Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Study Outcomes
2.3.1. Anthropometric Measures
2.3.2. Cardiovascular Measures
2.3.3. Biochemical Analyses
2.3.4. Oral Glucose Tolerance Test
2.3.5. 24-h Free-Living Appetite Ratings
2.3.6. Acceptance and Palatability Ratings
2.4. Statistical Analyses
3. Results
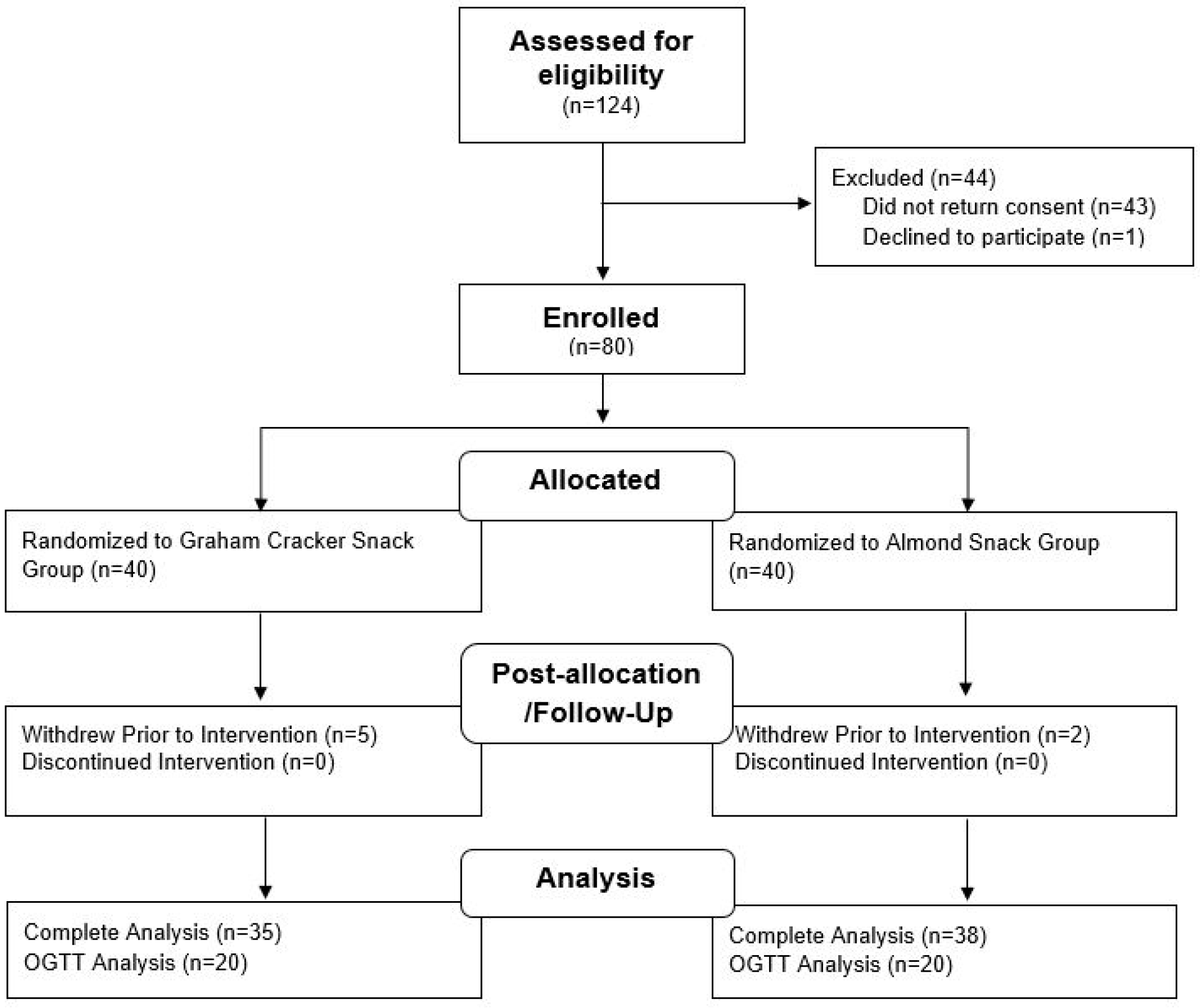
3.1. Participant Characteristics
3.2. Overall Breakfast Consumption and Sleep Habits
3.3. Compliance with Snack Consumption
3.4. BM and Fat-Free Mass Increased over 8 Weeks in Both Snack Groups
3.5. Cardiovascular Outcomes Did Not Change Significantly over 8 Weeks in Both Snack Groups
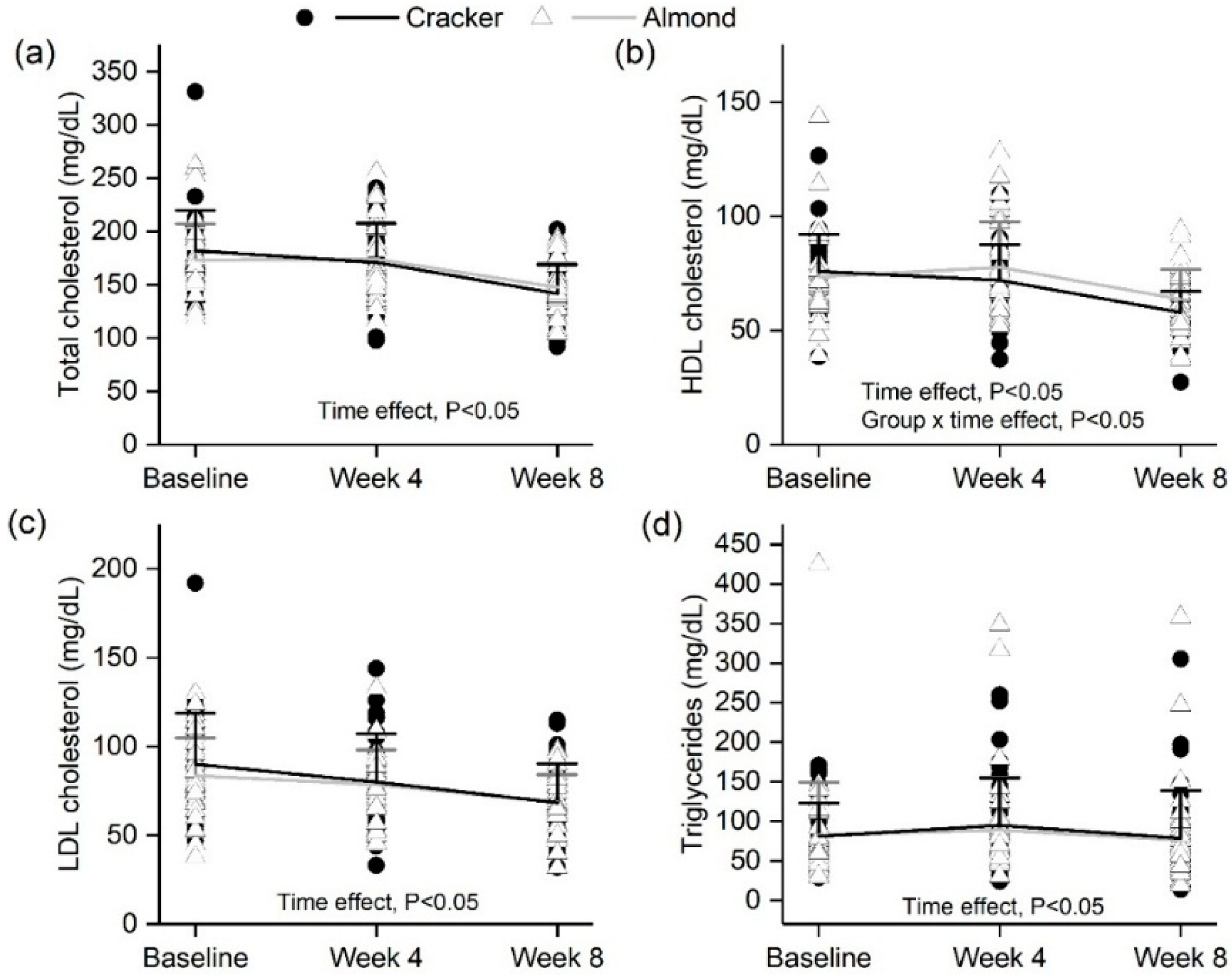
3.6. Almond Group Had a Smaller Decline in Fasting HDL Cholesterol over 8 Weeks Compared to the Cracker Group
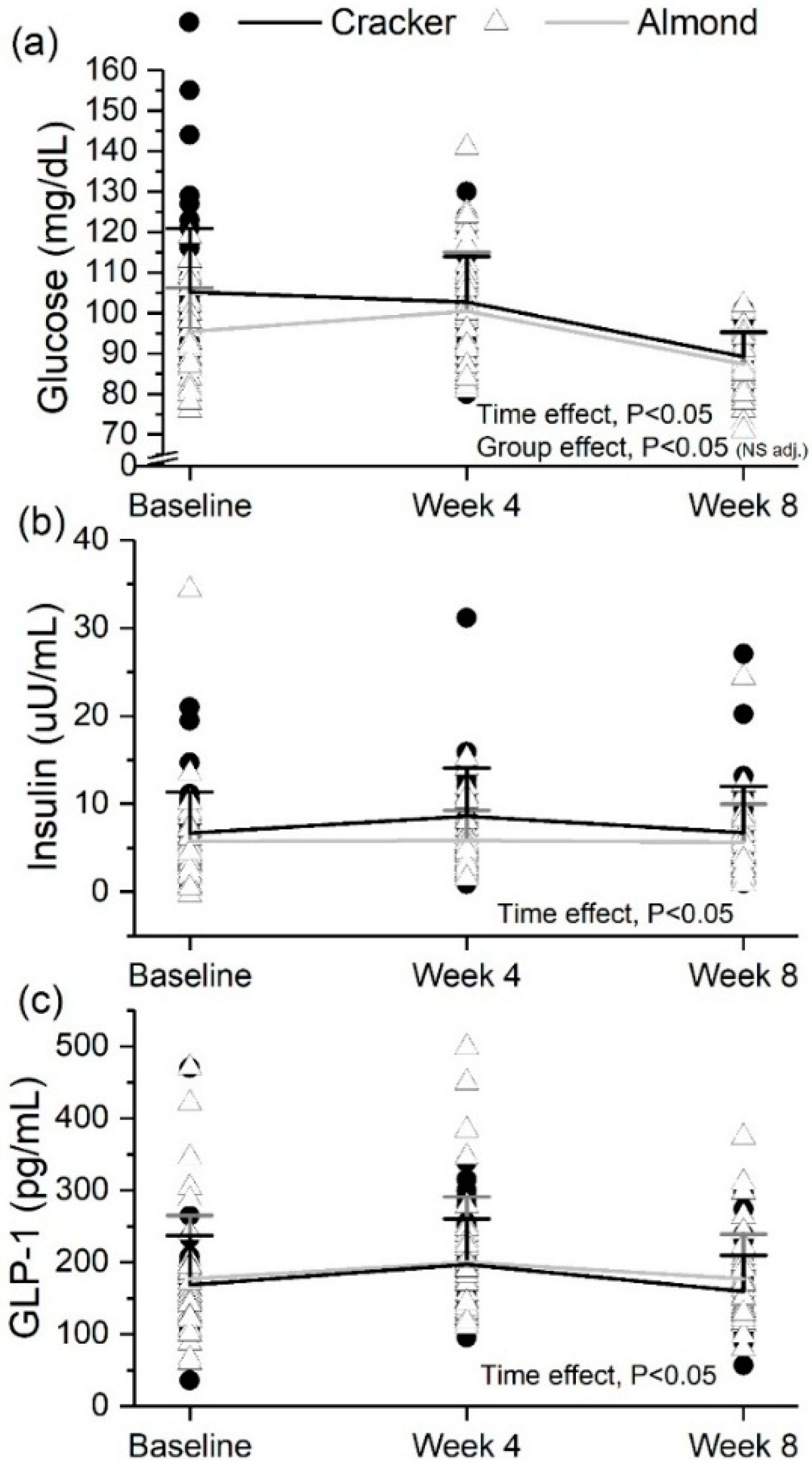
3.7. Fasting Glucose Decreased but Insulin and GLP-1 Did Not Change Significantly over 8 Weeks in Both Snack Groups
3.8. Fasting β-Cell Function Increased over 8 Weeks in Both Snack Groups
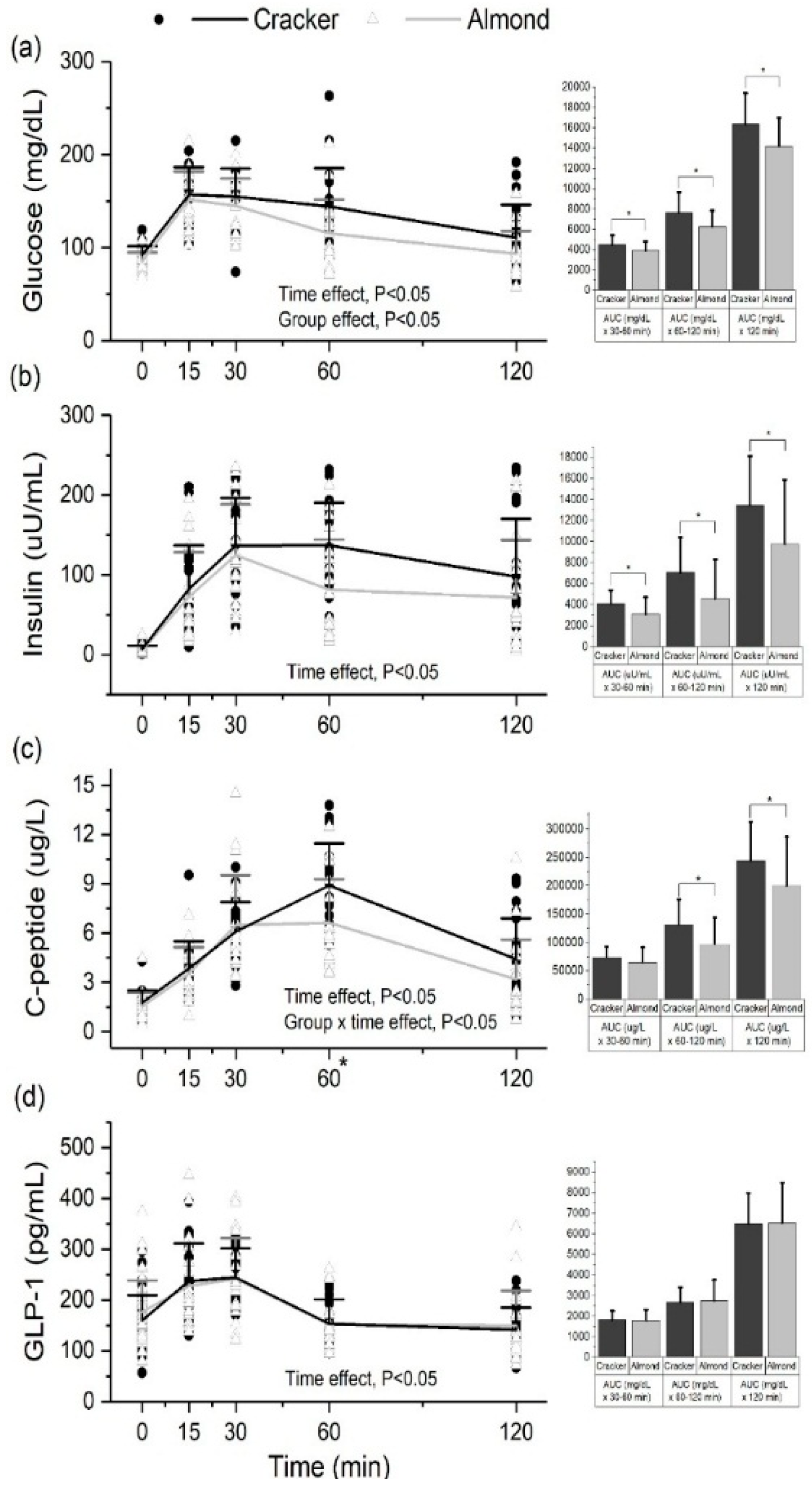
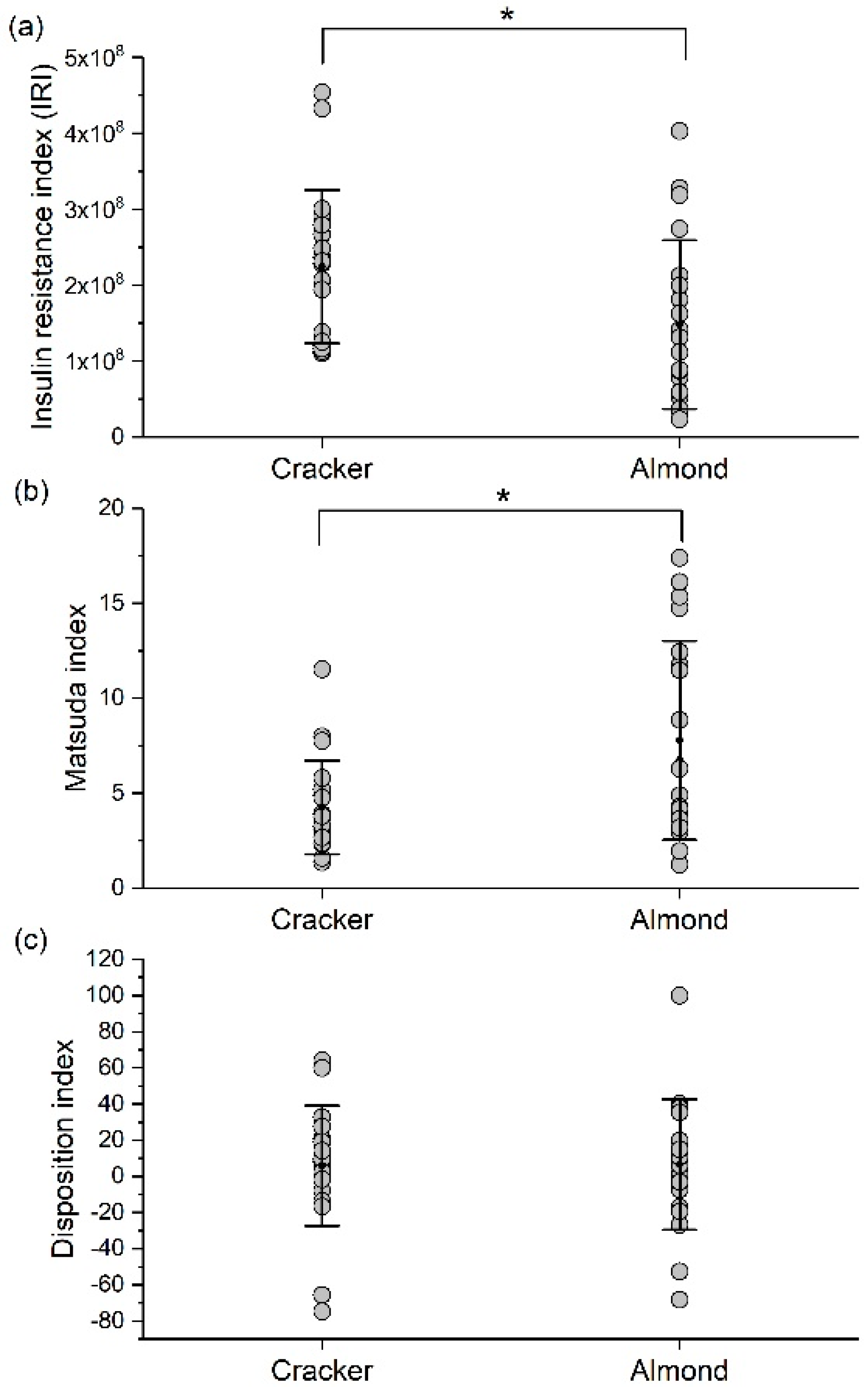
3.9. Almond Group Had Lower Glucose, Insulin and C-Peptide AUC0–120min during the OGTT Compared to the Cracker Group at the End of the 8-Week Intervention
3.10. Almond Group Had Higher Insulin Sensitivity during the OGTT Compared to the Cracker Group at the End of the 8-Week Intervention
3.11. 24-h Free-Living Appetite Ratings Did Not Change Significantly over 8 Weeks in Both Snack Groups
3.12. Snack Palatability and Acceptance Ratings Declined over 8 Weeks but Almonds Had Higher Acceptance Than Crackers
3.13. Activity Energy Expenditure Decreased on the Weekends over the 8-Week Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. Nuts, metabolic syndrome and diabetes. Br. J. Nutr. 2010, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M.; Chen, C.-Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Josse, A.R.; Kendall, C.W.C.; Augustin, L.S.A.; Ellis, P.R.; Jenkins, D.J.A. Almonds and postprandial glycemia—A dose-response study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef] [PubMed]
- MacKay, A.; Duran, C. Adolescent Health in the United States. Available online: https://www.cdc.gov/nchs/data/misc/adolescent2007.pdf (accessed on 1 July 2018).
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- Pendergast, F.J.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Correlates of meal skipping in young adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.E.; Laska, M.N. Balancing healthy meals and busy lives: Associations between work, school and family responsibilities and perceived time constraints among young adults. J. Nutr. Educ. Behav. 2012, 44, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Brevard, P.B.; Ricketts, C.D. Residence of College Students Affects Dietary Intake, Physical Activity, and Serum Lipid Levels. J. Acad. Nutr. Diet. 1996, 96, 35–38. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Song, W.O.; Schemmel, R.A.; Hoerr, S.M. What do college students eat? Food selection and meal pattern. Nutr. Res. 1994, 14, 1143–1153. [Google Scholar] [CrossRef]
- Heatherton, T.; Nichols, P.; Mahamedi, F.; Keel, P. Body weight, dieting, and eating disorder symptoms among college students, 1982 to 1992. Am. J. Psychiatry 1995, 152, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Driskell, J.A.; Kim, Y.-N.; Goebel, K.J. Few differences found in the typical eating and physical activity habits of lower-level and upper-level university students. J. Am. Diet. Assoc. 2005, 105, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.S.; Rockett, H.R.H.; Gillman, M.W.; Field, A.E.; Colditz, G.A. Longitudinal study of skipping breakfast and weight change in adolescents. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Vadeboncoeur, C.; Townsend, N.; Foster, C. A meta-analysis of weight gain in first year university students: Is freshman 15 a myth? BMC Obes. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Crombie, A.P.; Ilich, J.Z.; Dutton, G.R.; Panton, L.B.; Abood, D.A. The freshman weight gain phenomenon revisited. Nutr. Rev. 2009, 67, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.S.; Lofgren, I.E.; Burke, J.D.; Reilly, R.A. Metabolic syndrome, obesity, and related risk factors among college men and women. J. Am. Coll. Health J. ACH 2012, 60, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Abazarfard, Z.; Salehi, M.; Keshavarzi, S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: A randomized controlled clinical trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 457–464. [Google Scholar]
- IDF Consensus Definition of Metabolic Syndrome in Children and Adolescents. Available online: https://www.idf.org/e-library/consensus-statements/61-idf-consensus-definition-of-metabolic-syndrome-in-children-and-adolescents.html (accessed on 1 July 2018).
- Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool. Available online: https://epi.grants.cancer.gov/asa24/ (accessed on 23 November 2017).
- Rowlands, A.V.; Thomas, P.W.M.; Eston, R.G.; Topping, R. Validation of the RT3 triaxial accelerometer for the assessment of physical activity. Med. Sci. Sports Exerc. 2004, 36, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Peripheral Signals Conveying Metabolic Information to the Brain: Short-Term and Long-Term Regulation of Food Intake and Energy Homeostasis. Exp. Biol. Med. 2001, 226, 963–977. [Google Scholar] [CrossRef]
- Havel, P.J. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Jacob, S.; Kinnick, T.R.; Teachey, M.K.; Krekler, M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 2001, 38, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.Y.; Torgan, C.E.; Brozinick, J.T.; Ivy, J.L. Insulin resistance of obese Zucker rats exercise trained at two different intensities. Am. J. Physiol. 1991, 261, E613–E619. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D.; Abdul-Ghani, M.; Musi, N.; Gastaldelli, A. The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J. Clin. Endocrinol. Metab. 2014, 99, 3774–3781. [Google Scholar] [CrossRef] [PubMed]
- A Critical Review of Visual Analogue Scales in the Measurement of Clinical Phenomena—Wewers—1990—Research in Nursing & Health—Wiley Online Library. Available online: http://onlinelibrary.wiley.com/doi/10.1002/nur.4770130405/abstract (accessed on 23 February 2018).
- Schutz, H.G. Food Action Rating Scale for Measuring Food Acceptance. J. Food Sci. 1965, 30, 365–374. [Google Scholar] [CrossRef]
- Kalva, J.J.; Sims, C.A.; Puentes, L.A.; Snyder, D.J.; Bartoshuk, L.M. Comparison of the hedonic general Labeled Magnitude Scale with the hedonic 9-point scale. J. Food Sci. 2014, 79, S238–S245. [Google Scholar] [CrossRef] [PubMed]
- About Child & Teen BMI | Healthy Weight | CDC. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (accessed on 1 July 2018).
- High Blood Cholesterol | National Heart, Lung, and Blood Institute (NHLBI). Available online: https://www.nhlbi.nih.gov/health-topics/high-blood-cholesterol (accessed on 29 June 2018).
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes with A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.A.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Björck, I.M.E. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Most, M.M.; Lefevre, M.; Greenway, F.L.; Rood, J.C. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2005, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Paulionis, L.; Poon, T.; Lee, H.Y. The effects of almond consumption on fasting blood lipid levels: A systematic review and meta-analysis of randomised controlled trials. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Inclusion of Almonds in a Cholesterol-Lowering Diet Improves Plasma HDL Subspecies and Cholesterol Efflux to Serum in Normal-Weight Individuals with Elevated LDL Cholesterol. J. Nutr. 2017, 147, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Cupples, L.A.; Demissie, S.; Horvath, K.V.; Cox, C.E.; Batista, M.C.; Schaefer, E.J. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Voronova, N.V.; Nikitin, A.G.; Chistiakov, A.P.; Chistiakov, D.A. Skipping breakfast is correlated with impaired fasting glucose in apparently healthy subjects. Cent. Eur. J. Med. 2012, 7, 376–382. [Google Scholar] [CrossRef]
- Lazarou, C.; Matalas, A.-L. Breakfast intake is associated with nutritional status, Mediterranean diet adherence, serum iron and fasting glucose: The CYFamilies study. Public Health Nutr. 2015, 18, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Bray, S.R.; Martin Ginis, K.A. Behavior change and the freshman 15: Tracking physical activity and dietary patterns in 1st-year university women. J. Am. Coll. Health J. ACH 2008, 56, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.M.; Black, D.R.; Blue, C.L.; Gretebeck, R.J. Change in diet, physical activity, and body weight in female college freshman. Am. J. Health Behav. 2004, 28, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.J.; Moncur, C. Change in diet, physical activity, and body weight among young-adults during the transition from high school to college. Nutr. J. 2009, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Mialich, M.S.; Sicchieri, J.M.F.; Junior, A.A.J. Analysis of Body Composition: A Critical Review of the Use of Bioelectrical Impedance Analysis. Int. J. Clin. Nutr. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Coppini, L.Z.; Waitzberg, D.L.; Campos, A.C.L. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsu, Y.-L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Rojas-Rueda, D.; Basora, J.; Ros, E.; Salas-Salvadó, J. Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2013, 97, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S.J. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100, 412S–422S. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The Macronutrients, Appetite, and Energy Intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, E.H.; de Graaf, C.; van Trijp, H.C. Effects of variety and repeated in-home consumption on product acceptance. Appetite 2000, 35, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Gray, A.R.; Chisholm, A.W.; Delahunty, C.M.; Brown, R.C. The Dose of Hazelnuts Influences Acceptance and Diet Quality but Not Inflammatory Markers and Body Composition in Overweight and Obese Individuals. J. Nutr. 2013, 143, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Tan, S.-Y.; Mattes, R.D. Almond Consumption during Energy Restriction Lowers Truncal Fat and Blood Pressure in Compliant Overweight or Obese Adults. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cracker (n = 35) | Almond a (n = 38) |
|---|---|---|
| Sex, n (%) | ||
| Male | 16 (45.7) | 16 (42.1) |
| Female | 19 (54.3) | 22 (57.9) |
| Age, years (range) | ||
| 18 | 34 (97) | 38 (100) |
| 19 | 1 (3) | 0 (0) |
| Race/Ethnicity, n (%) | ||
| Hispanic | 16 (45.7) | 15 (39.5) |
| Asian/Pacific Islander | 13 (37.1) | 14 (36.8) |
| African American | 2 (5.7) | 5 (13.2) |
| Caucasian White | 4 (11.4) | 4 (10.5) |
| BMI, kg/m2 | 25.3 ± 4.5 | 25.6 ± 5.0 |
| BMI Category, n (%) | ||
| Normal weight (5th–85th percentile) | 22 (63) | 28 (74) |
| Overweight (85th–95th percentile) | 8 (23) | 6 (16) |
| Obese (≥95th percentile) | 5 (14) | 4 (11) * |
| Waist circumference, cm | ||
| ≥94 (M), ≥80 (F) | 9 (26) | 10 (26) |
| <94 (M), <80 (F) | 26 (74) | 28 (74) |
| Fasting glucose ** | ||
| <100 mg/dL | 13 (37) | 23 (62) |
| ≥100 mg/dL | 22 (63) | 14 (38) |
| HOMA-IR | ||
| <1.0 | 9 (26) | 18 (49) |
| 1.0–2.0 | 15 (43) | 11 (30) |
| ≥2.0 | 11 (31) | 8 (22) |
| Total cholesterol | ||
| <170 mg/dL | 12 (34) | 21 (57) |
| ≥170 mg/dL | 23 (66) | 16 (43) |
| LDL cholesterol | ||
| <100 mg/dL | 23 (66) | 29 (78) |
| ≥100 mg/dL | 12 (34) | 8 (22) |
| HDL cholesterol | ||
| <45 mg/dL | 1 (3) | 1 (3) |
| ≥45 mg/dL | 34 (97) | 36 (97) |
| Triglycerides | ||
| <150 mg/dL | 32 (91) | 36 (97) |
| ≥150 mg/dL | 3 (9) | 1 (3) |
| Systolic BP | ||
| <130 mmHg | 33 (94) | 32 (84) |
| ≥130 mmHg | 2 (6) | 6 (16) |
| Diastolic BP | ||
| <85 mmHg | 35 (100) | 37 (97) |
| ≥85 mmHg | 0 (0) | 1 (3) |
| IDF Metabolic syndrome risk factors | ||
| 0 | 10 (29) | 13 (34) |
| 1 | 14 (40) | 18 (47) |
| 2 | 10 (29) | 6 (16) |
| 3 | 1 (3) | 1 (3) |
| Nutrients | Cracker (n = 35) | Almond (n = 38) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Group | Week | Group × Week | |
| Energy, kcal | 1618.3 ± 524.1 | 1650.4 ± 603.9 | 1624.4 ± 470.2 | 1677.6 ± 714.4 | 1774.3 ± 772.6 | 1722.2 ± 603.4 | 0.366 | 0.793 | 0.942 |
| Carbohydrate, g/day | 205.1 ± 12.9 | 210.9 ± 78.3 | 217.1 ± 70.6 | 188.9 ± 86.6 | 208.9 ± 109.3 | 183.7 ± 69.7 | 0.214 | 0.523 | 0.441 |
| Carbohydrate, % energy | 52 ± 1.8 | 51.8 ± 10.1 | 53.7 ± 9.9 | 45.5 ± 10.4 | 46.3 ± 11.2 | 42.7 ± 10.2 | <0.001 | 0.834 | 0.158 |
| Fat Ɨ, g/day | 62.2 ± 5.4 | 63.3 ± 32.7 | 61 ± 23.1 | 65.9 ± 35 | 74.5 ± 41.8 | 79.9 ± 32.6 | 0.047 | 0.261 | 0.342 |
| Fat, % energy | 33.3 ± 1.4 | 33.5 ± 7.6 | 33.5 ± 7.2 a | 34.8 ± 8.4 | 38 ± 9.7 | 41.7 ± 8.1 | 0.002 | 0.013d | 0.021 |
| Protein Ɨ, g/day | 64.2 ± 7.2 | 62.6 ± 34.9 | 56.3 ± 26.6 | 83.6 ± 51.1 | 73 ± 35.5 | 72 ± 34.2 | 0.014 (NS adj.) | 0.365 | 0.823 |
| Protein Ɨ, % energy | 15.5 ± 1.1 | 15.5 ± 6.5 | 13.9 ± 5.4 | 19.9 ± 8.3 | 17.2 ± 7 | 16.9 ± 6.5 | 0.006 (NS adj.) | 0.04d | 0.504 |
| Dietary fiber, g/day | 13.6 ± 1 | 15.2 ± 7.3 | 13.9 ± 7.3 | 12.5 ± 6.3 | 16.2 ± 8.2 | 13.8 ± 6.2 | 0.979 | 0.016b | 0.542 |
| Total MUFAs Ɨ, g/day | 20.9 ± 2.2 | 22.9 ± 12 | 22 ± 8.6 | 23.3 ± 14.7 | 29.3 ± 17.1 | 31.8 ± 14.1 | 0.013 | 0.014d | 0.224 |
| Total PUFAs Ɨ, g/day | 17.2 ± 1.7 | 15.8 ± 9.2 | 15.2 ± 7.7 | 16.3 ± 10.1 | 17.7 ± 10.6 | 20.1 ± 9.9 | 0.191 | 0.511 | 0.207 |
| Total SFAs Ɨ, g/day | 19.1 ± 1.8 | 19.7 ± 12.1 | 19.2 ± 9.8 | 21.1 ± 12.1 | 21.8 ± 15.3 | 22.2 ± 11.7 | 0.357 | 0.909 | 0.963 |
| Oleic acid Ɨ, g/day | 19.5 ± 2 | 21.4 ± 11.2 | 20.7 ± 8.1 | 21.5 ± 13.6 | 27.6 ± 16.5 | 30.1 ± 13.6 | 0.013 | 0.009bc | 0.223 |
| Linoleic acid Ɨ, g/day | 15.2 ± 1.6 | 14.1 ± 8.3 | 13.7 ± 7 | 14.1 ± 8.9 | 15.8 ± 9.7 | 18.1 ± 8.9 | 0.205 | 0.339 | 0.189 |
| Total alpha-tocopherol, mg/day | 6.5 ± 0.5 | 7.2 ± 5.2 a | 6.5 ± 5.4 a | 6.3 ± 3.7 | 13.2 ± 9.7 | 12.6 ± 8.4 | <0.001 | 0.001 | 0.005 |
| Magnesium, mg/day | 220.9 ± 16.9 | 219.4 ± 81.8 a | 211.1 ± 86 | 230.6 ± 119.4 | 305.6 ± 133 | 261.7 ± 106.6 | 0.01 | 0.020b | 0.027 |
| Selenium Ɨ, mg/day | 88.4 ± 9.7 | 83.7 ± 39.8 | 79.3 ± 36.2 | 119.3 ± 70 | 95.5 ± 57.3 | 95.9 ± 50.3 | 0.040 (NS adj.) | 0.046 | 0.394 |
| Phosphorous Ɨ, mg/day | 998.4 ± 86.9 | 1019 ± 432.9 | 953.8 ± 377.1 | 1167.7 ± 572.3 | 1234.3 ± 538.8 | 1105.7 ± 478.8 | 0.056 | 0.298 | 0.893 |
| Sodium Ɨ, mg/day | 2962.3 ± 248.2 | 2987 ± 1226.7 | 2780.7 ± 1304.3 | 3210.4 ± 1599.3 | 3048.6 ± 1539.7 | 2861.3 ± 1357.9 | 0.6879 | 0.403 | 0.764 |
| Peanuts, tree nuts, and seeds; excludes coconut (oz. eq.) | 0.1 ± 0 | 0.5 ± 1.2 a | 0.5 ± 1.5 a | 0.1 ± 0 bd | 2.2 ± 2.5 | 2.1 ± 2.3 | - | - | <0.001 * |
| Characteristic | Cracker (n = 35) | Almond (n = 38) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Group | Week | Group × Week | |
| Body mass Ɨ, kg | 71.3 ± 15.1 | 71.3 ± 14.7 | 71.9 ± 15 | 71.5 ± 18.6 | 72 ± 18.8 | 72.5 ± 19 | 0.781 | <0.001abc | 0.386 |
| Total fat Ɨ, % | 27.9 ± 9 | 27.5 ± 9.2 | 27.8 ± 9.4 | 26.9 ± 9.5 | 27.2 ± 9.6 | 27 ± 9.4 | 0.926 | 0.461 | 0.856 |
| Total fat mass, kg | 19.9 ± 10.5 | 20 ± 10.1 | 20.5 ± 10.2 | 19.8 ± 11.2 | 20.1 ± 11.1 | 20.1 ± 11.1 | 0.969 | 0.202 | 0.239 |
| Total fat-free mass Ɨ, kg | 50.9 ± 10 | 51.2 ± 9.9 | 51.4 ± 10 | 51.7 ± 12.7 | 51.9 ± 12.7 | 52.4 ± 13 | 0.882 | 0.011bc | 0.233 |
| Trunk fat Ɨ, % | 25.6 ± 9.1 | 25.7 ± 9.4 | 25.9 ± 9.5 | 24 ± 10.8 | 24.8 ± 10.9 | 24.2 ± 10.3 | 0.465 | 0.529 | 0.634 |
| Trunk fat mass Ɨ, kg | 10.2 ± 5.4 | 10.2 ± 5.5 | 10.9 ± 6.4 | 9.7 ± 6.7 | 9.9 ± 6.4 | 9.9 ± 6.2 | 0.462 | 0.539 | 0.747 |
| Trunk fat-free mass, kg | 28.6 ± 5.4 | 27.8 ± 6.9 | 28.7 ± 5.5 | 28.9 ± 6.5 | 28.7 ± 6.4 | 29.1 ± 6.7 | 0.716 | 0.223 | 0.741 |
| Waist circumference Ɨ, cm | 80.4 ± 10.3 | 80.5 ± 10.1 | 80.6 ± 10.5 | 80.5 ± 11.1 | 81.3 ± 11.3 b | 80.2 ± 11.2 | 0.942 | 0.081 | 0.024 |
| HOMA-IR Ɨ | 1.8 ± 1.3 | 2.2 ± 1.4 | 1.5 ± 1.2 | 1.4 ± 1.4 | 1.5 ± 1 | 1.2 ± 1.1 | 0.040 (NS adj.) | <0.001ab | 0.369 |
| HOMA-β Ɨ, % | 58.7 ± 44.7 | 80.9 ± 50.6 | 89.5 ± 61.3 | 68 ± 60 | 61.4 ± 40.2 | 84.3 ± 53.9 | 0.495 | <0.001bc | 0.125 |
| QUICKI Ɨ | 0.38 ± 0.09 | 0.35 ± 0.04 | 0.38 ± 0.05 | 0.39 ± 0.06 | 0.37 ± 0.04 | 0.39 ± 0.05 | 0.052 | <0.001ab | 0.399 |
| Resting systolic blood pressure, mmHg | |||||||||
| Systolic BP | 107.8 ± 15.4 | 106.2 ± 14.6 | 108.5 ± 12.5 | 112.4 ± 14.4 | 109.9 ± 14.3 | 108.2 ± 14.9 | 0.366 | 0.285 | 0.175 |
| Diastolic BP | 67.6 ± 6.5 | 68.6 ± 6.4 | 68.4 ± 6.9 | 67.9 ± 8.5 | 67.1 ± 6.4 | 67.7 ± 7.2 | 0.625 | 0.927 | 0.502 |
| Mean arterial pressure | 81 ± 8.6 | 81.1 ± 8.3 | 81.8 ± 8.1 | 82.7 ± 8.6 | 81.3 ± 8.1 | 81.2 ± 8.1 | 0.797 | 0.727 | 0.321 |
| Reactive hyperemia index (RHI) | - | - | 2.0 ± 0.7 | - | - | 1.9 ± 0.6 | 0.558 * | - | - |
| Augmentation index (AI) | - | - | −8.7 ± 5.7 | - | - | −6.5 ± 10.8 | 0.193 * | - | - |
| Appetite ratings, mm | |||||||||
| Hunger | 31.1 ± 13.3 | 27.4 ± 14.7 | 30.5 ± 13.9 | 34.7 ± 13.7 | 29.2 ± 15.5 | 34.5 ± 12.6 | 0.284 | 0.014ab | 0.721 |
| Fullness | 48.4 ± 16 | 48.5 ± 17.1 | 48.6 ± 16.8 | 47.5 ± 16.5 | 52.5 ± 15.5 | 48.6 ± 14 | 0.731 | 0.343 | 0.347 |
| Desire to eat | 27.8 ± 13.2 | 23 ± 12.5 | 27 ± 15.4 | 32.6 ± 13.2 | 26 ± 14.8 | 30 ± 13.6 | 0.202 | 0.001ab | 0.807 |
| Prospective consumption | 29.5 ± 12.6 | 26.4 ± 14.3 | 29.9 ± 15.8 | 36.4 ± 17.9 | 30.1 ± 15.3 | 33 ± 13.8 | 0.147 | 0.011a | 0.469 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, J.; Thorwald, M.; De La Cruz, N.; Vu, E.; Asghar, S.A.; Kuse, Q.; Diaz Rios, L.K.; Ortiz, R.M. Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial. Nutrients 2018, 10, 960. https://doi.org/10.3390/nu10080960
Dhillon J, Thorwald M, De La Cruz N, Vu E, Asghar SA, Kuse Q, Diaz Rios LK, Ortiz RM. Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial. Nutrients. 2018; 10(8):960. https://doi.org/10.3390/nu10080960
Chicago/Turabian StyleDhillon, Jaapna, Max Thorwald, Natalie De La Cruz, Emily Vu, Syed Asad Asghar, Quintin Kuse, L. Karina Diaz Rios, and Rudy M. Ortiz. 2018. "Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial" Nutrients 10, no. 8: 960. https://doi.org/10.3390/nu10080960
APA StyleDhillon, J., Thorwald, M., De La Cruz, N., Vu, E., Asghar, S. A., Kuse, Q., Diaz Rios, L. K., & Ortiz, R. M. (2018). Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial. Nutrients, 10(8), 960. https://doi.org/10.3390/nu10080960





