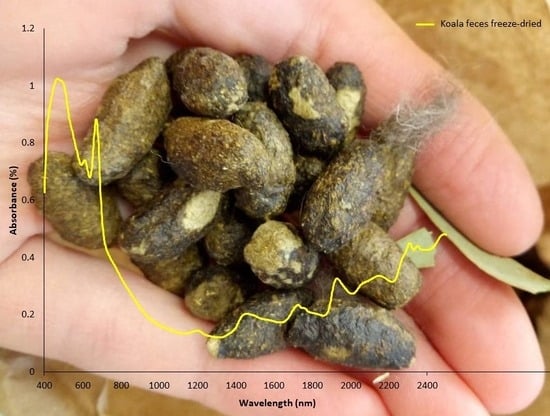
The Application of NIRS to Determine Animal Physiological Traits for Wildlife Management and Conservation
Abstract
1. Introduction
2. NIRS in Practice
3. Measuring Animal Traits with NIRS
3.1. Animal Health and Disease
3.2. Population Demographics
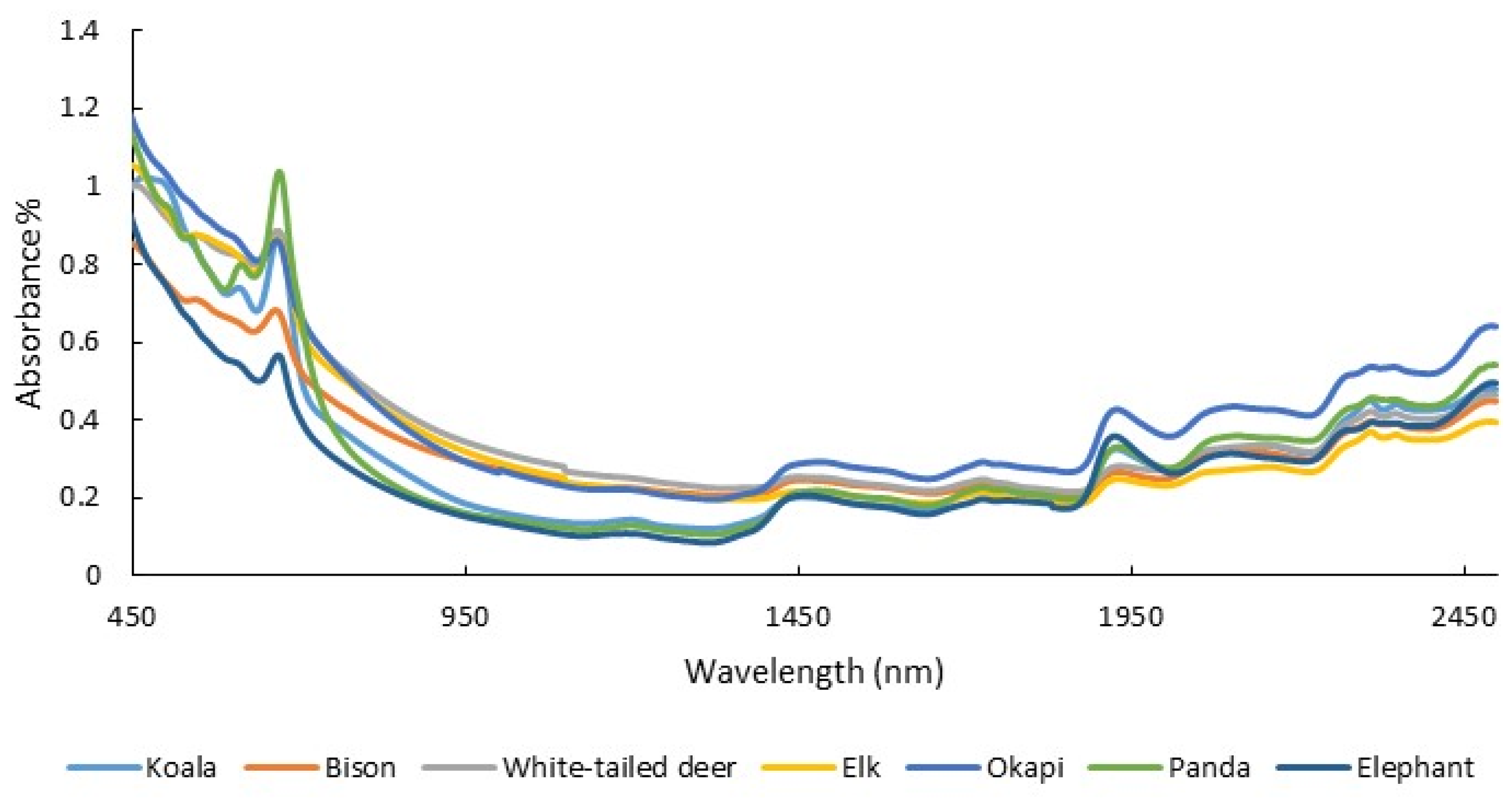
3.3. Diet Quality
4. Considerations for the Application of NIRS to Wildlife Health, Population, and Diet Assessments
4.1. Data Collection
4.2. Pre-Treatment of Samples
4.3. Data Sharing and Building Widely Applicable Calibration Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arnemo, J.M.; Ahlqvist, P.; Andersen, R.; Berntsen, F.; Ericsson, G.; Odden, J.; Brunberg, S.; Segerström, P.; Swenson, J.E. Risk of capture-related mortality in large free-ranging mammals: Experiences from Scandinavia. Wildl. Biol. 2006, 12, 109–113. [Google Scholar] [CrossRef]
- Beja-Pereira, A.; Oliveira, R.; Alves, P.C.; Schwartz, M.; Luikart, G. Advancing ecological understandings through technological transformations in noninvasive genetics. Mol. Ecol. Resour. 2009, 9, 1279–1301. [Google Scholar] [CrossRef]
- Eyvindson, K.; Hakanen, J.; Mönkkönen, M.; Juutinen, A.; Karvanen, J. Value of information in multiple criteria decision making: An application to forest conservation. Stoch. Environ. Res. Risk Assess. 2019, 33, 2007–2018. [Google Scholar] [CrossRef]
- McMahon, C.R.; Hindell, M.; Harcourt, R. Publish or perish: Why it’s important to publicise how, and if, research activities affect animals. Wildl. Res. 2012, 39, 375–377. [Google Scholar] [CrossRef]
- Alacs, E.; Alpers, D.; De Tores, P.J.; Dillon, M.; Spencer, P.B.S. Identifying the presence of quokkas (Setonix brachyurus) and other macropods using cytochrome b analyses from faeces. Wildl. Res. 2003, 30, 41–47. [Google Scholar] [CrossRef]
- Taberlet, P.; Waits, L.P.; Luikart, G. Noninvasive genetic sampling: Look before you leap. Trends Ecol. Evol. 1999, 14, 323–327. [Google Scholar] [CrossRef]
- Kohn, M.H.; Wayne, R.K. Facts from feces revisited. Trends Ecol. Evol. 1997, 12, 223–227. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Z.; Jiang, G.; Fang, J. Seasonal Changes of Reproductive Behavior and Fecal Steroid Concentrations in Père David’s Deer. Horm. Behav. 2001, 40, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Goymann, W. Noninvasive Monitoring of Hormones in Bird Droppings: Physiological Validation, Sampling, Extraction, Sex Differences, and the Influence of Diet on Hormone Metabolite Levels. Ann. N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef]
- Wiedower, E.E.; Kouba, A.J.; Vance, C.K.; Hansen, R.L.; Stuth, J.W.; Tolleson, D.R. Fecal Near Infrared Spectroscopy to Discriminate Physiological Status in Giant Pandas. PLoS ONE 2012, 7, e38908. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, N.; Zaky, W.I.; Abrams, B.P.; Chadee, D.D.; Williams, S. A Novel Xenomonitoring Technique Using Mosquito Excreta/Feces for the Detection of Filarial Parasites and Malaria. PLOS Negl. Trop. Dis. 2016, 10, e0004641. [Google Scholar] [CrossRef]
- Jean, P.-O.; Bradley, R.L.; Giroux, M.-A.; Tremblay, J.-P.; Côté, S.D. Near Infrared Spectroscopy and Fecal Chemistry as Predictors of the Diet Composition of White-Tailed Deer. Rangel. Ecol. Manag. 2014, 67, 154–159. [Google Scholar] [CrossRef]
- Valentini, A.; Miquel, C.; Nawaz, M.A.; Bellemain, E.; Coissac, E.; Pompanon, F.; Gielly, L.; Cruaud, C.; Nascetti, G.; Wincker, P.; et al. New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: ThetrnL approach. Mol. Ecol. Resour. 2009, 9, 51–60. [Google Scholar] [CrossRef]
- Foley, W.J.; McIlwee, A.; Lawler, I.; Aragones, L.; Woolnough, A.P.; Berding, N. Ecological applications of near infrared reflectance spectroscopy-a tool for rapid, cost-effective prediction of the composition of plant and animal tissues and aspects of animal performance. Oecologia 1998, 116, 293–305. [Google Scholar] [CrossRef]
- Bailey, D.W.; Thomas, M.G.; Walker, J.W.; Witmore, B.K.; Tolleson, D. Effect of Previous Experience on Grazing Patterns and Diet Selection of Brangus Cows in the Chihuahuan Desert. Rangel. Ecol. Manag. 2010, 63, 223–232. [Google Scholar] [CrossRef]
- Dixon, R.; Coates, D.; Jackson, D. Utilizing faecal near infrared spectroscopy to improve nutritional management of grazing cattle in the tropics of northern Australia. Adv. Anim. Biosci. 2010, 1, 432–433. [Google Scholar] [CrossRef]
- Landau, S.; Dvash, L.; Roudman, M.; Muklada, H.; Barkai, D.; Yehuda, Y.; Ungar, E. Faecal near-IR spectroscopy to determine the nutritional value of diets consumed by beef cattle in east Mediterranean rangelands. Animal 2016, 10, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Jancewicz, L.J.; Penner, G.; Swift, M.L.; Waldner, C.; Gibb, D.; McAllister, T.A. Predictability of growth performance in feedlot cattle using fecal near infrared spectroscopy. Can. J. Anim. Sci. 2017, 97, 701–720. [Google Scholar] [CrossRef]
- Johnson, J.R.; Carstens, G.E.; Prince, S.D.; Ominski, K.H.; Wittenberg, K.M.; Undi, M.; Forbes, T.A.; Hafla, A.N.; Tolleson, D.R.; Basarab, J.A. Application of fecal near-infrared reflectance spectroscopy profiling for the prediction of diet nutritional characteristics and voluntary intake in beef cattle. J. Anim. Sci. 2017, 95, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hu, W.; Zhou, Y.; Zhang, X.; Xu, S.; Guo, Q.; Qi, P.; Chen, L.; Yang, X.; Zhang, F.; et al. Use of near-infrared spectroscopy for the rapid evaluation of soybean [Glycine max (L.) Merri.] water soluble protein content. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117400. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S.; Toyoda, K.; Ozaki, Y.; Itoh, K.; Fearn, T. Near-Infrared Spectroscopy for Dairy Management: Measurement of Unhomogenized Milk Composition. J. Dairy Sci. 1999, 82, 2344–2351. [Google Scholar] [CrossRef]
- Engelhard, S.; Löhmannsröben, H.-G.; Schael, F. Quantifying Ethanol Content of Beer Using Interpretive Near-Infrared Spectroscopy. Appl. Spectrosc. 2004, 58, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.M.; Domingos, E.; Tosato, F.; dos Santos, N.A.; Leite, J.D.A.; da Silva, M.; Marcelo, M.C.A.; Ortiz, R.S.; Filgueiras, P.; Romão, W. Portable near infrared spectroscopy applied to abuse drugs and medicine analyses. Anal. Methods 2018, 10, 593–603. [Google Scholar] [CrossRef]
- Leite, E.; Stuth, J. Fecal NIRS equations to assess diet quality of free-ranging goats. Small Rumin. Res. 1995, 15, 223–230. [Google Scholar] [CrossRef]
- Landau, S.; Nitzan, R.; Barkai, D.; Dvash, L. Excretal Near Infrared Reflectance Spectrometry to Monitor the Nutrient Content of Diets of Grazing Young Ostriches (Struthio Camelus). S. Afr. J. Anim. Sci. 2006, 36, 248–256. [Google Scholar]
- Showers, S.E.; Tolleson, D.R.; Stuth, J.W.; Kroll, J.C.; Koerth, B.H. Predicting Diet Quality of White-Tailed Deer via NIRS Fecal Profiling. Rangel. Ecol. Manag. 2006, 59, 300–307. [Google Scholar] [CrossRef]
- Glasser, T.; Landau, S.; Ungar, E.D.; Perevolotsky, A.; Dvash, L.; Muklada, H.; Kababya, D.; Walker, J.W. A fecal near-infrared reflectance spectroscopy-aided methodology to determine goat dietary composition in a Mediterranean shrubland1. J. Anim. Sci. 2008, 86, 1345–1356. [Google Scholar] [CrossRef]
- Rothman, J.M.; Chapman, C.A.; Hansen, J.L.; Cherney, D.J.R.; Pell, A.N. Rapid Assessment of the Nutritional Value of Foods Eaten by Mountain Gorillas: Applying Near-Infrared Reflectance Spectroscopy to Primatology. Int. J. Primatol. 2009, 30, 729–742. [Google Scholar] [CrossRef]
- Marsh, K.J.; Moore, B.D.; Wallis, I.R.; Foley, W.J. Feeding rates of a mammalian browser confirm the predictions of a ‘foodscape’ model of its habitat. Oecologia 2013, 174, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Au, J.; Clark, R.G.; Allen, C.; Marsh, K.; Foley, W.J.; Youngentob, K.N. A nutritional mechanism underpinning folivore occurrence in disturbed forests. For. Ecol. Manag. 2019, 453, 117585. [Google Scholar] [CrossRef]
- Vance, C.K.; Tolleson, D.R.; Kinoshita, K.; Rodriguez, J.; Foley, W.J. Near Infrared Spectroscopy in Wildlife and Biodiversity. J. Near Infrared Spectrosc. 2016, 24, 1–25. [Google Scholar] [CrossRef]
- Esperança, P.M.; Blagborough, A.M.; Da, D.F.; Dowell, F.E.; Churcher, T.S. Detection of Plasmodium berghei infected Anopheles stephensi using near-infrared spectroscopy. Parasites Vectors 2018, 11, 377. [Google Scholar] [CrossRef]
- Tolleson, D.; Teel, P.; Stuth, J.; Strey, O.; Welsh, T.; Carstens, G. Fecal NIRS: Detection of tick infestations in cattle and horses. Vet. Parasitol. 2007, 144, 146–152. [Google Scholar] [CrossRef]
- Tolleson, D.R. Determination of Sex in Ungulate Herbivores via near Infrared Spectroscopy of Hair: Growing Cattle as a Surrogate Model. J. Anim. Sci. 2021, 99, 23–24. [Google Scholar] [CrossRef]
- Tolleson, D.; Randel, R.; Stuth, J.; Neuendorff, D. Determination of sex and species in red and fallow deer by near infrared reflectance spectroscopy of the faeces. Small Rumin. Res. 2005, 57, 141–150. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Dowell, F.E.; Broce, A.B.; Throne, J.E.; Wirtz, R.A.; Xie, F.; Fabrick, J.A.; Baker, J.E. Chronological age-grading of house flies by using near-infrared spectroscopy. J. Med. Èntomol. 2002, 39, 499–508. [Google Scholar] [CrossRef][Green Version]
- Tolleson, D.R.; Schafer, D.W. Detection of Pregnancy in Arizona Range Cattle Using near Infrared Spectroscopy of Feces. J. Anim. Sci. 2012, 90, 3442–3450. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Morita, H.; Miyazaki, M.; Hama, N.; Kanemitsu, H.; Kawakami, H.; Wang, P.; Ishikawa, O.; Kusunoki, H.; Tsenkova, R. Near infrared spectroscopy of urine proves useful for estimating ovulation in giant panda (Ailuropoda melanoleuca). Anal. Methods 2010, 2, 1671–1675. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kuze, N.; Kobayashi, T.; Miyakawa, E.; Narita, H.; Inoue-Murayama, M.; Idani, G.; Tsenkova, R. Detection of urinary estrogen conjugates and creatinine using near infrared spectroscopy in Bornean orangutans (Pongo pygmaeus). Primates 2015, 57, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tallo-Parra, O.; Albanell, E.; Carbajal, A.; Monclús, L.; Sabes-Alsina, M.; Riba, C.; Martin, M.; Abelló, M.; Lopez-Bejar, M. Prediction of Faecal Cortisol Metabolites from Western Lowland Gorilla (Gorilla Gorilla Gorilla) by near Infrared Reflectance Spectroscopy (NIRS). In Proceedings of the 5th ISWE CONFERENCE, Berlin, Germany, 12–14 October 2015; p. 61. [Google Scholar]
- Tallo-Parra, O.; Albanell, E.; Carbajal, A.; Monclús, L.; Manteca, X.; Lopez-Bejar, M. Prediction of Cortisol and Progesterone Concentrations in Cow Hair Using Near-Infrared Reflectance Spectroscopy (NIRS). Appl. Spectrosc. 2017, 71, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Ancin-Murguzur, F.J.; Tarroux, A.; Bråthen, K.A.; Bustamante, P.; Descamps, S. Using near-infrared reflectance spectroscopy (NIRS) to estimate carbon and nitrogen stable isotope composition in animal tissues. Ecol. Evol. 2021, 11, 10483–10488. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G.; Masoero, G. NIRS of body and tissues in growing rabbits fed diets with different fat sources and supplemented with Curcuma longa. World Rabbit Sci. 2013, 21, 85–90. [Google Scholar] [CrossRef][Green Version]
- Windley, H.R.; Wallis, I.R.; DeGabriel, J.L.; Moore, B.D.; Johnson, C.N.; Foley, W.J. A faecal index of diet quality that predicts reproductive success in a marsupial folivore. Oecologia 2013, 173, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, C. Near Infrared Spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef]
- Dixon, R.; Coates, D. Review: Near Infrared Spectroscopy of Faeces to Evaluate the Nutrition and Physiology of Herbivores. J. Near Infrared Spectrosc. 2009, 17, 1–31. [Google Scholar] [CrossRef]
- Kokaly, R. Spectroscopic Determination of Leaf Biochemistry Using Band-Depth Analysis of Absorption Features and Stepwise Multiple Linear Regression. Remote Sens. Environ. 1999, 67, 267–287. [Google Scholar] [CrossRef]
- Shenk, J.S.; Workman, J.J., Jr.; Westerhaus, M.O. Application of NIR Spectroscopy to Agricultural Products. In Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2020; pp. 365–404. [Google Scholar]
- Moore, B.D.; Lawler, I.R.; Wallis, I.R.; Beale, C.M.; Foley, W. Palatability mapping: A koala’s eye view of spatial variation in habitat quality. Ecology 2010, 91, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innov. Food Sci. Emerg. Technol. 2013, 19, 1–14. [Google Scholar] [CrossRef]
- Tolleson, D.R.; Teel, P.D.; Carstens, G.E.; Welsh, T.H. The physiology of tick-induced stress in grazing animals. In Ticks: Disease, Management and Control; Nova Science Publishers, Inc: Hauppauge, NY, USA, 2012; pp. 1–18. ISBN 9781620811368. [Google Scholar]
- Ziegel, E.R. Chemometrics: Statistics and Computer Application in Analytical Chemistry. Technometrics 2001, 43, 240. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; IM Publications Open LLP: Chichester, UK, 2017; Volume 6. [Google Scholar]
- Wold, H. Soft Modelling by Latent Variables: The Non-Linear Iterative Partial Least Squares (NIPALS) Approach. J. Appl. Probab. 1975, 12, 117–142. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Populations Structuring of Near Infrared Spectra and Modified Partial Least Squares Regression. Crop. Sci. 1991, 31, 1548–1555. [Google Scholar] [CrossRef]
- Lawler, I.R.; Aragones, L.; Berding, N.; Marsh, H.; Foley, W. Near-Infrared Reflectance Spectroscopy is a Rapid, Cost-Effective Predictor of Seagrass Nutrients. J. Chem. Ecol. 2006, 32, 1353–1365. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Garrido-Varo, A.; Guerrero, J.E.; Gutiérrez-Estrada, J.C. Use of Artificial Neural Networks in Near-Infrared Reflectance Spectroscopy Calibrations for Predicting the Inclusion Percentages of Wheat and Sunflower Meal in Compound Feedingstuffs. Appl. Spectrosc. 2006, 60, 1062–1069. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemom. 2003, 17, 16–33. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Au, J.; Youngentob, K.N.; Foley, W.J.; Moore, B.; Fearn, T. Sample selection, calibration and validation of models developed from a large dataset of near infrared spectra of tree leaves. J. Near Infrared Spectrosc. 2020, 28, 186–203. [Google Scholar] [CrossRef]
- Elisseeff, A.; Pontil, M. Leave-one-out error and stability of learning algorithms with applications. In Advances in Learning Theory: Methods, Models, and Applications; Suykens, J.A.K., Ed.; IOS Press: Leuven, Belgium, 2002; p. 415. [Google Scholar]
- Youngentob, K.N.; Renzullo, L.J.; Held, A.A.; Jia, X.; Lindenmayer, D.B.; Foley, W. Using imaging spectroscopy to estimate integrated measures of foliage nutritional quality. Methods Ecol. Evol. 2011, 3, 416–426. [Google Scholar] [CrossRef]
- Anderson, K.; Norby, B.; Prince, S.; Ball, G.; Tolleson, D. Detection of Paratuberculosis in Dairy Cattle via near Infrared Reflectance Spectroscopy of Feces. J. Anim. Sci. 2007, 85, 42. [Google Scholar]
- Vance, C.K.; Kouba, A.J.; Zhang, H.-X.; Zhao, H.; Wang, Q.; Willard, S.T. Near Infrared Reflectance Spectroscopy Studies of Chinese Giant Salamanders in Aquaculture Production. NIR News 2015, 26, 4–7. [Google Scholar] [CrossRef]
- Hing, S.; Narayan, E.; Thompson, R.C.A.; Godfrey, S. The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildl. Res. 2016, 43, 51–60. [Google Scholar] [CrossRef]
- Scott, M.E. The Impact of Infection and Disease on Animal Populations: Implications for Conservation Biology. Conserv. Biol. 1988, 2, 40–56. [Google Scholar] [CrossRef]
- Andrew, A.; Edward, D.; Solomon, A.; Isaac, A.; Ibrahim, Y. The Cortisol Steroid Levels as a Determinant of Health Status in Animals. J. Proteomics Bioinform. 2017, 10, 277–283. [Google Scholar] [CrossRef]
- Hanger, J.; Loader, J.; Wan, C.; Beagley, K.W.; Timms, P.; Polkinghorne, A. Comparison of antigen detection and quantitative PCR in the detection of chlamydial infection in koalas (Phascolarctos cinereus). Vet. J. 2013, 195, 391–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennett, A.; Hayssen, V. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef]
- Sachsenröder, J.; Twardziok, S.; Hammerl, J.A.; Janczyk, P.; Wrede, P.; Hertwig, S.; Johne, R. Simultaneous Identification of DNA and RNA Viruses Present in Pig Faeces Using Process-Controlled Deep Sequencing. PLoS ONE 2012, 7, e34631. [Google Scholar] [CrossRef]
- Yamazaki, W.; Makino, R.; Nagao, K.; Mekata, H.; Tsukamoto, K. New Micro-amount of Virion Enrichment Technique (Mi VET ) to detect influenza A virus in the duck faeces. Transbound. Emerg. Dis. 2019, 66, 341–348. [Google Scholar] [CrossRef]
- Norby, B.; Tolleson, D.; Ball, G.; Jordan, E.; Stuth, J. Near Infrared Spectroscopy: A New Approach to Diagnosis of Paratuberculosis in Cattle. In Proceedings of the International Epidemiology Conference, Cairns, Australia; 2006; pp. 2003–2005. Available online: http://www.sciquest.org.nz/node/64094 (accessed on 8 September 2021).
- Tolleson, D.R.; Schafer, D.W. Evaluation of non-invasive bioforensic techniques for determining the age of hot-iron brand burn scars in cattle. Transl. Anim. Sci. 2021, 5, txab108. [Google Scholar] [CrossRef]
- Masoero, G.; Sala, G.; Meineri, G.; Cornale, P.; Tassone, S.; Peiretti, P.G. Nir Spectroscopy and Electronic Nose Evaluation on Live Rabbits and on the Meat of Rabbits Fed Increasing Levels of Chia (Salvia hispanica L.) Seeds. J. Anim. Vet. Adv. 2008, 7, 1394–1399. [Google Scholar]
- Tolleson, D.R.; Hollingsworth, K.; Barboza, P.S. Determination of Species and Sex in Deer via near Infrared Spectroscopy of Liver Tissue. In Proceedings of the Society for Range Management Annual Meetings, Denver, CO, USA, 20 January 2020. [Google Scholar]
- Tolleson, D.R.; Garza, N.; Hollingsworth, K.; Diaz, J.M.; Welsh, T.H. 157 Spectroscopic analysis of tissues collected from male goats differing in genetic propensity to consume juniper. J. Anim. Sci. 2020, 98, 119–120. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, M.; Wang, Y.; Peng, S.; Chen, Y.; Zhang, D.; Zhang, D.; Guo, Y.; Wang, X.; Luo, H.; et al. Fast Screening and Primary Diagnosis of COVID-19 by ATR–FT-IR. Anal. Chem. 2021, 93, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Kochan, K.; Bedolla, D.E.; Salazar-Quiroz, N.; Grimley, S.L.; Perez-Guaita, D.; Baker, M.J.; Vongsvivut, J.; Tobin, M.J.; Bambery, K.R.; et al. Infrared Based Saliva Screening Test for COVID-19. Angew. Chem. 2021, 133, 17239–17244. [Google Scholar] [CrossRef]
- Kimura, M. Introduction to Population Genetics. Evol. Hum. Genome I 2020, 3, 85–101. [Google Scholar] [CrossRef]
- Johnson-Ulrich, L.; Vance, C.K.; Kouga, A.J.; Willard, S.T. Fecal Near Infrared Reflectance FNIR Spectroscopy for Discrimination of Species and Gender for Amur Leopards and Snow Leopards. NIR-2013 Proc. 2013, 1438, 495–505. [Google Scholar]
- Passerotti, M.S.; E Helser, T.; Benson, I.M.; Barnett, B.K.; Ballenger, J.C.; Bubley, W.J.; Reichert, M.J.M.; Quattro, J.M. Age estimation of red snapper (Lutjanus campechanus) using FT-NIR spectroscopy: Feasibility of application to production ageing for management. ICES J. Mar. Sci. 2020, 77, 2144–2156. [Google Scholar] [CrossRef]
- Rigby, C.L.; Wedding, B.B.; Grauf, S.; Simpfendorfer, C.A. Novel method for shark age estimation using near infrared spectroscopy. Mar. Freshw. Res. 2016, 67, 537. [Google Scholar] [CrossRef]
- Aw, W.C.; E Dowell, F.; O Ballard, J.W. Using Near-Infrared Spectroscopy to Resolve the Species, Gender, Age, and the Presence of Wolbachia Infection in Laboratory-Reared Drosophila. G3 Genes Genomes Genet. 2012, 2, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Sikulu-Lord, M.T.; Mayagaya, V.S.; Devine, G.; Dowell, F.; Churcher, T.S. Monitoring the Age of Mosquito Populations Using Near-Infrared Spectroscopy. Sci. Rep. 2018, 8, 5274. [Google Scholar] [CrossRef]
- Dowell, F.; Parker, A.; Benedict, M.; Robinson, A.; Broce, A.; Wirtz, R. Sex separation of tsetse fly pupae using near-infrared spectroscopy. Bull. Èntomol. Res. 2005, 95, 249–257. [Google Scholar] [CrossRef]
- Peberdy, J.F.; Moore, P.M. Chitin Synthase in Mortierella vinacea: Properties, Cellular Location and Synthesis in Growing Cultures. J. Gen. Microbiol. 1975, 90, 228–236. [Google Scholar] [CrossRef][Green Version]
- Tolleson, D.; Willard, S.; Gandy, B.; Stuth, J. Determination of Reproductive Status in Dairy Cattle Using near Infrared Reflectance Spectroscopy of Feces. J. Anim. Sci. 2001, 79, 21. [Google Scholar]
- Johnson, J. Near-infrared spectroscopy (NIRS) for taxonomic entomology: A brief review. J. Appl. Èntomol. 2020, 144, 241–250. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Tulli, F.; Tibaldi, E. Prediction of chemical composition and origin identification of european sea bass (Dicentrarchus labrax L.) by near infrared reflectance spectroscopy (NIRS). Food Chem. 2004, 86, 275–281. [Google Scholar] [CrossRef]
- Benson, I.M.; Barnett, B.K. In Proceedings of the Research Workshop on the Rapid Estimation of Fish Age Using Fourier Transform Near-Infrared Spec-Troscopy (FT-NIRS) Age and Growth Program, September 2019. Available online: https://www.fisheries.noaa.gov/alaska/science-data/alaska-fisheries-science-center-publications (accessed on 12 September 2021).
- Wedding, B.B.; Forrest, A.J.; Wright, C.; Grauf, S.; Exley, P.; Poole, S.E. A novel method for the age estimation of Saddletail snapper (Lutjanus malabaricus) using Fourier Transform-near infrared (FT-NIR) spectroscopy. Mar. Freshw. Res. 2014, 65, 894–900. [Google Scholar] [CrossRef]
- Robins, J.; Wedding, B.B.; Wright, C.; Grauf, S.; Sellin, M.; Fowler, A.; Saunders, T.; Newman, S. Fisheries Research & Development Corporation (Australia); Queensland. Department of Agriculture and Fisheries. In Revolutionising Fish Ageing: Using Near Infrared Spectroscopy to Age Fish; State of Queensland through Department of Agriculture and Fisheries: Queensland, Australia, 2015; ISBN 9780734504494. [Google Scholar]
- DeGabriel, J.L.; Moore, B.; Foley, W.; Johnson, C. The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 2009, 90, 711–719. [Google Scholar] [CrossRef] [PubMed]
- McArt, S.H.; Spalinger, D.E.; Collins, W.B.; Schoen, E.R.; Stevenson, T.; Bucho, M. Summer dietary nitrogen availability as a potential bottom-up constraint on moose in south-central Alaska. Ecology 2009, 90, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.K.; Stuth, J.W. Fecal NIRS Equations for Predicting Diet Quality of Free-Ranging Cattle. J. Range Manag. 1992, 45, 238. [Google Scholar] [CrossRef]
- Landau, S.; Glasser, T.; Muklada, H.; Dvash, L.; Perevolotsky, A.; Ungar, E.; Walker, J. Fecal NIRS prediction of dietary protein percentage and in vitro dry matter digestibility in diets ingested by goats in Mediterranean scrubland. Small Rumin. Res. 2005, 59, 251–263. [Google Scholar] [CrossRef]
- Tolleson, D.; Osborn, R.G.; Stuth, J.W.; Ginnet, T.F.; Applegath, M.T. Determination of Dietary Tannin Concentration in White-Tailed Deer via Near Infrared Reflectance Spectroscopy of Feces. In Proceedings of the National Conference on Grazinglands, Las Vegas, NV, USA, 5–8 December 2000; pp. 727–733. [Google Scholar]
- Landau, S.; Glasser, T.; Dvash, L.; Perevolotsky, A. Faecal NIRS to Monitor the Diet of Mediterranean Goats. S. Afr. J. Anim. Sci. 2004, 34, 76–80. [Google Scholar]
- Li, H.; Tolleson, D.; Stuth, J.; Bai, K.; Mo, F.; Kronberg, S. Faecal near infrared reflectance spectroscopy to predict diet quality for sheep. Small Rumin. Res. 2007, 68, 263–268. [Google Scholar] [CrossRef]
- Kidane, N.F.; Stuth, J.W.; Tolleson, D.R. Predicting Diet Quality of Donkeys via Fecal-NIRS Calibrations. Rangel. Ecol. Manag. 2008, 61, 232–239. [Google Scholar] [CrossRef]
- Kamler, J.; Homolka, M. Faecal Nitrogen: A Potential Indicator of Red and Roe Deer Diet Quality in Forest Habitats. Folia Zool. 2005, 54, 89–98. [Google Scholar]
- Leslie, D.M.; Bowyer, R.T.; Jenks, J.A. Facts From Feces: Nitrogen Still Measures Up as a Nutritional Index for Mammalian Herbivores. J. Wildl. Manag. 2008, 72, 1420–1433. [Google Scholar] [CrossRef]
- Dhiman, T.; Satter, L. Protein as the First-Limiting Nutrient for Lactating Dairy Cows Fed High Proportions of Good Quality Alfalfa Silage. J. Dairy Sci. 1993, 76, 1960–1971. [Google Scholar] [CrossRef]
- DeGabriel, J.L.; Wallis, I.R.; Moore, B.D.; Foley, W.J. A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia 2008, 156, 107–116. [Google Scholar] [CrossRef]
- Wu, H.; Levin, N.; Seabrook, L.; Moore, B.D.; McAlpine, C. Mapping Foliar Nutrition Using WorldView-3 and WorldView-2 to Assess Koala Habitat Suitability. Remote Sens. 2019, 11, 215. [Google Scholar] [CrossRef]
- Yang, Z.; Han, L.; Zhu, R. Near-Infrared Sensing of Pig Manure Nutrients. In Livestock Environment VII, 18–20 May 2005, Beijing, China; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013; Volume 43, pp. 309–315. [Google Scholar] [CrossRef]
- Reeves, J.; Van Kessel, J. Determination of Ammonium-N, Moisture, Total C and Total N in Dairy Manures Using a near Infrared Fibre-Optic Spectrometer. J. Near Infrared Spectrosc. 2000, 8, 151–160. [Google Scholar] [CrossRef]
- Reeves, J.B.; Van Kessel, J.A.S.; Reeves, I.J. Spectroscopic Analysis of Dried Manures. Near- versus Mid-Infrared Diffuse Reflectance Spectroscopy for the Analysis of Dried Dairy Manures. J. Near Infrared Spectrosc. 2002, 10, 93–101. [Google Scholar] [CrossRef]
- Villamuelas, M.; Serrano, E.; Espunyes, J.; Fernández, N.; López-Olvera, J.R.; Garel, M.; e Santos, J.P.V.; Parra-Aguado, M. Ángeles; Ramanzin, M.; Aguilar, X.F.; et al. Predicting herbivore faecal nitrogen using a multispecies near-infrared reflectance spectroscopy calibration. PLoS ONE 2017, 12, e0176635. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, D.R.; Angerer, J.P. The application of near infrared spectroscopy to predict faecal nitrogen and phosphorus in multiple ruminant herbivore species. Rangel. J. 2020, 42, 415–423. [Google Scholar] [CrossRef]
- Leite, E.R.; Stuth, J.W. Influence of Duration of Exposure to Field Conditions on Viability of Fecal Samples for NIRS Analysis. J. Range Manag. 1994, 47, 312. [Google Scholar] [CrossRef]
- Hadinger, U.; Haymerle, A.; Knauer, F.; Schwarzenberger, F.; Walzer, C. Faecal cortisol metabolites to assess stress in wildlife: Evaluation of a field method in free-ranging chamois. Methods Ecol. Evol. 2015, 6, 1349–1357. [Google Scholar] [CrossRef]
- Kaneko, H.; Lawler, I.R. Can Near Infrared Spectroscopy be Used to Improve Assessment of Marine Mammal Diets via Fecal Analysis? Mar. Mammal Sci. 2006, 22, 261–275. [Google Scholar] [CrossRef]
- Corlatti, L. Anonymous fecal sampling and NIRS studies of diet quality: Problem or opportunity? Ecol. Evol. 2020, 10, 6089–6096. [Google Scholar] [CrossRef]
- Fernández-Cabanás, V.M.; Garrido-Varo, A.; Pérez-Marín, D.; Dardenne, P. Evaluation of Pretreatment Strategies for Near-Infrared Spectroscopy Calibration Development of Unground and Ground Compound Feedingstuffs. Appl. Spectrosc. 2006, 60, 17–23. [Google Scholar] [CrossRef]
- Giordanengo, T.; Charpentier, J.-P.; Roger, J.-M.; Roussel, S.; Brancheriau, L.; Chaix, G.; Baillères, H. Correction of moisture effects on near infrared calibration for the analysis of phenol content in eucalyptus wood extracts. Ann. For. Sci. 2008, 65, 803. [Google Scholar] [CrossRef][Green Version]
- Ji, W.; Rossel, R.A.V.; Shi, Z. Accounting for the effects of water and the environment on proximally sensed vis-NIR soil spectra and their calibrations. Eur. J. Soil Sci. 2015, 66, 555–565. [Google Scholar] [CrossRef]
- Bastianelli, D.; Bonnal, L.; Jaguelin-Peyraud, Y.; Noblet, J. Predicting feed digestibility from NIRS analysis of pig faeces. Animal 2015, 9, 781–786. [Google Scholar] [CrossRef]
- McArthur, L.; Greensill, C. Comparison of two NIR systems for quantifying kaolinite in Weipa bauxites. Meas. Sci. Technol. 2007, 18, 3463–3470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, L.R.; Marsh, K.J.; Tolleson, D.R.; Youngentob, K.N. The Application of NIRS to Determine Animal Physiological Traits for Wildlife Management and Conservation. Remote Sens. 2021, 13, 3699. https://doi.org/10.3390/rs13183699
Morgan LR, Marsh KJ, Tolleson DR, Youngentob KN. The Application of NIRS to Determine Animal Physiological Traits for Wildlife Management and Conservation. Remote Sensing. 2021; 13(18):3699. https://doi.org/10.3390/rs13183699
Chicago/Turabian StyleMorgan, Laura R., Karen J. Marsh, Douglas R. Tolleson, and Kara N. Youngentob. 2021. "The Application of NIRS to Determine Animal Physiological Traits for Wildlife Management and Conservation" Remote Sensing 13, no. 18: 3699. https://doi.org/10.3390/rs13183699
APA StyleMorgan, L. R., Marsh, K. J., Tolleson, D. R., & Youngentob, K. N. (2021). The Application of NIRS to Determine Animal Physiological Traits for Wildlife Management and Conservation. Remote Sensing, 13(18), 3699. https://doi.org/10.3390/rs13183699