Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants and Data Collection
2.3. Assessment and Definition of Probiotic Exposure
2.4. Definition of Metabolic Comorbidities, Smoking, and Physical Activity
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics According to Probiotic Consumption
3.2. Prevalence of Metabolic Comorbidities According to Probiotic Consumption
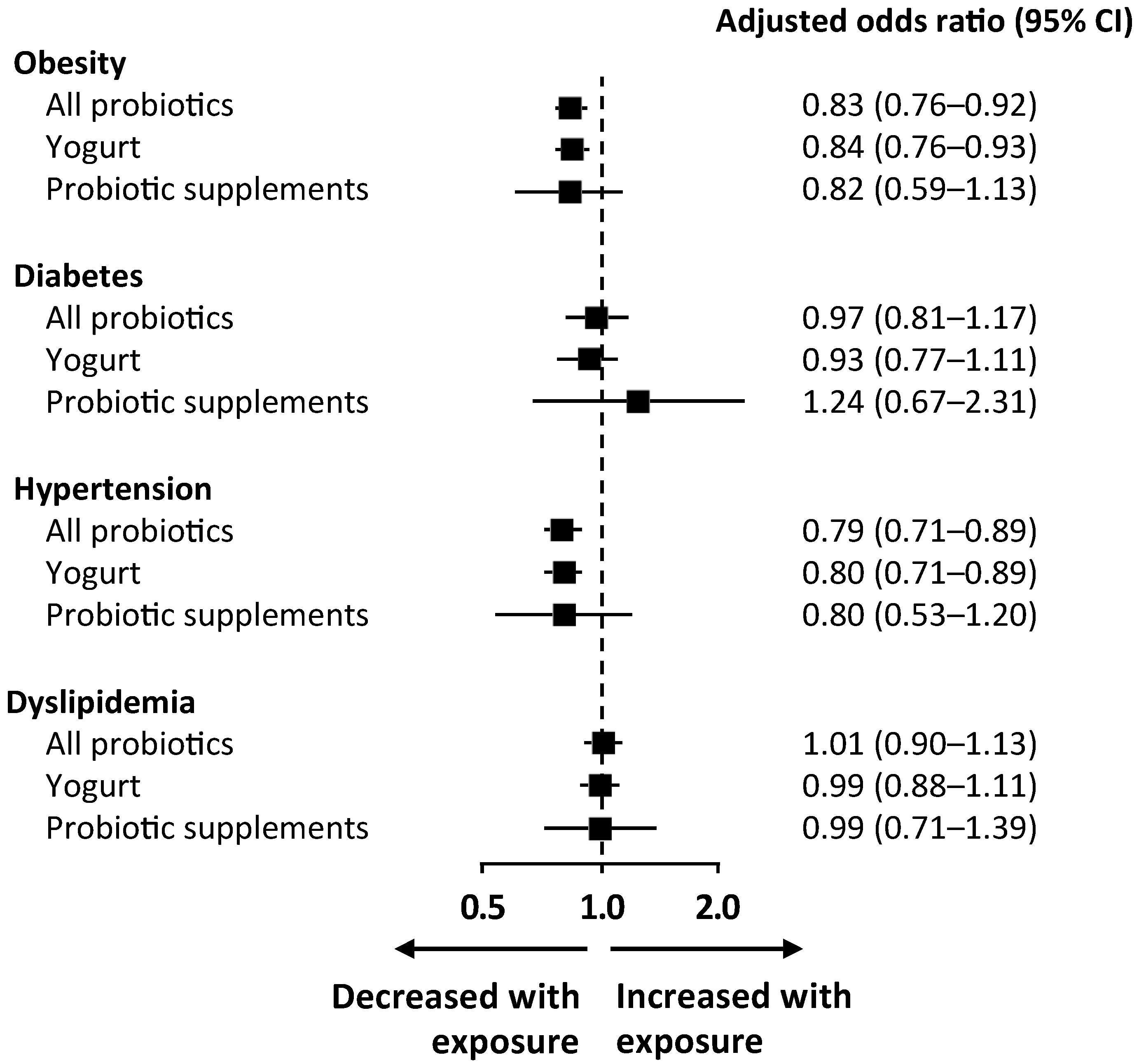
3.3. Modulation of Metabolic Comorbidities According to Probiotic Consumption
4. Discussion
4.1. Obesity
4.2. Diabetes
4.3. Dyslipidemia
4.4. Hypertension
4.5. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar]
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biologics 2011, 5, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The Role of the Gut Microbiota in Energy Metabolism and Metabolic Disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab (Lond) 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guna, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr. Rev. 2018, 39, 133–153. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.J. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- NHANES—National Health and Nutrition Examination Survey Homepage. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 7 June 2018).
- NHANES—Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx. (accessed on 10 June 2018).
- Kim, H.; Andrade, F.C.D. Diagnostic status of hypertension on the adherence to the Dietary Approaches to Stop Hypertension (DASH) diet. Prev. Med. Rep. 2016, 4, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Menke, A.; Rust, K.F.; Fradkin, J.; Cheng, Y.J.; Cowie, C.C. Associations Between Trends in Race/Ethnicity, Aging, and Body Mass Index With Diabetes Prevalence in the United States. Ann. Intern. Med. 2014, 161, 328. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Akbari, V.; Hendijani, F. Effects of probiotic supplementation in patients with type 2 diabetes: Systematic review and meta-analysis. Nutr. Rev. 2016, 74, 774–784. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J. Effect of Probiotics on Blood Lipid Concentrations. Medicine (Baltimore) 2015, 94, e1714. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertens (Dallas Tex. 1979) 2014, 64, 897–903. [Google Scholar] [CrossRef]
- Upadrasta, A.; Madempudi, R.S. Probiotics and blood pressure: Current insights. Integr. Blood Press. Control 2016, 9, 33–42. [Google Scholar]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampe, J.W.; Navarro, S.L.; Hullar, M.A.J.; Shojaie, A. Inter-individual differences in response to dietary intervention: Integrating omics platforms towards personalised dietary recommendations. Proc. Nutr. Soc. 2013, 72, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
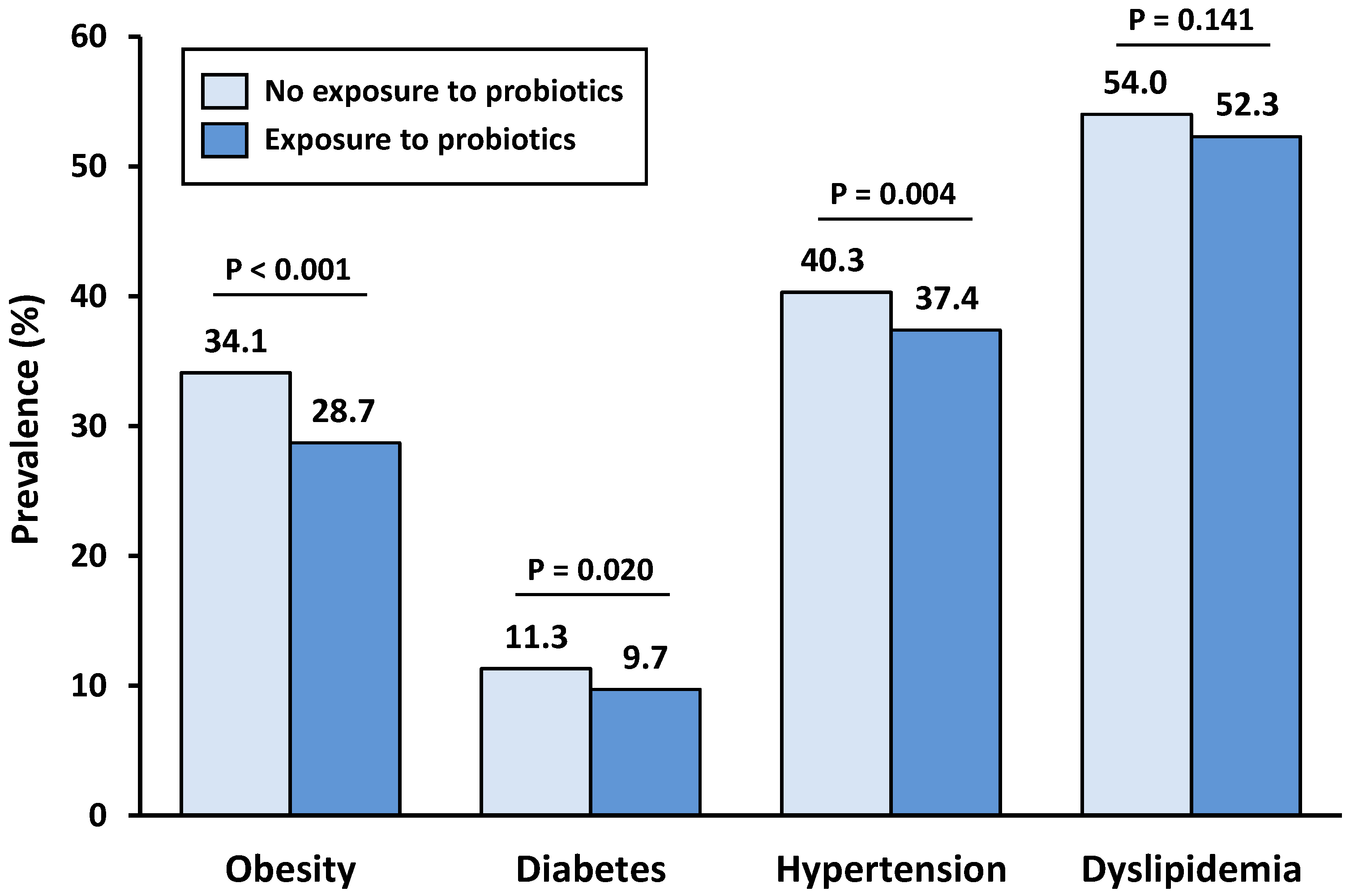
| No Exposure to Probiotics | Exposure to Probiotics | p Value | |
|---|---|---|---|
| Participants, n (%) | 33,719 (86.9%) | 5083 (13.1%) | n.a. |
| Socio-economic characteristics | |||
| Male gender, % | 50.2 % | 35.0 % | <0.001 * |
| Age, years ± SD | 46.0 ± 15.3 | 48.9 ± 13.2 | <0.001 * |
| Annual family income <$25000, % | 30.7% | 19.6% | <0.001 * |
| Education level less than 9th grade, % | 6.4% | 2.7% | <0.001 * |
| Ethnicity | <0.001 * | ||
| Non-Hispanic White, % | 68.4% | 79.5% | |
| Non-Hispanic Black, % | 11.9% | 5.4% | |
| Mexican American, % | 8.5% | 5.0% | |
| Other Hispanic, % | 5.2% | 4.1% | |
| Other ethnicities, % | 6.0% | 5.9% | |
| Risk factors | |||
| Current smokers, % | 20.2% | 7.6% | <0.001 * |
| Former smokers, % | 28.3% | 31.7% | 0.002 * |
| Alcohol consumption >20 g/day, % | 15.7% | 13.9% | 0.028 * |
| Physical activity level # | 0.028 * | ||
| Low | 28.8% | 26.9% | |
| Intermediate | 36.6% | 39.3% | |
| High | 34.6% | 33.8% | |
| Nutritional characteristics | |||
| Kcal/day, kcal ± SD | 2060.7 ± 648.9 | 2042.4 ± 529.3 | 0.163 |
| Carbohydrates/day, g/100 kcal ± SD | 12.3 ± 2.3 | 12.6 ± 1.8 | <0.001 * |
| Protein/day, g/100 kcal ± SD | 3.9 ± 0.9 | 4.2 ±0.8 | <0.001 * |
| Fiber/day, g/100 kcal ± SD | 0.8 ± 0.3 | 0.9 ± 0.3 | <0.001 * |
| Polyunsaturated/saturated fatty acids ratio ± SD | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.001 * |
| Sodium per day, mg | 3389.2 ± 1272.3 | 3282.3 ± 1024.4 | <0.001 * |
| DASH score (0–9) | 2.66 ± 1.15 | 3.33 ± 1.12 | <0.001 * |
| Cardiometabolic parameters | |||
| BMI, kg/m2 | 28.5 ± 5.8 | 27.8 ± 5.1 | <0.001 * |
| HbA1c, % | 5.6 ± 0.8 | 5.5 ± 0.6 | <0.001 * |
| Glucose, mg/dL | 97.9 ± 28.4 | 96.0 ± 22.4 | 0.001 * |
| Systolic BP, mmHg | 122.8 ± 15.7 | 120.5 ± 13.9 | <0.001 * |
| Diastolic BP, mmHg | 71.2 ± 10.1 | 70.2 ± 8.4 | <0.001 * |
| HDL, mg/dL | 51.9 ± 13.6 | 56.7 ± 12.6 | <0.001 * |
| LDL, mg/dL | 116.5 ± 29.5 | 115.5 ± 25.5 | 0.398 |
| Triglycerides, mg/dL | 139.1 ± 100.1 | 121.1 ± 61.5 | <0.001 * |
| Unadjusted, OR (95% CI) | p Value | Model 1, OR (95% CI) | p Value | Model 2, OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Obesity | 0.78 (0.71–0.86) | <0.001 | 0.82 (0.75–0.90) | <0.001 | 0.83 (0.76–0.92) | <0.001 * |
| Diabetes | 0.84 (0.73–0.97) | 0.020 | 0.96 (0.82–1.13) | 0.650 | 0.97 (0.81–1.17) | 0.783 |
| Hypertension | 0.88 (0.81–0.96) | 0.004 | 0.76 (0.68–0.84) | <0.001 | 0.79 (0.71–0.89) | <0.001 * |
| Dyslipidemia | 0.93 (0.85–1.02) | 0.141 | 0.95 (0.86–1.05) | 0.356 | 1.01 (0.90–1.13) | 0.863 |
| Unadjusted | p Value | Model 1 | p Value | Model 2 | p Value | |
|---|---|---|---|---|---|---|
| BMI, kg/m2 | −0.74 (−1.01 to −0.46) | <0.001 * | −0.47 (−0.75 to –0.20) | 0.001 * | −0.41 (−0.67 to −0.15) | 0.002 * |
| HbA1c a, % | −0.03 (−0.05 to −0.01) | 0.003 * | −0.01 (−0.03 to 0.01) | 0.382 | 0.01 (−0.01 to 0.03) | 0.590 |
| Glucose a, mg/dL | −0.94 (−1.71 to −0.18) | 0.016 * | −0.55 (−1.29 to 0.19) | 0.146 | −0.17 (−0.90 to −0.55) | 0.641 |
| Systolic BP b, mmHg | −2.43 (−3.33 to −1.53) | <0.001 * | −1.99 (−2.83 to −1.16) | <0.001 * | −1.48 (−2.31 to −0.66) | <0.001 * |
| Diastolic BP b, mmHg | −0.92 (−1.46 to −0.38) | 0.001 * | −1.13 (−1.71 to −0.55) | <0.001 * | −0.86 (−1.45 to −0.27) | 0.005 * |
| LDL c, mg/dL | 0.03 (−2.53 to 2.59) | 0.980 | −0.93 (−3.50 to 1.63) | 0.472 | −0.02 (−2.59 to 2.55) | 0.988 |
| HDL c, mg/dL | 4.93 (4.10 to 5.75) | <0.001 * | 1.89 (1.12 to 2.66) | <0.001 * | 1.43 (0.69 to 2.17) | <0.001 * |
| Triglycerides c, mg/dL | −16.82 (−22.64 to −10.99) | <0.001 * | −11.74 (−18.14 to −5.33) | <0.001 * | −8.52 (−15.18 to −1.86) | 0.013 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, E.; Neves, J.S.; Ferreira-Magalhães, M.; Carvalho, D.; Freitas, P. Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014. Nutrients 2019, 11, 1482. https://doi.org/10.3390/nu11071482
Lau E, Neves JS, Ferreira-Magalhães M, Carvalho D, Freitas P. Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014. Nutrients. 2019; 11(7):1482. https://doi.org/10.3390/nu11071482
Chicago/Turabian StyleLau, Eva, João Sérgio Neves, Manuel Ferreira-Magalhães, Davide Carvalho, and Paula Freitas. 2019. "Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014" Nutrients 11, no. 7: 1482. https://doi.org/10.3390/nu11071482
APA StyleLau, E., Neves, J. S., Ferreira-Magalhães, M., Carvalho, D., & Freitas, P. (2019). Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014. Nutrients, 11(7), 1482. https://doi.org/10.3390/nu11071482





