Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Substance Questionnaire
2.3. MRI Data Acquisition
2.4. Statistical Analysis of Demographic and Substance Data
2.5. MR Data Analysis
2.5.1. Whole-Brain Voxel-Based Morphometry (VBM)
2.5.2. Arterial Spin Labelling (ASL)
2.5.3. Diffusion Tensor Imaging (DTI) Tract Based Spatial Statistics (TBSS)
2.5.4. GM Region of Interest (ROI) Analysis
3. Results
3.1. Clinical, Demographic and Substance Data
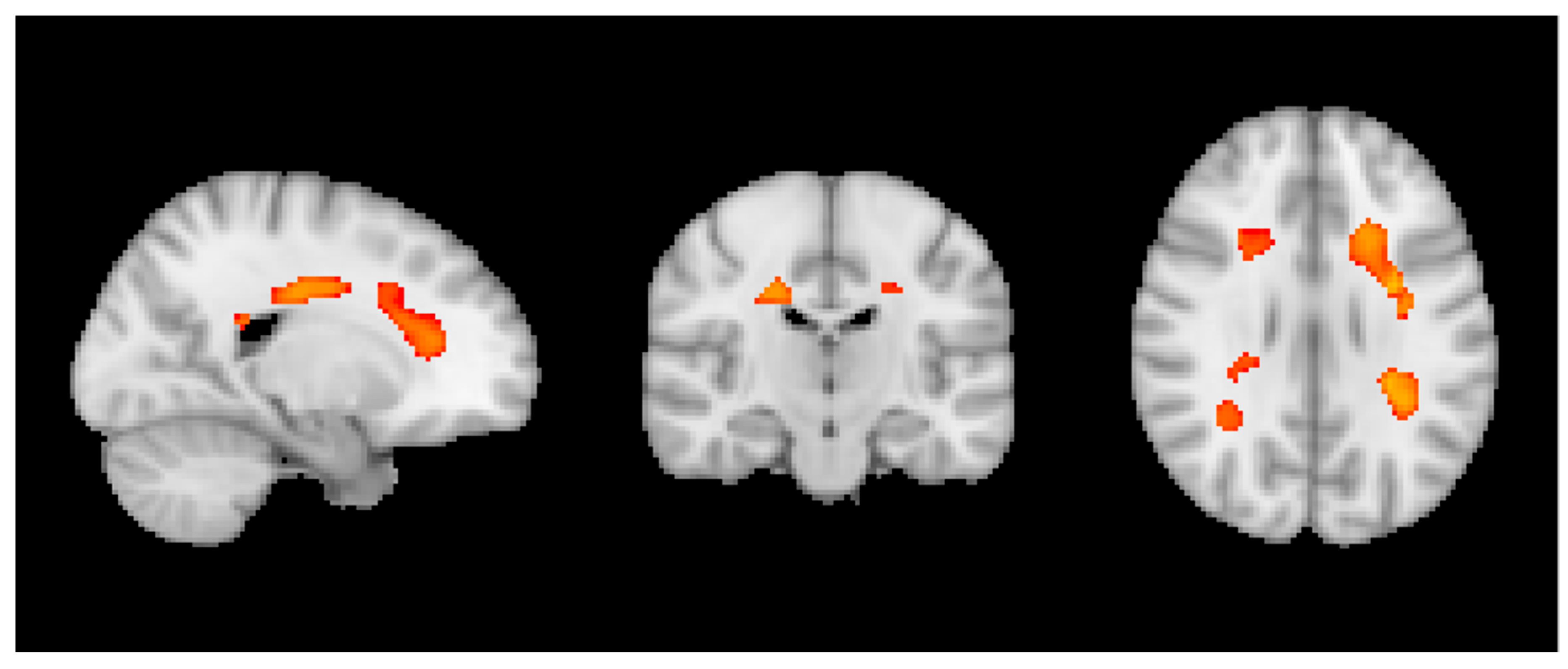
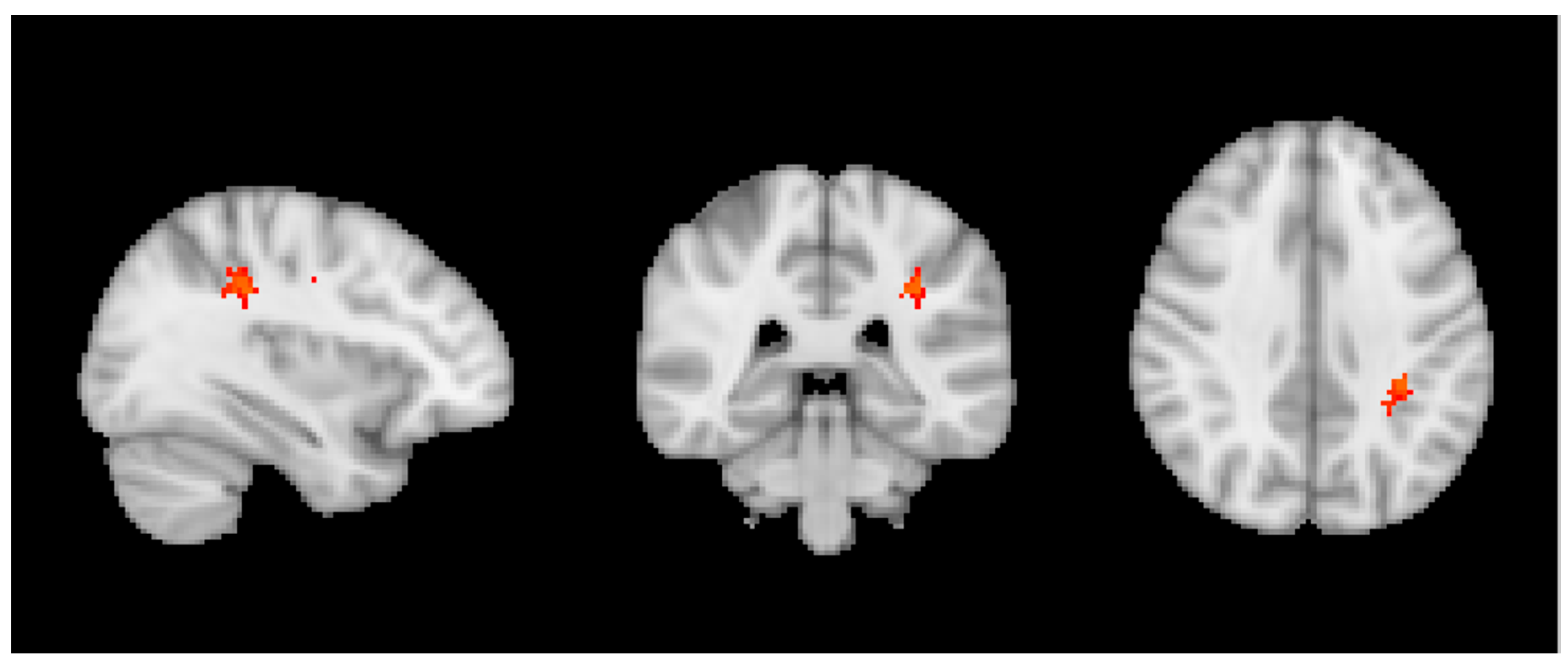
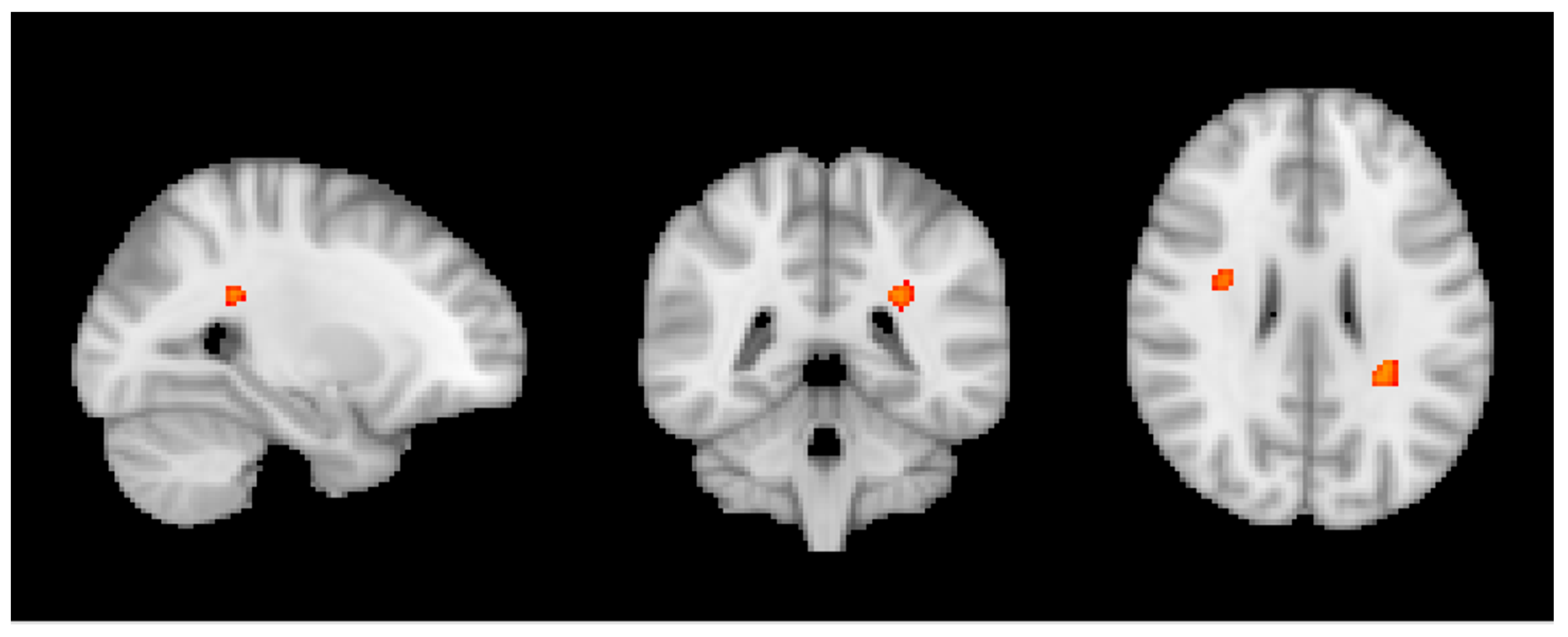
3.2. MRI Analysis across the Entire Group
3.3. Group MRI Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.; de Mendonca, A. Does caffeine intake protect from Alzheimer’s disease? Eur. J. Neurol. 2002, 9, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, B.M.; Buijsse, B.; Tijhuis, M.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Coffee consumption is inversely associated with cognitive decline in elderly european men: The fine study. Eur. J. Clin. Nutr. 2006, 61, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimers Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Artero, S.; Portet, F.; Brickman, A.; Muraskin, J.; Beanino, E.; Ancelin, M.; Carrière, I. Caffeine, cognitive functioning, and white matter lesions in the elderly: Establishing causality from epidemiological evidence. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S161–S166. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Chang, W.-H.; Black, R.M.; Liu, J.-R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid β1–42 levels in an alzheimer’s mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F.; Imbimbo, B.P.; D’Introno, A.; Galluzzo, L.; Gandin, C.; Misciagna, G.; Guerra, V.; Osella, A.; Baldereschi, M.; et al. Coffee consumption habits and the risk of mild cognitive impairment: The Italian longitudinal study on aging. J. Alzheimers Dis. 2015, 47, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Ilomaki, J.; Jokanovic, N.; Tan, E.C.; Lonnroos, E. Alcohol consumption, dementia and cognitive decline: An overview of systematic reviews. Curr. Clin. Pharmacol. 2015, 10, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Santamato, A.; Imbimbo, B.P.; Scafato, E.; Pilotto, A.; Solfrizzi, S. Alcohol consumption in mild cognitive impairment and dementia: Harmful or neuroprotective. Int. J. Geriatr. Psychiatry 2012, 27, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Ebmeier, K.P. Effects of drinking on late-life brain and cognition. Evid. Based Ment. Health 2018, 21, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing human cognition with cocoa flavonoids. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, E.B.; de Groot, R.H.M.; Evers, E.A.T.; Snel, J.; Veerman, E.C.I.; Ligtenberg, A.J.M.; Jolles, J.; Veltman, D.J. The effect of caffeine on working memory load-related brain activation in middle-aged males. Neuropharmacology 2013, 64, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, R.; Greyling, A.; Draijer, R.; Corfield, D.R.; Parkes, L.M. The effect of black tea and caffeine on regional cerebral blood flow measured with arterial spin labeling. J. Cereb. Blood Flow Metab. 2013, 33, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral blood flow measurements in adults: A review on the effects of dietary factors and exercise. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J. Alzheimers Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine A2a receptor antagonists prevent β-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O.; et al. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J. Alzheimers Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.Q. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2013, 8, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, L.; Lin, X.; Mamcarz, M.; Zhang, C.; Bai, G.; Nong, J.; Sussman, S.; Arendash, G. Caffeine synergizes with another coffee component to increase plasma GCSF: Linkage to cognitive benefits in Alzheimer’s mice. J. Alzheimers Dis. 2011, 25, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Laurienti, P.J.; Field, A.S.; Burdette, J.H.; Maldjian, J.A.; Yen, Y.-F.; Moody, D.M. Dietary caffeine consumption modulates fmri measures. NeuroImage 2002, 17, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Rack-Gomer, A.L.; Liau, J.; Liu, T.T. Caffeine reduces resting-state bold functional connectivity in the motor cortex. NeuroImage 2009, 46, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Olafsson, V.; Tal, O.; Liu, T.T. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional mri. NeuroImage 2012, 63, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Tal, O.; Diwakar, M.; Wong, C.-W.; Olafsson, V.; Lee, R.; Huang, M.-X.; Liu, T.T. Caffeine-induced global reductions in resting-state bold connectivity reflect widespread decreases in meg connectivity. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Urrutia, J.C.; Burgos, C.F.; Silva, V.; Aguilar, F.; Sama, M.; Yeh, H.H.; Opazo, C.; Aguayo, L.G. Low concentrations of ethanol protect against synaptotoxicity induced by aβ in hippocampal neurons. Neurobiol. Aging 2015, 36, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Vergara, V.M.; Liu, J.; Claus, E.D.; Hutchison, K.; Calhoun, V. Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage 2017, 151, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Spagnolli, F.; Cerini, R.; Cardobi, N.; Barillari, M.; Manganotti, P.; Storti, S.; Mucelli, R.P. Brain modifications after acute alcohol consumption analyzed by resting state fMRI. Magn. Reson. Imaging 2013, 31, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Varghese, M.; Ono, K.; Yamada, M.; Levine, S.; Tzavaras, N.; Gong, B.; Hurst, W.J.; Blitzer, R.D.; Pasinetti, G.M. Cocoa extracts reduce oligomerization of amyloid-β: Implications for cognitive improvement in Alzheimer’s disease. J. Alzheimers Dis. 2014, 41, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa powder triggers neuroprotective and preventive effects in a human alzheimer’s disease model by modulating bdnf signaling pathway. J. Cell. Biochem. 2013, 114, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Verbaten, M.N. Chronic effects of low to moderate alcohol consumption on structural and functional properties of the brain: Beneficial or not? Hum. Psychopharmacol. Clin. Exp. 2009, 24, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Jorm, A.F.; Réglade-Meslin, C.; Maller, J.; Kumar, R.; von Sanden, C.; Windsor, T.D.; Rodgers, B.; Wen, W.; Sachdev, P. Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosom. Med. 2006, 68, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Debruin, E.; Hulshoffpol, H.; Schnack, H.; Janssen, J.; Bijl, S.; Evans, A.; Leonkenemans, J.; Kahn, R.; Verbaten, M. Focal brain matter differences associated with lifetime alcohol intake and visual attention in male but not in female non-alcohol-dependent drinkers. NeuroImage 2005, 26, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Chen, X.; Wen, W.; Anstey, K.J.; Anstry, K.J. Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: A voxel-based morphometric study. Psychiatry Res. 2008, 163, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Addicott, M.A.; Yang, L.L.; Peiffer, A.M.; Burnett, L.R.; Burdette, J.H.; Chen, M.Y.; Hayasaka, S.; Kraft, R.A.; Maldjian, J.A.; Laurienti, P.J. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum. Brain Mapp. 2009, 30, 3102–3114. [Google Scholar] [CrossRef] [PubMed]
- Pelligrino, D.A.; Xu, H.L.; Vetri, F. Caffeine and the control of cerebral hemodynamics. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S51–S62. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Commenges, D.; Gagnon, M.; Letenneur, L.; Sauvel, C.; Dartigues, J.-F. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J. Am. Geriatr. Soc. 1992, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D.A. Wechsler Memory Scale, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- REITAN, R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Wechsler, D. Manual for the Wechsler Adult Intelligence Scale; Psychological Corporation: New York, NY, USA, 1955. [Google Scholar]
- Milner, B. Interhemispheric differences in the localization of psychological processes in man. Columbia Méd. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef]
- Buschke, H.; Sliwinski, M.J.; Kuslansky, G.; Lipton, R.B. Diagnosis of early dementia by the double memory test: Encoding specificity improves diagnostic sensitivity and specificity. Neurology 1997, 48, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Baddley, A.; Emslie, H.; Nimmo-Smith, I. Doors and People: A Test of Visual and Verbal Recall and Recognition; Bury St Edmunds: St Edmundsbury, UK, 1994. [Google Scholar]
- Kaplan, E.F.; Goodglass, H.; Weintraub, S. The Boston Naming Test, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Schnider, A.; Hanlon, R.E.; Alexander, D.N.; Benson, D.F. Ideomotor apraxia: Behavioral dimensions and neuroanatomical basis. Brain Lang. 1997, 58, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Poeck, K. Clues to the Nature of disruption to limb Praxis. In Neuropsychological Studies of Apraxia and Related Disorders; Elsevier: Amsterdam, The Netherlands, 1985; pp. 99–109. [Google Scholar]
- Welsh, K.A.; Butters, N.; Mohs, R.C.; Beekly, D.; Edland, S.; Fillenbaum, G.; Heyman, A. The consortium to establish a registry for alzheimer’s disease (cerad). Part V. A normative study of the neuropsychological battery. Neurology 1994, 44, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Columbia J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Xekardaki, A.; Rodriguez, C.; Montandon, M.-L.; Toma, S.; Tombeur, E.; Herrmann, F.R.; Zekry, D.; Lovblad, K.-O.; Barkhof, F.; Giannakopoulos, P.; et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015, 274, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Huybrechts, I.; Verschraegen, M.; De Henauw, S.; Van de Wiele, T. Validity and reproducibility of a self-administered semi-quantitative food-frequency questionnaire for estimating usual daily fat, fibre, alcohol, caffeine and theobromine intakes among belgian post-menopausal women. Int. J. Environ. Res. Public Heal. 2009, 6, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Harland, B.F. Caffeine and nutrition. Nutrition 2000, 16, 522–526. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- FSL Software Package. Available online: http://www.fmrib.ox.ac.uk/fsl/ (accessed on 20 February 2018).
- Brain Extraction Tool. Available online: http://www.fmrib.ox.ac.uk/fsl/fslwiki/BET (accessed on 20 February 2018).
- FMRIB’s Automated Segmentation Tool. Available online: http://www.fmrib.ox.ac.uk/fsl/fslwiki/fast (accessed on 20 February 2018).
- Smith, S.; Nichols, T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef] [PubMed]
- Combinostics cMRI Software Package. Available online: https://www.cneuro.com (accessed on 20 February 2018).
- Sabia, S.; Elbaz, A.; Britton, A.; Bell, S.; Dugravot, A.; Shipley, M.; Kivimaki, M.; Singh-Manoux, A. Alcohol consumption and cognitive decline in early old age. Neurology 2014, 82, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, X.; Yin, Q.; Zhu, W.; Zhang, R.; Fan, X. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin. Neurosci. 2009, 63, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.; Jia, X.; Brett, C.E.; Gow, A.J.; Starr, J.M.; Kyle, J.A.M.; McNeill, G.; Deary, I.J. Alcohol intake and cognitive abilities in old age: The lothian birth cohort 1936 study. Neuropsychology 2011, 25, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Huntley, J.; Corbett, A.; Wesnes, K.; Brooker, H.; Stenton, R.; Hampshire, A.; Ballard, C. Online assessment of risk factors for dementia and cognitive function in healthy adults. Int. J. Geriatr. Psychiatry 2018, 33, e286–e293. [Google Scholar] [CrossRef] [PubMed]
- Lobo, E.; Dufouil, C.; Marcos, G.; Quetglas, B.; Saz, P.; Guallar, E.; Lobo, A. Is There an Association Between Low-to-Moderate Alcohol Consumption and Risk of Cognitive Decline? Am. J. Epidemiol. 2010, 172, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J. Alcohol consumption and abnormalities of brain structure and vasculature. Am. J. Geriatr. Cardiol. 2004, 13, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Montandon, M.L.; Rodriguez, C.; Moser, D.; Toma, S.; Hofmeister, J.; Sinanaj, I.; Lovblad, K.O.; Giannakopoulos, P. Acute caffeine administration effect on brain activation patterns in mild cognitive impairment. J. Alzheimers Dis. 2014, 41, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Agostinho, P.M. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S95–S116. [Google Scholar] [CrossRef] [PubMed]
| sCON (Stable-Stable/Stable-Improved) | iCON (Stable-Progressed/Progressed-Stable/Progressed-Improved) | dCON (Progressed-Progressed) | Total | p Value | |
|---|---|---|---|---|---|
| N | 52 | 61 | 32 | 145 | |
| Age | 73.6 ± 3.4 | 73.9 ± 3.3 | 74.0 ± 3.8 | 73.8 ± 3.5 | 0.898 |
| Gender | 0.321 | ||||
| Female | 33 (63.5%) | 30 (49.2%) | 18 (56.3%) | 81 (55.9%) | |
| Male | 19 (36.5%) | 31 (50.8%) | 14 (43.8%) | 64 (44.1%) | |
| Education (year) | 0.315 | ||||
| <9 | 10 (19.2%) | 5 (8.2%) | 6 (18.8%) | 21 (14.5%) | |
| 9–12 | 20 (38.5%) | 29 (47.5%) | 16 (50.0%) | 65 (44.8%) | |
| >12 | 22 (42.3%) | 27 (44.3%) | 10 (31.3%) | 59 (40.7%) | |
| MMSE | 28.6 ± 1.2 | 28.3 ± 1.3 | 28.5 ± 1.7 | 28.5 ± 1.4 | 0.534 |
| Chocolate (serving/month) | 61.3 ± 58.5 | 56.0 ± 49.2 | 46.4 ± 44.4 | 55.8 ± 51.7 | 0.443 |
| Coffee (cup/month) | 56.3 ± 32.6 | 50.6 ± 36.1 | 58.7 ± 43.2 | 54.4 ± 36.5 | 0.535 |
| Wine (glass/month) | 18.6 ± 18.3 | 28.1 ± 29.9 | 34.5 ± 43.7 | 26.1 ± 30.7 | 0.054 |
| Chocolate (tertile) | 0.689 | ||||
| Light | 18 (34.6%) | 20 (32.8%) | 15 (46.9%) | 53 (36.6%) | |
| Moderate | 17 (32.7%) | 22 (36.1%) | 7 (21.9%) | 46 (31.7%) | |
| Heavy | 17 (32.7%) | 19 (31.1%) | 10 (31.3%) | 46 (31.7%) | |
| Coffee (tertile) | 0.228 | ||||
| Light | 12 (23.1%) | 25 (41.0%) | 13 (40.6%) | 50 (34.5%) | |
| Moderate | 21 (40.4%) | 19 (31.1%) | 7 (21.9%) | 47 (32.4%) | |
| Heavy | 19 (36.5%) | 17 (27.9%) | 12 (37.5%) | 48 (33.1%) | |
| Wine (tertile) | 0.154 | ||||
| Light | 24 (46.2%) | 17 (27.9%) | 12 (37.5%) | 53 (36.6%) | |
| Moderate | 19 (36.5%) | 30 (49.2%) | 8 (25.0%) | 57 (39.3%) | |
| Heavy | 9 (17.3%) | 14 (23.0%) | 12 (37.5%) | 35 (24.1%) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, S.; Montandon, M.-L.; Rodriguez, C.; Herrmann, F.R.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. https://doi.org/10.3390/nu10101391
Haller S, Montandon M-L, Rodriguez C, Herrmann FR, Giannakopoulos P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients. 2018; 10(10):1391. https://doi.org/10.3390/nu10101391
Chicago/Turabian StyleHaller, Sven, Marie-Louise Montandon, Cristelle Rodriguez, François R. Herrmann, and Panteleimon Giannakopoulos. 2018. "Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age" Nutrients 10, no. 10: 1391. https://doi.org/10.3390/nu10101391
APA StyleHaller, S., Montandon, M.-L., Rodriguez, C., Herrmann, F. R., & Giannakopoulos, P. (2018). Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients, 10(10), 1391. https://doi.org/10.3390/nu10101391





