Abstract
The consumption of fresh produce is steadily increasing and chlorine washing is the most commonly used method of disinfecting fresh produce. However, chlorine washing possesses a potential risk. Hence, this study used ozone microbubble (OMB) water to disinfect Salmonella Enteritidis, S. Typhimurium, Staphylococcus aureus, and Escherichia coli on tomatoes. After injecting ozone into the microbubble generator, OMB was fulfilled in a 10 L tank for 10 or 20 min. The inoculated tomatoes were washed for 30 or 60 s. Control groups included unwashed, water-washed, microbubble-only, and ozone-only. The microbial populations were significantly lower on the OMB-treated tomatoes than controls (p < 0.05), but not between various fulfilling or treatment time (p > 0.05). When tomatoes were treated with OMB with 10 min fulfilling and 30-s washing, the differences of tested bacteria and water washing, ozone-only, and microbubble-only were: S. Enteritidis: 4.11, 3.37, 2.54 log CFU/tomato; S. Typhimurium: 4.83, 4.50, 2.78 log CFU/tomato; E. coli: 4.31, 4.08, 2.09 log CFU/tomato; S. aureus: 4.12, 3.93, 2.82 log CFU/tomato. In addition, significant higher ozone concentrations and conductivity were detected in OMB water than other groups (p < 0.05). Color, texture, and sensory characteristics of the OMB-treated tomatoes were not significantly different from other groups (p > 0.05). This study demonstrated that OMB effectively inactivated bacteria on tomatoes and did not affect the physical and sensory characteristics of tomatoes.
1. Introduction
The consumption of fresh produce has been steadily increasing due to modern health trends; the major risk of fresh produce is microbial contamination. The major pathogens are Salmonella spp., pathogenic Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes [1,2,3]. Among fresh produce, tomato is a popular item which is a common ingredient in salads, sandwiches, or consumed alone as fruit. According to the Centers for Disease Control and Prevention (CDC) survey in 2015, the fresh produce with the most associated outbreaks were tomatoes [4]. In the United States, 15 outbreaks associated with tomatoes caused 1959 illnesses, 384 hospitalizations, and 3 deaths from 1990 to 2010 [5]. Among those outbreaks, multistate outbreaks associated with tomatoes occurred several times from 1996–2008, and all those outbreaks were caused by Salmonella spp. [6]. For example, several Salmonella serovars, including Typhimurium, Javiana, Anatum, Thompson, and Muenchen, were associated with a Roma tomato outbreak in 2004 and resulted in 427 illnesses [7]. Furthermore, four large outbreaks associated with tomatoes and Salmonella occurred in 2005 and 2006, totaling 459 cases; the involved Salmonella serovars included Newport, Braenderup, and Typhimurium, which caused 187, 82, and 190 cases, respectively [8]. In addition to Salmonella spp., S. aureus also caused outbreaks associated with tomatoes, such as the recent outbreak in Vietnam [9].
Presently, the most common practice for disinfecting microorganisms on tomatoes is washing by chlorine-based disinfectants, including sodium hypochlorite, calcium hypochlorite, and chlorine dioxide [1,2]. Although the chlorine disinfectants are commercially affordable and effective, a potential carcinogen, trihalomethane, could be formed by the reaction of chlorine and the organic substances in the washing solution [3]. Several techniques were tested to sanitize tomatoes without using chlorine, which included ozone gas and e-beam irradiation [1], ultraviolet [4], corona plasma [5], plasma-activated water (PAW) [6], and fixed multi-frequency sonication [7]. These techniques were effective, but also have some disadvantages that included no water washing to remove debris on tomato surface or a limited amount of water. Thus, a new technique which is able to produce a large amount of water and possesses an effective antimicrobial ability is needed.
Microbubbles (MB) are defined as bubbles with a diameter below 50 μm [8,9], whose size and density are closely associated with the inputted air pressure, generator power, water amount, and temperatures [9,10]. When microbubbles come into contact with the sample, the bubbles burst and release shear force and surface tension, which trigger substances to detach from the sample surface and achieve cleaning effects [11]. When combined with disinfectants, microbubbles could enhance the antimicrobial activity of the disinfectants on food items [12,13,14,15,16,17,18]. Among them, ozone was the most commonly combined disinfectant with microbubbles since the tiny bubbles greatly increase the surface/volume ratio, extend retention time, and solubility of ozone in water [13,14,15,16,17]. Ozone microbubble (OMB) was demonstrated to be effective in removing pesticides [14,19,20] and microorganisms on the food surface. Kwack et al. (2014) washed alfalfa seeds by water, microbubble alone, ozone water (3.5 ppm), OMB (5.3 ppm), and hypochlorite (5000 ppm) for 5 min. The greatest microbial reductions were obtained from OMB and hypochlorite [15]. However, the high concentration of hypochlorite negatively affected the seed germination and sprout weight. In contrast, no significant difference was found between OMB and water control for germination and growing weight. OMB was also shown to be superior to hot water and hypochlorite in reducing microbial load on cantaloupe [21]. In another study, leafy vegetables, such as sweet basil and mint, were washed by OMB (0.5, 1.0, or 2.0 ppm) or hypochlorite (50 or 100 ppm). Results showed that greater reduction of E. coli was achieved by OMB (1.0 ppm) when compared with hypochlorite (100 ppm) [17]. Additionally, color and texture were not altered after OMB treatment. This study also showed that the antibacterial effect at 10 °C was higher than at 30 °C, but not statistically significant. Similarly, Chuajedton et al. (2017) tested OMB against E. coli O157:H7 suspension and significantly higher antibacterial effect and ozone concentration was obtained at 13 °C rather than at 28 °C [13]. Microbubble was also used as the water of a hydroponic culture [22]. Compared with regular water, more than 3 log CFU/mL reduction for fungi (Fusarium oxysporum f. sp. melonis) and bacterium (Pectobacterium carotovorum subsp. carotovorum) was obtained. The water quantities in the aforementioned studies of MB were between 1 and 10 L, which were larger than for other new techniques, such as PAW. Therefore, OMB possesses a potential to sanitize tomatoes on a commercial scale. Until now, OMB has not been used to inactivate bacterial pathogens on tomatoes and its efficacy against Gram-positive bacteria has not been tested. Additionally, the optimal operative parameters and the physiochemical properties of OMB water and the effects on the sensory characteristics of tomatoes was not determined.
Therefore, this study aimed to determine the efficacy of OMB against S. Enteritidis, S. Typhimurium, E. coli, or S. aureus on tomato surfaces with various combinations of activation and treatment time periods. Several control groups, including water washing, ozone only, and microbubble only, were conducted. Physicochemical characteristics of the OMB water and the controls’ water, such as oxidation reduction potential (ORP), pH, and conductivity, were determined to understand the combined effect of ozone and microbubble for the antibacterial mechanism. Lastly, the texture, color, and sensory qualities of the treated tomatoes were verified.
2. Material and Methods
2.1. Preparation of Bacterial Suspension
The common pathogens associated with tomatoes, Salmonella enterica subsp. enterica serovars Enteritidis (ATCC 13076) and Typhimurium (ATCC 14028), Staphylococcus aureus (ATCC 12600), as well as the most used sanitation indicator, Escherichia coli (ATCC 23815), were used. All bacteria were maintained on tryptic soy agar (TSA) and confirmed by the selective media, such as xylose lysine deoxycholate agar (XLD), Baird-Parker (BP) agar, and eosin methylene blue (EMB) agar, respectively. The working culture was freshly prepared by inoculating in tryptic soy broth (TSB), then incubated at 37 °C for 18–20 h twice consecutively. All bacterial cultures were maintained at a 1.0–1.5 optical density at 600 nm (OD600), which was approximately 9 log CFU/mL. All media were purchased from Difco Laboratories (Detroit, MI, USA).
2.2. Preparation of Ozone Microbubble (OMB)
Ozone was generated on site by an oxygen tank and plasma-ozone generator, then injected into the motor (Nikuni, KTM20ND07Z, Tokyo, Japan), which mixed the ozone and water to generate ozone microbubbles (Figure 1). The ozone generator was set to produce ozone concentration at 20 ppm and saturation of ozone (>10 ppm) in OMB water was achieved in 7–9 min. Therefore, 10 and 20 min of activation was chosen. The OMB water flew through a tank which separated the microbubbles and large bubbles. The microbubble delivering tube was placed into the bottom of a plastic beaker containing 10 L of water, and OMB water was circulated back the motor. Based on the preliminary study, the air flow rate of injecting air was 2.5 L/min. The pressures of releasing and retaking water were 4.5 and 2.719 kg/cm2, respectively. The diameter of the microbubbles was determined by a light microscope (Leica, DM500, Wetzlar, Germany) connected to a high-resolution camera (Leica, ICC50 HD, Wetzlar, Germany) and computer with the relevant software (Leica Application Suite V3.4.0). The diameter of the microbubbles ranged from 3.84–11.99 μm, which fitted the definition of microbubbles.
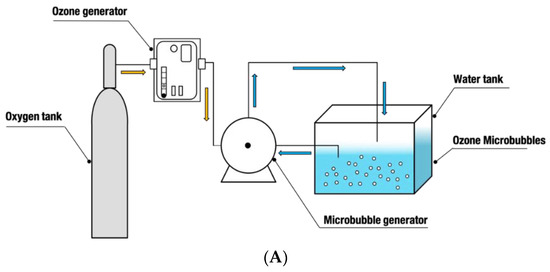
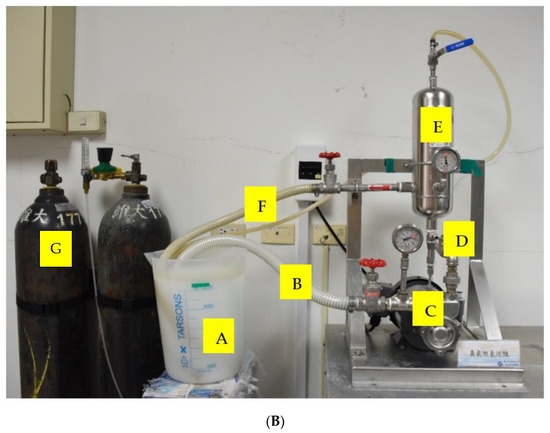
Figure 1.
(A) The diagram of the ozone microbubble device. (B) The microbubble system: A: the plastic beaker containing 10 L water; B: water circulating into motor, pressure: 2.719 kg/cm2; C: motor; D: air input, flow rate: 2.5 L/min; E: bubble mixing tank; F: microbubble water entering beaker, pressure: 4.5 kg/cm2; G: oxygen tank. The flow direction of microbubble water is indicated by the arrows.
2.3. Inoculation and Inactivation of Bacteria on Tomatoes
Beefsteak tomato (Lycopersicon esculentum cv. Beefsteak), which is the most common cultivar for sandwiches and salads in Taiwan, was purchased from nearby grocery stores. Each tomato (about 80–90 g) was washed thoroughly by running tap water, then 100 ppm sodium hypochlorite (NaClO) for 30 s, then sterile water for another 30 s. Reagent-grade NaClO (Nihon Shiyaku, Tokyo, Japan) was used and the concentration of the available chlorine was confirmed by (ISO 7393-3:1990). After wiping with sterile paper, tomatoes were air-dried in a laminar hood. The bacterial culture was centrifuged at 5000× g for 5 min at 4 °C and resuspended in new TSB, then 100 μL of the TSB suspension was delivered in 30 drops onto the surface of one tomato. After being air-dried for 20–30 min, inoculated tomatoes were placed into the beaker containing 10 L OMB water for 30 or 60 s. Before treatment, the OMB device was activated to fulfill the 10 L container for 10 or 20 min. Hence, four combinations of OMB treatments were used. After OMB treatment, a treated tomato was transferred into a sterile bag with 50 mL of phosphate buffer saline (PBS, pH 7.2), then gently rubbed by hand for 2 min. After decimally serial dilution, 0.1 mL of the suitable dilutions was spread onto plate count agar (PCA). Triplicate plates were used for each dilution and the plates were incubated at 37 °C for 18–24 h. The dilutant was also spread onto XLD, EMB, or BP agar to confirm that the recovered bacteria were Salmonella sp., E. coli, or S. aureus. Four controls were applied, which included unwashed, washed by sterile water, microbubble only, or ozone only. The operational parameters for the controls of microbubble and ozone only were the same as the OMB group. Additionally, a membrane filtration method was used to enumerate the bacterial population in the treated water. One hundred mL of water was collected after testing and drawn through the membrane (0.45 μm, cellulose nitrate, Sartorius, Göttingen, Germany). This membrane was placed onto PCA, then incubated at 37 °C for 18–24 h.
2.4. Measurement of pH, Oxidation Reduction Potential (ORP), Electrical Conductivity (EC), and Ozone (O3) of OMB Water
The values of pH and conductivity were measured by a pH/conductivity probe (serial 100 probe, Cole-Parmer, Vernon Hills, IL, USA) connected with a pH/conductivity meter (PC-200, Cole-Parmer, Vernon Hills, IL, USA). The values of ORP were measured by an ORP probe (ORP-148G, TECPEL, Taipei, Taiwan) connected with a pH/ORP meter (SP-2300, SUNTEX CO. LTD, New Taipei, Taiwan). Ozone concentrations were determined by a handheld ozone meter (Twinno DOZ30, CLEAN Instruments Co., New Taipei, Taiwan).
2.5. Analyses of Tomato Texture, Color, and Sensory Characteristics
Among the different combinations of OMB activation and washing time, the combination that showed the best microbial reduction was to be used for texture, color, and sensory analysis. However, no significant difference between bacterial populations was found from the different combinations of activation and washing time. Hence, the shortest time period, 10 min activation following 30 s washing, was used. Uninoculated tomatoes were used for these analyses. Tomatoes washed by tap water were used as negative controls. Tomatoes washed by chlorine (100 ppm) and rinsed with water were used as commercial controls.
2.5.1. Analyses of Tomato Texture
Characteristics of the texture of treated and control tomatoes were analyzed by a texture analyzer (CR-500DX, Sun Scientific Co., Tokyo, Japan). The shear force was measured by a No. 13 probe. The breaking force and chewiness of the tomatoes was measured by a No. 34 probe. The descending speed of the probes was 1 mm/s, and the starting distance between the probes and the samples was 2.3 mm, 1.6 mm, and 2.4 mm for breaking force, chewiness, and shear force, respectively. Each test contained at least triplicate samples.
2.5.2. Analyses of Tomato Color
The CIE-Lab values of the tomatoes were measured by a colorimeter (NE 4000, Nippon Denshoku, Tokyo, Japan). L*a*b* values were indexes for brightness, red/green, and yellow/blue, respectively. Greater L* values indicated higher brightness. Greater a* and b* values indicated more red and yellow. In contrast, negative a* and b* values indicated more green and blue. The colorimeter was calibrated with the standard black/white plates, then the slices of treated and control tomatoes were placed into the enclosed chamber of the colorimeter for measurement. Triplicate samples were used for each test. The color difference (ΔE) was calculated according to the following formula: ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2.
2.5.3. Sensory Evaluation of Tomatoes
A total of 20 people, 12 females and 8 males, in the range of age from 21 to 56, were trained with fresh tomatoes to standardize the sensory characteristics. After training, these panelists were selected to evaluate the aroma, taste, texture, color, and overall acceptance. The pair comparison method was used based on the protocols of the sensory evaluation guidelines. The fresh tomatoes washed by water and chlorine (100 ppm) were presented as the negative control and commercial washing, respectively. During sensory evaluation, the tomatoes washed by water, chlorine (100 ppm), or OMB were labeled with random numbers and served as testing samples. A group of water-washed tomatoes was served as the standard. The scores were from 1 to 9 (1 = extremely dislike, 9 = extremely like) and the score of the standard group was set at 7. All tomatoes were cut into 4 equal pieces longitudinally and each panelist tasted 3 pieces for each sample.
2.6. Statistical Analyses
Triplicate samples were used for each treatment and all experiments were conducted at least twice. After obtaining the average and standard deviation of each treatment, data were analyzed by one-way ANOVA and Duncan’s test. The significant differences between treatments were set at p = 0.05. All statistical analyses were performed by IBM SPSS program (version 22.0, St. Armonk, NY, USA).
3. Results and Discussion
3.1. Antibacterial Effects of Various Treatments
OMB treatments showed significantly higher reduction (p < 0.05) against all the tested bacteria than other treatments (Table 1). When compared with samples washed by water, 4.05–4.45, 4.83–4.91, 4.06–4.35, and 4.12–4.38 log CFU/tomato reductions were obtained for S. Enteritidis, S. Typhimurium, E. coli, and S. aureus, respectively, on the tomatoes treated with OMB. Hence, S. Typhimurium was the most susceptible and E. coli showed the highest resistance to OMB treatment but no significant difference was observed among the tested bacteria. Longer activation and washing time elevated the antibacterial activity and the greatest reduction was obtained from the combination of 20 min activation time and 60 s washing time. However, the reductions were not significantly lower when the combinations of shorter activation and washing time were used (p > 0.05). Antibacterial activity of microbubble-only was significantly lower than OMB treatment but higher (p < 0.05) than applying ozone-only. The bacterial populations in the OMB-treated water was below detection level (1 CFU/100 mL) and the water in the ozone-only treatment contained approximately 1 log CFU/100 mL. In contrast, the water of microbubble-only contained around 2 log CFU/100 mL of the tested bacteria, which was significantly higher than those of the ozone-only and OMB treatments. These results indicated that the antibacterial mechanism of microbubble mainly detaching the adhesive bacteria from the food surface and ozone only was not adequate in inactivating the bacteria on the tomato surface. Combining ozone and microbubbles, adhesive bacteria were detached from tomato surface and inactivated by ozone in the water. Thus, a greater reduction of bacterial population was achieved.

Table 1.
The populations (log CFU/tomato) of Salmonella Enteritidis, Salmonella Typhimurium, Escherichia coli, and Staphylococcus aureus on tomatoes with various operative treatments.
Kwack et al. (2014) reported 2.66 log reduction of natural microorganisms on alfalfa seeds treated by OMB when compared with water washing [17]. Around 0.5 log reduction was shown on alfalfa seeds treated with ozone or microbubble only compared with water washing control. Phaephiphat & Mahakarnchanakul (2018) reported 2.8 and 2.6 log CFU/g reduction of S. Typhimurium and E. coli, respectively, obtained from OMB treatment on sweet basil [19]. However, this study showed there was no significant difference in bacterial reduction between microbubble-only and water treatment. Moreover, the results of scanning electron microscope showed no obvious morphological damage of bacteria cells after microbubble only treatment. A recent study [23] reported that microbubbles effectively removed soil from spinach, but did not enhance the antibacterial activity of NaOCl (100 ppm) significantly against E. coli O157:H7.
This is the first study using a Gram-positive bacterium, S. aureus, for antibacterial activity of microbubbles on food and no significant difference (p > 0.05) was obtained between Gram-positive and negative bacteria. In the previous studies [19,23,24], only E. coli and Salmonella spp. were tested. Moreover, the effect of activation time was also first described in this study. Although there was no significant difference between 10 and 20 min of activation time in this study, an ongoing study in our laboratory showed the longer activation time (20 min) presenting greater antibacterial effects in a large volume (100 L).
3.2. Physiochemical Characteristics of Treatment Water at Various Parameters
Among the physiochemical characteristics of treated water, the values of pH and conductivity were not significantly different between treatments (p > 0.05) (Table 2). However, ORP and ozone concentrations were significantly higher in the water of OMB and ozone-only treatments, particularly in the OMB group. Furthermore, the OMB group showed much higher concentrations of ozone than the ozone-only group. This indicated that microbubbles allowed to increase the solubility and retention time of ozone in water, and subsequently increased ozone concentrations. Kwack et al. (2014) reported similar results [17], in which the ozone concentrations of the OMB group was 2–3-fold those in the ozone-only group. In addition, ozone concentrations increased much faster in the OMB group than in the group using ozone only.

Table 2.
The values of pH, conductivity (μs/cm), oxidation reduction potential (ORP, mV), and ozone (ppm) of water in various treatments.
When ozone dissolves in water, it reacts with water molecules to form hydrogen peroxide (H2O2) or degrades to oxygen molecule (O2) and singlet oxygen (O), which further reacts with water molecules to form hydroxyl radicals (OH.) [25]. These reactive oxygen species (ROS) increase ORP values and inactivate microorganisms. The bursting of microbubbles also generates free radicals and this extra production of OH. increases ORP values [26], which also contributes to its antimicrobial activity [27,28,29]. This study demonstrated a synergistic effect of combining microbubble and ozone to elevate ozone concentrations and ORP values, which were the key elements in inactivating microorganisms. Several studies have also presented similar results [15,16,18]. However, this study conducted more control experiments simultaneously, such as the treatments of microbubble and ozone only, to offer a detailed baseline for comparison.
3.3. The Texture, Color, and Sensory Characteristics of Treated Tomatoes
The texture and sensory characteristics of the OMB-treated tomatoes were not significantly different (p > 0.05) with water- and chlorine-washed tomatoes (Figure 2 and Figure 3). The tomatoes in the water washing group showed significantly higher L* values (p < 0.05) than those in the OMB and chlorine washing group in colorimeter analysis (Table 3). However, nonsignificant difference of the scores for color (p > 0.05) were obtained between these three groups in sensory evaluation. In addition, L* values and scores of color sensory characteristics were not significantly different (p > 0.05) between the chlorine and OMB groups. These results indicated that both chlorine and ozone bleached the tomatoes slightly, which was detected by colorimeter analysis, but not by human observation. Since ozone possesses a strong aroma, aroma sensory characteristics are a potential concern for ozone-treated food, which is not addressed in previous studies. This study showed that the aroma of the OMB-treated tomatoes was not discriminated from that of other groups. Since MB enhanced the antibacterial efficacy of ozone, a short washing time, 30 s, was adequate. Therefore, the aroma of tomatoes was not affected by the ozone in OMB water in such a short treatment. Moreover, physical texture and other sensory characteristics were also not different from those of the water washing control.
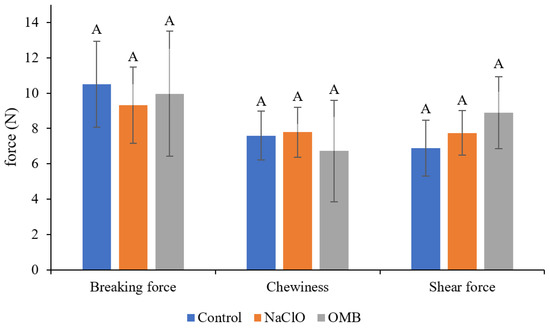
Figure 2.
The breaking force (N), chewiness (N), and shear force (N) of tomatoes after various treatments. There were three groups of tested tomatoes: Control: washed by water; NaClO: washed by 100 ppm NaClO, then rinsed by water; OMB: washed by ozone microbubble water for 10 min activation and 30 s washing time. Each group contained 3 sampling spots from 3 tomatoes (N = 9). The same letter on the top of column indicates no significant difference (p > 0.05).
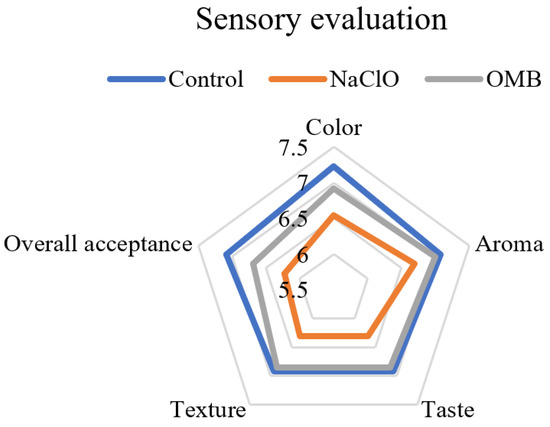
Figure 3.
Sensory evaluation of the tomato samples on treatment day. There were three groups of tested tomatoes: Control: washed by water; NaClO: washed by 100 ppm NaClO, then rinsed by water; OMB: washed by ozone microbubble water for 10 min activation and 30 s washing time. Each group contained 3 sampling spots from 3 tomatoes (N = 9).

Table 3.
CIE L*-a*-b* values of the tomato samples.
Water amount is a critical factor for applying novel techniques in practical use. The water amount (10 L) in this study was greater than most previous studies, such as 4 L tested in Klintham et al. (2017) [14] and Phaephiphat and Mahakanchanakul (2018) [19], or equal to the 10 L volume tested by Kobayashi et al. (2011) [30]. In addition, it offered a direct demonstration of the synergistic effect of microbubble and ozone, which provides a road map to understanding the antibacterial mechanism of ozone microbubble. The texture, color, and sensory characteristics of tomatoes were not altered by OMB treatment, which was seldom investigated in previous studies for inactivating microorganisms on fresh produce. Furthermore, a microbubble system capable of 100 L operation has been studied by this research group and promising results have been obtained.
4. Conclusions
A combining system of microbubble with ozone was used to inactivate S. Typhimurium, S. Enteritidis, E. coli, and S. aureus on tomato surface under various conditions. The susceptibility between the tested bacteria to OMB was not significantly different, though slightly lower reduction of E. coli was obtained. The capacity of the system was 10 L and it could be expanded to a larger volume. The results showed longer activation and washing time elevated the bactericidal activity, but no significant difference was observed between different activation and washing time periods. The largest values of ORP and conductivity were obtained in the water of the OMB group. Microbubbles were able to elevate ozone concentrations greatly and showed much higher antibacterial activity than the treatments of microbubble and ozone only. In addition, no negative effects on the texture, color, and sensory characteristics of tomatoes were observed after OMB treatment.
Author Contributions
Conceptualization, C.-Y.H., C.-M.L.; C.-P.H. and C.-T.L.; Data curation, Y.-R.C.; Funding acquisition, J.-S.W.; Investigation, Y.-R.C.; Methodology, C.-Y.H., Y.-R.C., H.-L.C., C.-P.H. and C.-M.L.; Project administration, C.-M.L.; Resources, H.-L.C.; Supervision, C.-M.L.; Writing—original draft, C.-M.L. and C.-Y.H.; Writing—review & editing, C.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST) under grant numbers MOST-109-2622-E-009-001-CC1, MOST-110-2637-E-992-013, MOST-110-2320-B-992-001-MY3 and MOST-110-2622-E-992-012-).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study is totally orginal and report no available data.
Acknowledgments
The authors would like to thank all of the individuals who volunteered for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wright, A.; Uprety, B.; Shaw, A.; Shama, G.; Iza, F.; Bandulasena, H. Effect of Humic Acid on E. coli Disinfection in a Microbubble-Gas Plasma Reactor. J. Water Process Eng. 2019, 31, 100881. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Elias, S.; Tombini Decol, L.; Tondo, E.C. Foodborne Outbreaks in Brazil Associated with Fruits and Vegetables: 2008 through 2014. Food Qual. Saf. 2018, 2, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Surveillance for Foodborne Disease Outbreaks; Annual Report; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2017. [Google Scholar]
- Bennett, S.D.; Littrell, K.W.; Hill, T.A.; Mahovic, M.; Behravesh, C.B. Multistate Foodborne Disease Outbreaks Associated with Raw Tomatoes, United States, 1990–2010: A Recurring Public Health Problem. Epidemiol. Infect. 2015, 143, 1352–1359. [Google Scholar] [CrossRef]
- Bartz, J.A.; Yuk, H.-G.; Mahovic, M.J.; Warren, B.R.; Sreedharan, A.; Schneider, K.R. Internalization of Salmonella Enterica by Tomato Fruit. Food Control 2015, 55, 141–150. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreaks of Salmonella Infections Associated with Eating Roma Tomatoes--United States and Canada, 2004. MMWR. Morb. Mortal. Wkly. Rep. 2005, 54, 325–328. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreaks of Salmonella Infections Associated with Raw Tomatoes Eaten in Restaurants—United States, 2005–2006. MMWR. Morb. Mortal. Wkly. Rep. 2007, 56, 909–911. [Google Scholar]
- Le, H.H.T.; Dalsgaard, A.; Andersen, P.S.; Nguyen, H.M.; Ta, Y.T.; Nguyen, T.T. Large-Scale Staphylococcus aureus Foodborne Disease Poisoning Outbreak among Primary School Children. Microbiol. Res. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Takahashi, M. Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas−Water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Tsuge, H. Micro-and Nanobubbles-Fundamentals and Applications; Jenny Stanford Publishing: New York, NY, USA, 2014; ISBN 9789814463102. [Google Scholar] [CrossRef]
- Parmar, R.; Majumder, S.K. Microbubble Generation and Microbubble-Aided Transport Process Intensification—A State-of-the-Art Report. Chem. Eng. Process. Process Intensif. 2013, 64, 79–97. [Google Scholar] [CrossRef]
- Sharma, P.K.; Gibcus, M.J.; van derMei, H.C.; Busscher, H.J. Influence of Fluid Shear and Microbubbles on Bacterial Detachment from a Surface. Appl. Environ. Microbiol. 2005, 71, 3668–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klintham, P.; Tongchitpakdee, S.; Chinsirikul, W.; Mahakarnchanakul, W. Two-Step Washing with Commercial Vegetable Washing Solutions, and Electrolyzed Oxidizing Microbubbles Water to Decontaminate Sweet Basil and Thai Mint: A Case Study. Food Control 2018, 94, 324–330. [Google Scholar] [CrossRef]
- Chuajedton, A.; Aoyagi, H.; Uthaibutra, J.; Pengphol, S.; Whangchai, K. Inactivation of Escherichia coli O157: H7 by Treatment with Different Temperatures of Micro-Bubbles Ozone Containing Water. Int. Food Res. J. 2017, 24, 1006–1010. [Google Scholar]
- Ikeura, H.; Hamasaki, S.; Tamaki, M. Effects of Ozone Microbubble Treatment on Removal of Residual Pesticides and Quality of Persimmon Leaves. Food Chem. 2013, 138, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kwack, Y.; Kim, K.; Hwang, H.; Chun, C. An Ozone Micro-Bubble Technique for Seed Sterilization in Alfalfa Sprouts. Korean J. Hortic. Sci. Technol. 2014, 32, 901–905. [Google Scholar] [CrossRef] [Green Version]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a Novel Emerging Technology for the Dissipation of Pesticide Residues in Foods–a Review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Phaephiphat, A.; Mahakarnchanakul, W. Surface Decontamination of Salmonella Typhimurium and Escherichia coli on Sweet Basil by Ozone Microbubbles. Cogent Food Agric. 2018, 4, 1558496. [Google Scholar] [CrossRef]
- Pongprasert, N.; Jitareerat, P.; Srilaong, V. Effect of Carbon Dioxide Micro Bubbles in Combination with Chlorine Dioxide Solution to Reduce Microbial Contamination and Browning of Fresh-Cut Cos Lettuce (Lactuca Sativa L.). Acta Hortic. 2020, 1275, 39–44. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Removal of Residual Pesticide, Fenitrothion, in Vegetables by Using Ozone Microbubbles Generated by Different Methods. J. Food Eng. 2011, 103, 345–349. [Google Scholar] [CrossRef]
- Li, P.; Wu, C.; Yang, Y.; Wang, Y.; Yu, S.; Xia, S.; Chu, W. Effects of Microbubble Ozonation on the Formation of Disinfection By-Products in Bromide-Containing Water from Tai Lake. Sep. Purif. Technol. 2018, 193, 408–414. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V. Air Microbubble Assisted Washing of Fresh Produce: Effect on Microbial Detachment and Inactivation. Postharvest Biol. Technol. 2021, 181, 111687. [Google Scholar] [CrossRef]
- Ushida, A.; Koyama, T.; Nakamoto, Y.; Narumi, T.; Sato, T.; Hasegawa, T. Antimicrobial Effectiveness of Ultra-Fine Ozone-Rich Bubble Mixtures for Fresh Vegetables Using an Alternating Flow. J. Food Eng. 2017, 206, 48–56. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of Ozone in Water: Rate of Initiation by Hydroxide Ions and Hydrogen Peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Seki, M.; Ishikawa, T.; Terada, H.; Nashimoto, M. Microbicidal Effects of Stored Aqueous Ozone Solution Generated by Nano-Bubble Technology. In Vivo 2017, 31, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Chiba, K.; Li, P. Formation of Hydroxyl Radicals by Collapsing Ozone Microbubbles under Strongly Acidic Conditions. J. Phys. Chem. B 2007, 111, 11443–11446. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, W.; Huo, M.; Zhao, H.; Lu, Y. Hydroxyl Radical Generation and Contaminant Removal from Water by the Collapse of Microbubbles Under Different Hydrochemical Conditions. Water Air Soil Pollut. 2018, 229, 86. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, B.; Zhu, W.; Tian, K.; Zhang, H.A. Review on Ultrasonic Catalytic Microbubbles Ozonation Processes: Properties, Hydroxyl Radicals Generation Pathway and Potential in Application. Catalysts 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, F.; Ikeura, H.; Ohsato, S.; Goto, T.; Tamaki, M. Disinfection Using Ozone Microbubbles to Inactivate Fusarium oxysporum f. Sp. melonis and Pectobacterium carotovorum Subsp. carotovorum. Crop Prot. 2011, 30, 1514–1518. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).