dietary iron and zinc levels in wheat. Theor. Appl. Genet. 2017, 130, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Von Wirén, N.; Klair, S.; Bansal, S.; Briat, J.-F.; Khodr, H.; Shioiri, T.; Leigh, R.A.; Hider, R.C. Nicotianamine Chelates Both FeIII and FeII. Implications for Metal Transport in Plants1. Plant Physiol. 1999, 119, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Tsednee, M.; Huang, Y.C.; Chen, Y.R.; Yeh, K.C. Identification of metal species by ESI-MS/MS through release of free metals from the corresponding metal-ligand complexes. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Khoo, H.-E. Biological Effects of Myricetin. Gen. Pharmac. 1997, 29, 508–527. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Carcea, M.; Narducci, V.; Turfani, V.; Giannini, V. Polyphenols in Raw and Cooked Cereals/Pseudocereals/Legume Pasta and Couscous. Foods 2017, 6, 80. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beasley, J.T.; Hart, J.J.; Tako, E.; Glahn, R.P.; Johnson, A.A.T. Investigation of Nicotianamine and 2′ Deoxymugineic Acid as Enhancers of Iron Bioavailability in Caco-2 Cells. Nutrients 2019, 11, 1502. https://doi.org/10.3390/nu11071502
Beasley JT, Hart JJ, Tako E, Glahn RP, Johnson AAT. Investigation of Nicotianamine and 2′ Deoxymugineic Acid as Enhancers of Iron Bioavailability in Caco-2 Cells. Nutrients. 2019; 11(7):1502. https://doi.org/10.3390/nu11071502
Chicago/Turabian StyleBeasley, Jesse T., Jonathan J. Hart, Elad Tako, Raymond P. Glahn, and Alexander A. T. Johnson. 2019. "Investigation of Nicotianamine and 2′ Deoxymugineic Acid as Enhancers of Iron Bioavailability in Caco-2 Cells" Nutrients 11, no. 7: 1502. https://doi.org/10.3390/nu11071502
APA StyleBeasley, J. T., Hart, J. J., Tako, E., Glahn, R. P., & Johnson, A. A. T. (2019). Investigation of Nicotianamine and 2′ Deoxymugineic Acid as Enhancers of Iron Bioavailability in Caco-2 Cells. Nutrients, 11(7), 1502. https://doi.org/10.3390/nu11071502





