Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Process and Storage Conditions
2.3. Physico-Chemical Determinations
2.4. Water Interaction and Oil Emulsifying Properties
2.5. Antioxidant Properties
2.6. Prebiotic Effect Preliminary Assay: Powders Influence on Lactic Acid Bacteria Growth
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Processing Condition on Functional Properties of Powdered Blueberry Pomace
3.1.1. Physico-Chemical Properties
3.1.2. Antioxidant Properties
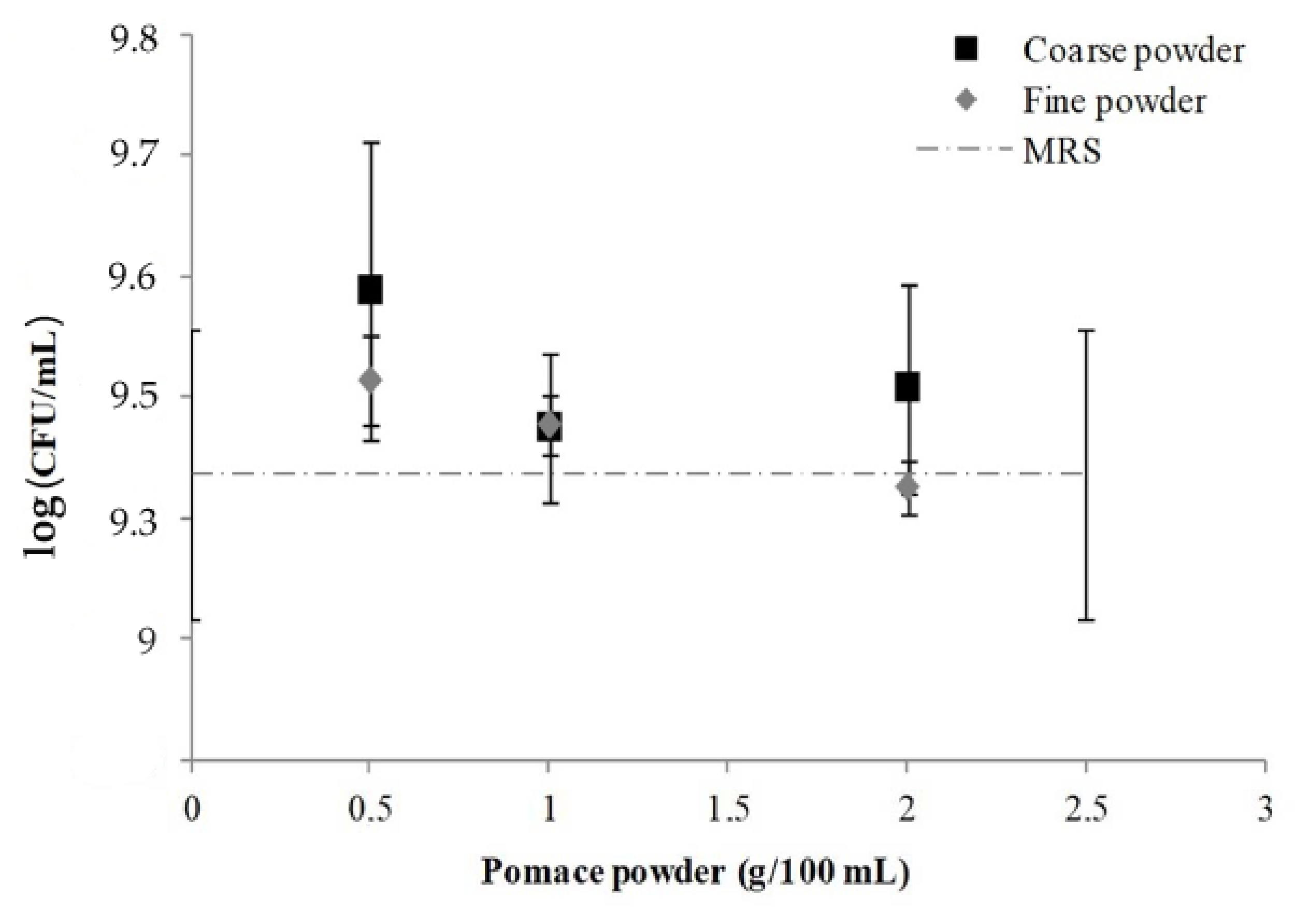
3.1.3. Potential Prebiotic Effect: Powders Influence on Lactic Acid Bacteria Growth
3.2. Evolution of Functional Properties of Blueberry Pomace Powders during Storage
3.2.1. Physico-Chemical Properties
3.2.2. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Food 2030 Pathways for Action: Research and Innovation Policy as a Driver for Sustainable, Healthy and Inclusive Food Systems; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- FAO. The State of the World’s Biodiversity for Food and Agriculture, The State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef] [Green Version]
- Khanal, R.C.; Howard, L.R.; Brownmiller, C.R.; Prior, R.L. Influence of extrusion processing on procyanidin composition and total anthocyanin contents of blueberry pomace. J. Food Sci. 2009, 74, 52–58. [Google Scholar] [CrossRef]
- Al-Awwadi, N.A.; Araiz, C.; Bornet, A.; Delbosc, S.; Cristol, J.P.; Linck, N.; Azay, J.; Teissedre, P.L.; Cros, G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005, 53, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Santos, M.C.P.; Moro, T.M.A.; Basto, G.J.; Andrade, R.M.S.; Gonçalves, E.C.B.A. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Plazzota, S.; Calligaris, A.; Manzocco, L. Application of different drying techniques to fresh-cut salad waste to obtain food ingredients rich in antioxidants and with high solvent loading capacity. LWT 2018, 89, 276–283. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning agri-food cooperative vegetable residues into functional powdered ingredients for the food industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, N.; Seguí, L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Djantou, E.B.; Mbofung, C.M.F.; Scher, J.; Phamba, N.; Morael, J.D. Alternation drying and grinding (ADG) technique, a novel approach for producing ripe mango powder. Food Sci. Technol. 2011, 4, 1585–1590. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Kammerer, D.R.; Carle, R. Matrix dependent impact of sugar and ascorbic acid addition on color and anthocyanin stability of black carrot, elderberry and strawberry single strength and from concentrate juices upon thermal treatment. Food Res. Int. 2009, 42, 1023–1033. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Lila, M.A. Comparison of berry juice concentrates and pomaces and alternative plant proteins to produce spray dried protein-polyphenol food ingredients. Food Funct. 2019, 10, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Tagliani, C.; Perez, C.; Curutchet, A.; Arcia, P.; Cozzano, S. Blueberry pomace, valorization of an industry by-product source of fibre with antioxidant capacity. Food Sci. Technol. 2019, 39, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Castagnini, J.M.; Betoret, N.; Betoret, E.; Fito, P. Vacuum impregnation and air drying temperature effect on individual anthocyanins and antiradical capacity of blueberry juice included into an apple matrix. LWT Food Sci. Technol. 2015, 64, 1289–1296. [Google Scholar] [CrossRef]
- AOAC. Official Method 973.18. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fibre in feeds with refluxing beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Process. Process Intensif. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agric. Biol. Chem. 1973, 36, 719–727. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1-F1.2.13. Available online: https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/0471142913.faf0102s00 (accessed on 1 December 2021). [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Garcia-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Impact of different stages of juice processing on the anthocyanin, flavonol, and procyanidin contents of cranberries. J. Agric. Food Chem. 2011, 59, 4692–4698. [Google Scholar] [CrossRef]
- Hinestroza-Córdoba, L.; Duarte, S.; Seguí, L.; Barrera, C.; Betoret, N. Characterization of powdered lulo (Solanum quitoense) bagasse as a functional food ingredient. Foods 2020, 9, 723. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Evaluation of Particle Size Influence on Proximate Composition, Physicochemical, Techno-Functional and Physio-Functional Properties of Flours Obtained from Persimmon (Diospyros kaki Trumb.) Coproducts. Plant Foods Hum. Nutr. 2017, 72, 67–73. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Rodríguez-González, S.; Pérez-Ramírez, I.F.; Loarca-Piña, G.; Amaya-Llano, S.; Gallegos-Corona, M.A.; Reynoso-Camacho, R. Juice by-products as a source of dietary fibre and antioxidants and their effect on hepatic steatosis. J. Funct. Foods 2015, 17, 93–102. [Google Scholar] [CrossRef]
- Lario, Y.; Sendra, E.; García-Pérez, J.; Fuentes, C.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Alvarez, J.A. Preparation of high dietary fiber powder from lemon juice by-products. Innov. Food Sci. Emerg. Technol. 2004, 5, 113–117. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Landines, E.F.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying process and particle size of persimmon fibre on its physicochemical, antioxidant, hydration and emulsifying properties. J. Food Sci. Technol. 2017, 54, 2902–2912. [Google Scholar] [CrossRef]
- Forni, S.; Aguilar, I.; Misztal, I. Different genomic relationship matrices for single-step analysis using phenotypic, pedigree and genomic information. Genet. Sel. Evol. 2011, 43, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De Moraes Crizel, T.; Hermes, V.S.; De Oliveira Rios, A.; Flôres, S.H. Evaluation of bioactive compounds, chemical and technological properties of fruits byproducts powder. J. Food Sci. Technol. 2016, 53, 4067–4075. [Google Scholar] [CrossRef] [Green Version]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of different drying techniques on physical properties, total polyphenols and antioxidant capacity of blackcurrant pomace powders. LWT Food Sci. Technol. 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Torres-León, C.; Ayala-Aponte, A. Evaluación de polvos alimentarios obtenidos de cáscaras de mango (Mangifera indica) como fuente de ingredientes funcionales. Inf. Tecnol. 2015, 26, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of extraction method on chemical composition and functional characteristics of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 113, 665–671. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Li, Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2016, 96, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Characterization of spray dried probiotic Sohiong fruit powder with Lactobacillus plantarum. LWT 2020, 117, 108699. [Google Scholar] [CrossRef]
- Kethireddipalli, P.; Hung, Y.C.; Mcwatters, K.H.; Phillips, R.D. Effect of Milling Method (Wet and Dry) on the Functional Properties of Cowpea (Vigna unguiculata) Pastes and End Product (Akara) Quality. J. Food Sci. 2002, 67, 48–52. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martínez, M.C.; Ortuño, J. Relationship between Physical and Hydration Properties of Soluble and Insoluble Fiber of Artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Farooq, Z.; Boye, J.I. Chapter 11. Novel food and industrial applications of pulse flours and fractions. In Pulse Foods; Brijesh, K., Tiwari Aoife, G., McKenna, B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 283–323. [Google Scholar]
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Moisture sorption isotherms and isosteric heat of sorption of dry persimmon leaves. Food Biosci. 2014, 7, 88–94. [Google Scholar] [CrossRef]
- Ramírez, A.; Delahaye, E.P. Propiedades funcionales de harinas altas en fibra dietética obtenidas de piña, guayaba y guanábana. Asoc. Intercienc. 2009, 34, 293–298. [Google Scholar]
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 5, 239–240. [Google Scholar] [CrossRef] [Green Version]
- Ehlenfeldt, M.K.; Camp, M.J.; Wang, S.Y. α-Glucosidase inhibitory activity and antioxidant capacity in the peel and pulp of mixed-species blueberry hybrids. Plan Genet. Resour. 2015, 13, 190–194. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colorants; Springer: Berlin/Heidelberg, Germany, 1996; pp. 244–309. [Google Scholar] [CrossRef]
- Kwok, B.H.L.; Hu, C.; Durance, T.; Kitts, D.D. Dehydration Techniques Affect Phytochemical Contents and Free Radical Scavenging Activities of Saskatoon berries (Amelanchier alnifolia Nutt.). J. Food Sci. 2004, 69, SNQ122–SNQ126. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. J. Food Compos. Anal. 2004, 17, 407–422. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.; De Pascual-Teresa, S.; León, A.E. The Influence of Different Air-Drying Conditions on Bioactive Compounds and Antioxidant Activity of Berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 126–151. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Rupérez, P. Indigestible fraction of okara from soybean: Composition, physicochemical properties and in vitro fermentability by pure cultures of Lactobacillus acidophilus and Bifidobacterium bifidum. Eur. Food Res. Technol. 2009, 228, 685–693. [Google Scholar] [CrossRef]
- Aly, A.A.; Ali, H.G.M.; Eliwa, N.E.R. Phytochemical screening, anthocyanins and antimicrobial activities in some berries fruits. J. Food Meas. Charact. 2019, 13, 911–920. [Google Scholar] [CrossRef]
- Rodríguez Sauceda, E.N. Uso de agentes antimicrobianos naturales en la conservación de frutas y hortalizas. Ra Ximhai 2011, 153–170. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Zafrilla, P.; Romero, F.; Abellan, P.; Artes, F.; Tomas-Barberan, F.A. Color Stability of Strawberry Jam as Affected by Cultivar and Storage Temperature. J. Food Sci. 1999, 64, 243–247. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Wrolstad, R.E. Comparison of the stability of pelargonidin-based Anthocyanins in strawberry juice and concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]

| P | DP60 | DP70 | CP | FP | |
| aw | 0.989 ± 0.003 d | 0.236 ± 0.004 c | 0.189 ± 0.004 a | 0.216 ± 0.004 b,c | 0.20 ± 0.06 b |
| xw (g water/g) | 0.722 ± 0.003 c | 0.032 ± 0.002 b | 0.017 ± 0.002 a | 0.017 ± 0.002 a | 0.019 ± 0.0006 a |
| Xss (g ss/g dm) | 0.283 ± 0.006 a | 0.289 ± 0.012 a | 0.281 ± 0.011 a | 0.340 ± 0.013 b | 0.451 ± 0.012 c |
| L* | 26.5 ± 1.0 a | 37.52 ± 0.15 b | 37.5 ± 0.3 b | 37.5 ± 0.2 b | 37.74 ± 0.13 b |
| a* | 3.0 ± 0.3 a | 4.3 ± 0.5 c | 3.80 ± 0.10 b | 3.8 ± 0.08 b | 3.37 ± 0.05 a |
| b* | 0.19 ± 0.3 a | 0.70 ± 0.10 b | 0.65 ± 0.09 b | 0.68 ± 0.08 b | 0.99 ± 0.05 c |
| ΔE | - | 11.1 ± 0.8 c | 11.0 ± 0.7 c | 0.06 ± 0.12 a | 0.46 ± 0.11 b |
| C | 3.0 ± 0.3 a | 4.3 ± 0.5 c | 3.86 ± 0.08 b | 3.9 ± 0.07 b | 3.93 ± 0.5 a,b |
| h | 3.7 ± 0.7 a | 9.2 ± 0.6 b | 9.8 ± 1.5 b | 10.1 ± 1.3 b | 14.6 ± 0.9 c |
| Cellulose (g/100 g dm) | - | - | - | 18.0 ± 0.4 b | 16.69 ± 0.14 a |
| Hemicellulose (g/100 g dm) | - | - | - | 12.846 ± 0.018 b | 10.444 ± 0.003 a |
| Lignin (g/100 g dm) | - | - | - | 7.6 ± 0.2 b | 6.6 ± 0.2 a |
| Insoluble fibre (g/100 g dm) | - | - | - | 25.6 ± 0.8 b | 23.24 ± 0.06 a |
| Total fibre (g/100 g dm) | - | - | - | 38.5 ± 0.8 b | 33.69 ± 0.06 a |
| CP | FP | |
|---|---|---|
| Specific volume (mL/g) | 9.60 ± 0.12 b | 7.57 ± 0.06 a |
| Solubility (%) | 31.6 ± 1.5 a | 33.1 ± 0.7 a |
| Water interaction properties | ||
| Hygroscopicity (g/100 g) | 61 ± 3 a | 62.7 ± 1.8 a |
| Wettability (s) | 175 ± 21 b | 77 ± 6 a |
| Swelling capacity (mL/g) | 2.88 ± 0.13 b | 2.56 ± 0.06 a |
| Water holding capacity (WHC) (g/g) | 5.1 ± 0.2 b | 4.63 ± 0.16 a |
| Water retention capacity (WRC) (g/g) | 3.4 ± 0.3 a | 3.08 ± 0.18 a |
| Emulsifying properties | ||
| Oil holding capacity (OHC) (g/g) | 2.7 ± 0.6 a | 2.9 ± 0.5 a |
| Emulsifying activity (%) | 0.41 ± 0.13 a | 0.53 ± 0.12 a |
| Emulsion stability (%) | 3± 2 b | 1.5 ± 0.7 a |
| P | DP60 | DP70 | CP | FP | |
|---|---|---|---|---|---|
| Total phenols (mg GAE/100 g dm) | 4.4 ± 0.2 d | 3.94 ± 0.10 c | 4.48 ± 0.12 d | 2.92 ± 0.11 a | 3.35 ± 0.10 b |
| Monomeric anthocyanins (mg glucosid-3-cyanidin/100 g dm) | 74.5 ± 0.4 d | 55.5 ± 0.4 c | 48.9 ± 0.7 b | 49.0 ± 0.9 b | 31 ± 4 a |
| DPPH (mg TE/g dm) | 145.7 ± 0.6 d | 118.6 ± 0.7 c | 102.4 ± 1.2 b | 101.6 ± 1.4 b | 84 ± 3 a |
| ABTS (mg TE/g dm) | 26.5 ± 1.0 a | 37.52 ± 0.15 b | 37.5 ± 0.3 b | 37.5 ± 0.2 b | 37.74 ± 0.13 b |
| 0 | 4 | 8 | 12 | 16 | 20 | ||
|---|---|---|---|---|---|---|---|
| aw | CP | 0.236 ± 0.004 b,c | 0.246 ± 0.003 c | 0.231 ± 0.003 a,b | 0.219 ± 0.004 a | 0.276 ± 0.014 d | 0.311 ± 0.008 e |
| FP | 0.198 ± 0.06 a | 0.218 ± 0.01 a,b | 0.254 ± 0.006 b,c | 0.305 ± 0.003 c,d | 0.315 ± 0.003 d | 0.293 ± 0.003 d | |
| xw (g w/g) | CP | 0.017 ± 0.002 a | 0.024 ± 0.005 b | 0.030 ± 0.005 b,c | 0.0264 ± 0.0013 b,c | 0.029 ± 0.004 b,c | 0.032 ± 0.001 c |
| FP | 0.0190 ± 0.001 a | 0.023 ± 0.003 a | 0.028 ± 0.002 b | 0.0300 ± 0.001 c | 0.0343 ± 0.001 d | 0.039 ± 0.002 e | |
| L* | CP | 37.51 ± 0.26 a | 38.04 ± 0.06 b | 38.10 ± 0.22 b | 37.72 ± 0.08 a | 37.49 ± 0.10 a | 37.74 ± 0.12 a |
| FP | 37.71 ± 0.12 c | 36.6 ± 0.2 a | 36.69 ± 0.04 ª,b | 36.71 ± 0.12 ª | 36.78 ± 0.03 ª,b | 36.85 ± 0.07 b | |
| a* | CP | 3.80 ± 0.09 c | 3.59 ± 0.03 a,b | 3.44 ± 0.08a | 3.73 ± 0.06 b,c | 3.67 ± 0.10 b,c | 3.81 ± 0.12 c |
| FP | 3.81 ± 0.12 c | 3.49 ± 0.07 a | 3.503 ± 0.012 a | 3.502 ± 0.012 a | 3.53 ± 0.04 a | 3.67 ± 0.01 b | |
| b* | CP | 0.65 ± 0.09 b | 0.44 ± 0.05 a | 0.46 ± 0.09 a | 0.77 ± 0.03 c | 0.91 ± 0.02 d | 0.99 ± 0.05 d |
| FP | 0.99 ± 0.05 d | 0.25 ± 0.2 a | 0.41 ± 0.4 b | 0.46 ± 0.03 b,c | 0.501 ± 0.012 b,c | 0.59 ± 0.03 c | |
| C | CP | 3.86 ± 0.082 b | 3.61 ± 0.03 a | 3.47 ± 0.08 a | 3.81 ± 0.06 b | 3.78 ± 0.09 b | 3.86 ± 0.13 b |
| FP | 3.93 ± 0.13 c | 3.45 ± 0.12 a | 3.511 ± 0.014 a | 3.533 ± 0.012 a | 3.721 ± 0.011 b | 3.724 ± 0.013 b | |
| h | CP | 14.6 ± 1.5 b | 7.0 ± 0.8 a | 7.6 ± 1.4 a | 11.67 ± 0.5 b | 14.0 ± 0.7 d | 14.6 ± 0.2 d |
| FP | 14.6 ± 0.2 d | 4 ± 3 a | 6.6 ± 1.3 b | 7.5 ± 0.5 b,c | 8.2 ± 0.3 b,c | 9.2 ± 0.5 c | |
| ΔE | CP | - | 0.6 ± 0.2 b | 0.72 ± 0.04 b | 0.25 ± 0.19 a | 0.29 ± 0.17 a | 0.4 ± 0.15 a,b |
| FP | - | 1.37 ± 0.18 b | 1.2 ± 0.4 a,b | 1.173 ± 0.110 a,b | 1.09 ± 0.13 a | 0.96 ± 0.12 a |
| 0 | 4 | 8 | 12 | 16 | 20 | ||
|---|---|---|---|---|---|---|---|
| Total phenols (mg GAE/100 g dm) | CP | 3.02 ± 0.12 a | 3.12 ± 0.15 a | 3.22 ± 0.03 a | 3.12 ± 0.2 a | 3.3 ± 0.2 a | 3.20 ± 0.13 a |
| FP | 3.36 ± 0.10 b | 3.27 ± 0.11 a,b | 3.05 ± 0.15 a | 3.00 ± 0.14 a,b | 3.03 ± 0.18 a,b | 3.01 ± 0.3 a | |
| Monomeric anthocyanins (mg glucosid-3-cyanidin/100 g dm) | CP | 49 ± 3 a | 48 ± 5 a | 47 ± 2 a | 45.1 ± 1.6 a | 47 ± 3 a | 49 ± 3 a |
| FP | 40 ± 2 a | 44 ± 3 a | 40 ± 3 a | 43 ± 2 a | 40 ± 5 a | 43 ± 3 a | |
| DPPH (mg TE/g dm) | CP | 101.1 ± 0.7 d | 85.2 ± 0.9 b | 91.6 ± 0.6 c | 104.9 ± 0.8 d,e | 107 ± 3 e | 82.7 ± 1.5 a |
| FP | 84.5 ± 1.9 b | 87 ± 2 b | 86.9 ± 1.4 b | 85.5 ± 0.5 b | 86.4 ± 1.5 b | 76.3 ± 0.3 a | |
| ABTS (mg TE/g dm) | CP | 58.0 ± 1.2 a | 61 ± 3 a | 60.3 ± 1.8 a | 58 ± 0.4 a | 60.4 ± 1.9 a | 59 ± 3a |
| FP | 62 ± 1.2 a | 62 ± 1.2 a | 63.1 ± 1.3 a | 62.6 ± 0.5 a | 63.37 ± 0.18 a | 61.8 ± 1.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabuig-Jiménez, L.; Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, N. Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace. Sustainability 2022, 14, 1839. https://doi.org/10.3390/su14031839
Calabuig-Jiménez L, Hinestroza-Córdoba LI, Barrera C, Seguí L, Betoret N. Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace. Sustainability. 2022; 14(3):1839. https://doi.org/10.3390/su14031839
Chicago/Turabian StyleCalabuig-Jiménez, Laura, Leidy Indira Hinestroza-Córdoba, Cristina Barrera, Lucía Seguí, and Noelia Betoret. 2022. "Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace" Sustainability 14, no. 3: 1839. https://doi.org/10.3390/su14031839
APA StyleCalabuig-Jiménez, L., Hinestroza-Córdoba, L. I., Barrera, C., Seguí, L., & Betoret, N. (2022). Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace. Sustainability, 14(3), 1839. https://doi.org/10.3390/su14031839









