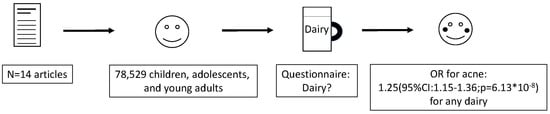
Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Procedure for Selection of Studies
2.4. Data Extraction and Management
2.5. Overall and Subgroup Analyses
2.6. Risk of Bias and Study Quality Assessment
2.7. Statistical Analyses
3. Results
3.1. Description of the Studies
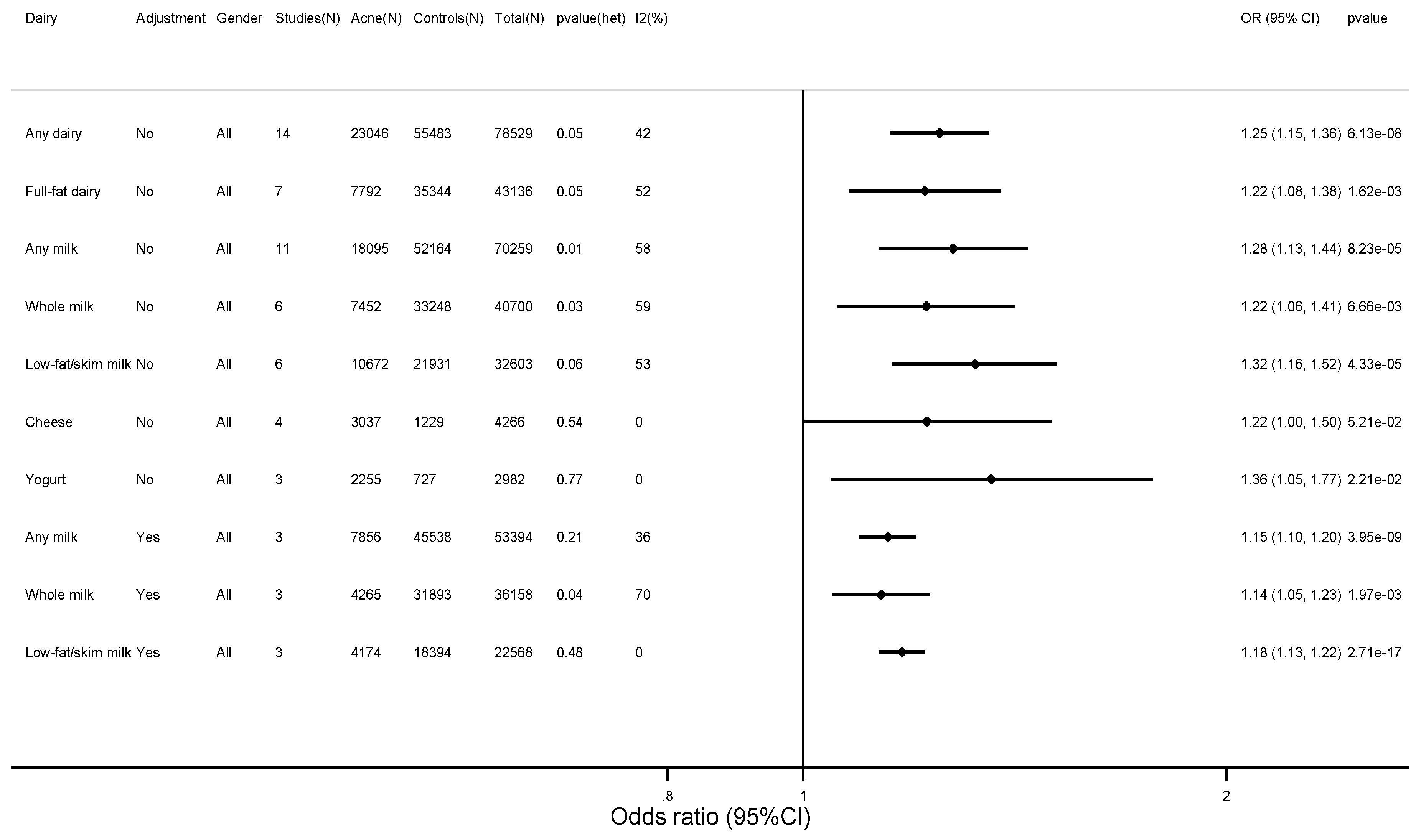
3.2. Findings
3.3. Sensitivity Analyses, Heterogeneity, Publication Bias, and Qualitative Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Schmitz, G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp. Dermatol. 2009, 18, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- White, G.M. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J. Am. Acad. Dermatol. 1998, 39, S34–S47. [Google Scholar] [CrossRef]
- Rademaker, M.; Garioch, J.J.; Simpson, N.B. Acne in schoolchildren: No longer a concern for dermatologists. BMJ 1989, 298, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, S.Z.; Orawa, H.; Zouboulis, C.C. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Bettoli, V.; Araviiskaia, E.; Sanchez Viera, M.; Bouloc, A. The influence of exposome on acne. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.H.; Ferdowsian, H.R.; Barnard, N.D. Diet and acne: A review of the evidence. Int. J. Dermatol. 2009, 48, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Ganmaa, D.; Pollak, M.N.; Nakamoto, E.K.; Kleinman, K.; Tserendolgor, U.; Willett, W.C.; Frazier, A.L. Milk consumption and the prepubertal somatotropic axis. Nutr. J. 2007, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Pollak, M.N.; Willett, W.C.; Hankinson, S.E. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 852–861. [Google Scholar] [PubMed]
- Rahaman, S.M.A.; De, D.; Handa, S.; Pal, A.; Sachdeva, N.; Ghosh, T.; Kamboj, P. Association of insulin-like growth factor (IGF)-1 gene polymorphisms with plasma levels of IGF-1 and acne severity. J. Am. Acad. Dermatol. 2016, 75, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadi, Y.; Thielitz, A.; Wiede, A.; Goihl, A.; Papakonstantinou, E.; Hartig, R.; Zouboulis, C.C.; Reinhold, D.; Simeoni, L.; Bommhardt, U.; et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol. Cell. Endocrinol. 2015, 415, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Tasli, L.; Turgut, S.; Kacar, N.; Ayada, C.; Coban, M.; Akcilar, R.; Ergin, S. Insulin-like growth factor-I gene polymorphism in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Spiegelman, D.; Danby, F.W.; Frazier, A.L.; Willett, W.C.; Holmes, M.D. High school dietary dairy intake and teenage acne. J. Am. Acad. Dermatol. 2005, 52, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Spiegelman, D.; Berkey, C.S.; Danby, F.W.; Rockett, H.H.; Colditz, G.A.; Willett, W.C.; Willett, W.C. Milk consumption and acne in teenaged boys. J. Am. Acad. Dermatol. 2008, 58, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Spiegelman, D.; Berkey, C.S.; Danby, F.W.; Rockett, H.H.; Colditz, G.A.; Willett, W.C.; Holmes, M.D. Milk consumption and acne in adolescent girls. Dermatol. Online J. 2006, 12, 1. [Google Scholar] [PubMed]
- Ismail, N.H.; Manaf, Z.A.; Azizan, N.Z. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: A case control study. BMC Dermatol. 2012, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Di Landro, A.; Cazzaniga, S.; Parazzini, F.; Ingordo, V.; Cusano, F.; Atzori, L.; Cutrì, F.T.; Musumeci, M.L.; Zinetti, C.; Pezzarossa, E.; et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J. Am. Acad. Dermatol. 2012, 67, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, P.; Misery, L.; Amici, J.M.; Maghia, R.; Branchoux, S.; Cazeau, C.; Voisard, J.-J.; Taïeb, C. Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: Results of a survey using a representative sample. Dermatology 2015, 230, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Okoro, E.O.; Ogunbiyi, A.O.; George, A.O.; Subulade, M.O. Association of diet with acne vulgaris among adolescents in Ibadan, southwest Nigeria. Int. J. Dermatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cerman, A.A.; Aktas, E.; Altunay, I.K.; Arici, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016. [Google Scholar] [CrossRef]
- Grossi, E.; Cazzaniga, S.; Crotti, S.; Naldi, L.; Di Landro, A.; Ingordo, V.; Cusano, F.; Atzori, L.; Cutrì, F.T.; Musumeci, M.L.; et al. The constellation of dietary factors in adolescent acne: A semantic connectivity map approach. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Yoon, M.Y.; Min, S.U.; Hong, J.S.; Choi, Y.S.; Suh, D.H. The influence of dietary patterns on acne vulgaris in Koreans. Eur. J. Dermatol. 2010, 20, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Ulvestad, M.; Bjertness, E.; Dalgard, F.; Halvorsen, J.A. Acne and dairy products in adolescence: Results from a Norwegian longitudinal study. J. Eur. Acad. Dermatol. Venereol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Balta, I.; Saricaoglu, H.; Kilic, S.; Kelekci, K.H.; Yildirim, M.; Arica, D.A.; Öztürk, S.; Karaman, G.; Cerman, A.A.; et al. The effect of personal, familial, and environmental characteristics on acne vulgaris: A prospective, multicenter, case controlled study from Turkey. G. Ital. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Machovcova, A.; Szepietowski, J.C.; Tennstedt, D.; Veraldi, S.; Delarue, A. Acne prevalence and associations with lifestyle: A cross-sectional online survey of adolescents/young adults in 7 European countries. J. Eur. Acad. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Veith, W.B.; Silverberg, N.B. The association of acne vulgaris with diet. Cutis 2011, 88, 84–91. [Google Scholar] [PubMed]
- Aghasi, M.; Golzarand, M.; Shab-Bidar, S.; Aminianfar, A.; Omidian, M.; Taheri, F. Dairy intake and acne development: A meta-analysis of observational studies. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tsoy, N. The Influence of Dietary Habits on Acne. World J. Med. Sci. 2013, 8, 212. [Google Scholar] [CrossRef]
- Tsoy, N.O. Effect of Milk and Dairy Products upon Severity of Acne for Young People. World Appl. Sci. J. 2013, 24, 403–417. [Google Scholar] [CrossRef]
- Burris, J.; Rietkerk, W.; Woolf, K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J. Acad. Nutr. Diet. 2014, 114, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Poli, F.; Auffret, N.; Beylot, C.; Chivot, M.; Faure, M.; Moyse, D.; Henri, P.; Jean, R.; Brigitte, D. Acne as seen by adolescents: Results of questionnaire study in 852 French individuals. Acta Derm. Venereol. 2011, 91, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Semedo, D.; Ladeiro, F.; Ruivo, M.; D’Oliveira, C.; De Sousa, F.; Gayo, M.; Amado, J.; Lima, C.; Magalhães, F.; Brandão, R.; et al. Adult Acne: Prevalence and Portrayal in Primary Healthcare Patients, in the Greater Porto Area, Portugal. Acta. Med. Port. 2016, 29, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Law, M.P.; Chuh, A.A.; Molinari, N.; Lee, A. An investigation of the association between diet and occurrence of acne: a rational approach from a traditional Chinese medicine perspective. Clin. Exp Dermatol. 2010, 35, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Seleit, I.; Bakry, O.A.; Abdou, A.G.; Hashim, A. Body mass index, selected dietary factors, and acne severity: Are they related to in situ expression of insulin-like growth factor-1? Anal. Quant. Cytopathol. Histpathol. 2014, 36, 267–278. [Google Scholar] [PubMed]
- Dreno, B.; Thiboutot, D.; Layton, A.M.; Berson, D.; Perez, M.; Kang, S. Large-scale international study enhances understanding of an emerging acne population: Adult females. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Di Landro, A.; Cazzaniga, S.; Cusano, F.; Bonci, A.; Carla, C.; Musumeci, M.L.; Patrizi, A.; Bettoli, V.; Pezzarossa, E.; Caproni, M.; et al. Adult female acne and associated risk factors: Results of a multicenter case-control study in Italy. J. Am. Acad. Dermatol. 2016, 75, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Skroza, N.; Tolino, E.; Semyonov, L.; Proietti, I.; Bernardini, N.; Nicolucci, F.; Viola, G.L.; Prete, G.D.; Saulle, R.; Potenza, C.; et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand. J. Public Health 2012, 40, 466–474. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, C.L.; Quach, K.A.; Koons, K.; Kunselman, A.R.; Zhu, J.; Thiboutot, D.M.; Zaenglein, A.L. Consumption of dairy in teenagers with and without acne. J. Am. Acad. Dermatol. 2016, 75, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Agamia, N.F.; Abdallah, D.M.; Sorour, O.; Mourad, B.; Younan, D.N. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016, 174, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Newcastle—Ottawa Quality Assessment Scale Case Control Studies. Available online: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed on 12 December 2017).
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Pereira Duquia, R.; da Silva Dos Santos, I.; de Almeida, H., Jr.; Martins Souza, P.R.; de Avelar Breunig, J.; Zouboulis, C.C. Epidemiology of Acne Vulgaris in 18-Year-Old Male Army Conscripts in a South Brazilian City. Dermatology 2017, 233, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kwon, H.H.; Min, S.; Yoon, J.Y.; Suh, D.H. Epidemiology and risk factors of childhood acne in Korea: A cross-sectional community based study. Clin. Exp. Dermatol. 2015, 40, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Itan, Y.; Jones, B.L.; Ingram, C.J.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://handbook-5-1.cochrane.org/ (accessed on 12 December 2017).
- Menon, C.; Gipson, K.; Bowe, W.P.; Hoffstad, O.J.; Margolis, D.J. Validity of subject self-report for acne. Dermatology 2008, 217, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.C. Foods as the cause of acne. Am. Fam. Physician 1971, 3, 102–113. [Google Scholar] [PubMed]
- Bergholdt, H.K.M.; Nordestgaard, B.G.; Varbo, A.; Ellervik, C. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98,529 Danish adults. Int. J. Epidemiol. 2015, 44, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Juhl, C.R.; Bergholdt, H.K.M.; Miller, I.M.; Jemec, G.B.; Kanters, J.K.; Ellervik, C. Lactase persistence, milk intake and adult acne: A Mendelian randomization study of 20,416 Danish adults. Nutrients 2018, 10, 1041. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Toward a new philosophy of preventive nutrition: From a reductionist to a holistic paradigm to improve nutritional recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.P.; de Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C.; et al. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Milk—A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. Int. J. Mol. Sci. 2015, 16, 17048–17087. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Mann, N.J.; Braue, A.; Makelainen, H.; Varigos, G.A. A low-glycemic-load diet improves symptoms in acne vulgaris patients: A randomized controlled trial. Am. J. Clin. Nutr. 2007, 86, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Lindeberg, S.; Hurtado, M.; Hill, K.; Eaton, S.B.; Brand-Miller, J. Acne vulgaris: A disease of Western civilization. Arch. Dermatol. 2002, 138, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Elmstahl, H.L.; Bjorck, I. Milk as a supplement to mixed meals may elevate postprandial insulinaemia. Eur. J. Clin. Nutr. 2001, 55, 994–1009. [Google Scholar] [CrossRef] [PubMed]

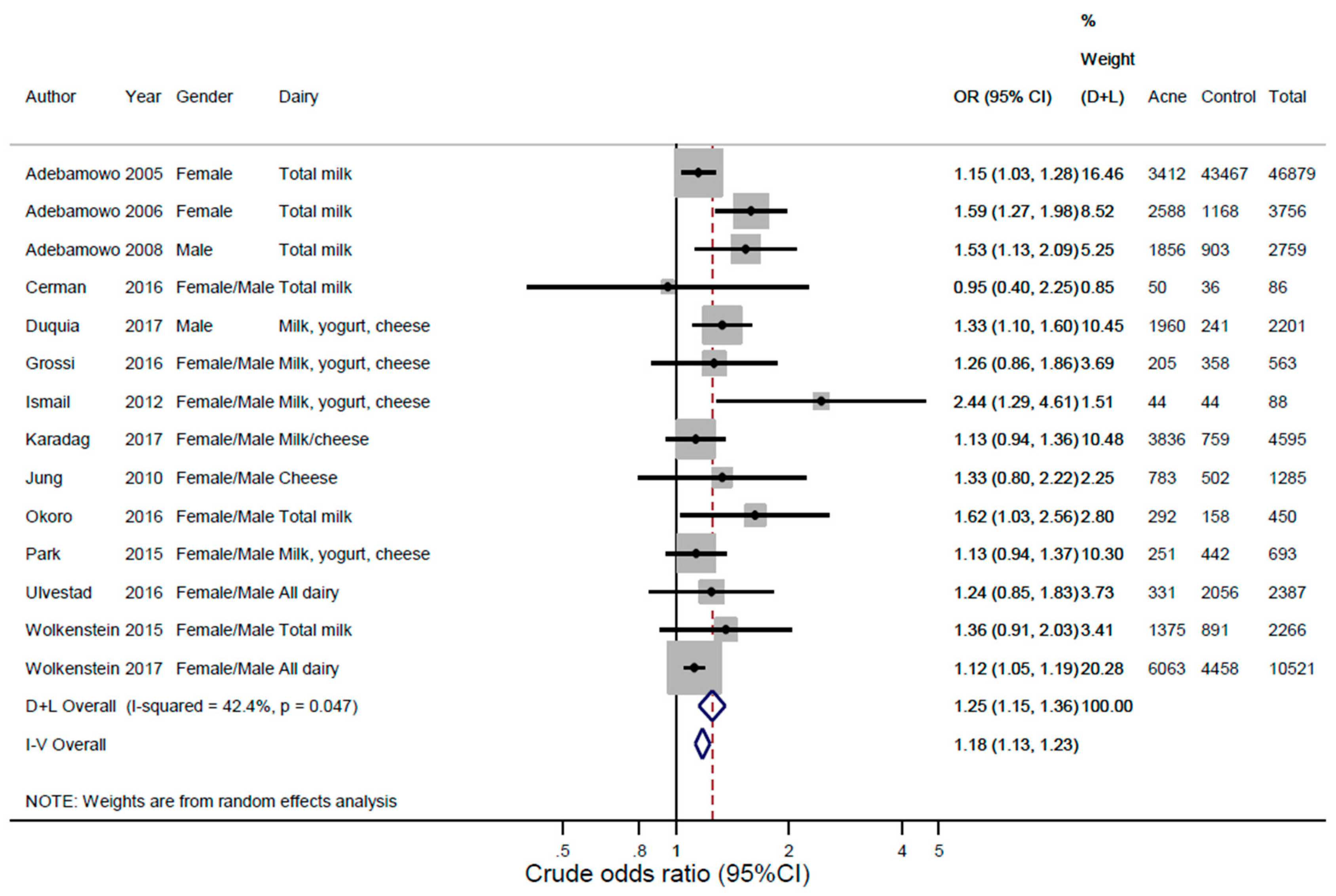
| First Author | Year | Study Population | Design | Age, Year | Gender | Country | Total n | Acne n | Acne (%) | No Acne n | Milk Variables | Acne Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adebamowo [15] | 2005 | Population cohort (Nurses’ Health Study II) | Retrospective | 13–18 | F | USA | 46,879 | 3412 | 7.28 | 43,467 | Any milk, whole milk, low-fat milk, skim milk | Q |
| Adebamowo [17] | 2006 | Population cohort (GUTS)—offspring of women in the Nurses’ Health Study II | Follow-up | 9–15 | F | USA | 3756 | 2588 | 68.9 | 1168 | Any milk, whole milk, low-fat milk, skim milk | Q |
| Adebamowo [16] | 2008 | Population cohort (GUTS)—offspring of women in the Nurses’ Health Study II | Follow-up | 9–15 | M | USA | 2759 | 1856 | 67.3 | 903 | Any milk, whole milk, low-fat milk, skim milk | Q |
| Cerman [22] | 2016 | Acne patients and healthy controls | Case-control | 19 | F/M | Turkey | 86 | 50 | 58.1 | 36 | Any milk | D |
| Duquia [46] | 2017 | Acne patients vs. healthy controls in the army | Cross-sectional | 18 | M | Brasil | 2201 | 1960 | 89.1 | 241 | Whole milk, low-fat milk, cheese, yogurt | P |
| Grossi [23] | 2016 | Acne patients and non-acne dermatology patient controls | Case-control | 10–24 | F/M | Italy | 563 | 205 | 36.4 | 358 | Any milk, whole milk, skim milk, cheese/yogurt combined | D |
| Ismail [18] | 2012 | Acne patients and healthy controls | Case-control | 18–30 | F/M | Malaysia | 88 | 44 | 50 | 44 | Any milk, yogurt, cheese | D |
| Karadag [26] | 2017 | Acne patients and non-acne dermatology patient controls | Case-control | 21 | M | Turkey | 4595 | 3836 | 83.5 | 759 | Milk/cheese combined | D |
| Jung [24] | 2010 | Acne patients and age-matched healthy controls | Case-control | 24 | F/M | South Korea | 1285 | 783 | 60.9 | 502 | Cheese | D |
| Okoro [21] | 2016 | Population cohort | Cross-sectional | 11–30 | F/M | Nigeria | 450 | 292 | 64.9 | 158 | Any milk | D |
| Park [47] | 2015 | Population cohort | Cross-sectional | 7–12 | F/M | South Korea | 693 | 251 | 36.2 | 442 | Any milk, cheese, yogurt | D |
| Ulvestad [25] | 2016 | Population cohort | Follow-up | 15–19 | F/M | Norway | 2387 | 331 | 13.9 | 2056 | Any milk, full-fat milk | Q |
| Wolkenstein [20] | 2015 | Population cohort | Cross-sectional | 15–24 | F/M | France | 2266 | 1375 | 60.7 | 891 | Any milk | Q |
| Wolkenstein [27] | 2017 | Population cohort | Cross-sectional | 15–24 | F/M | Europe * | 10521 | 6063 | 57.6 | 4458 | Whole milk, semi-skimmed milk, low-fat milk, dairy | Q |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhl, C.R.; Bergholdt, H.K.M.; Miller, I.M.; Jemec, G.B.E.; Kanters, J.K.; Ellervik, C. Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults. Nutrients 2018, 10, 1049. https://doi.org/10.3390/nu10081049
Juhl CR, Bergholdt HKM, Miller IM, Jemec GBE, Kanters JK, Ellervik C. Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults. Nutrients. 2018; 10(8):1049. https://doi.org/10.3390/nu10081049
Chicago/Turabian StyleJuhl, Christian R., Helle K. M. Bergholdt, Iben M. Miller, Gregor B. E. Jemec, Jørgen K. Kanters, and Christina Ellervik. 2018. "Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults" Nutrients 10, no. 8: 1049. https://doi.org/10.3390/nu10081049
APA StyleJuhl, C. R., Bergholdt, H. K. M., Miller, I. M., Jemec, G. B. E., Kanters, J. K., & Ellervik, C. (2018). Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults. Nutrients, 10(8), 1049. https://doi.org/10.3390/nu10081049