Use of Incinerated Eggshells to Produce Pidan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Incinerated Eggshell Powder
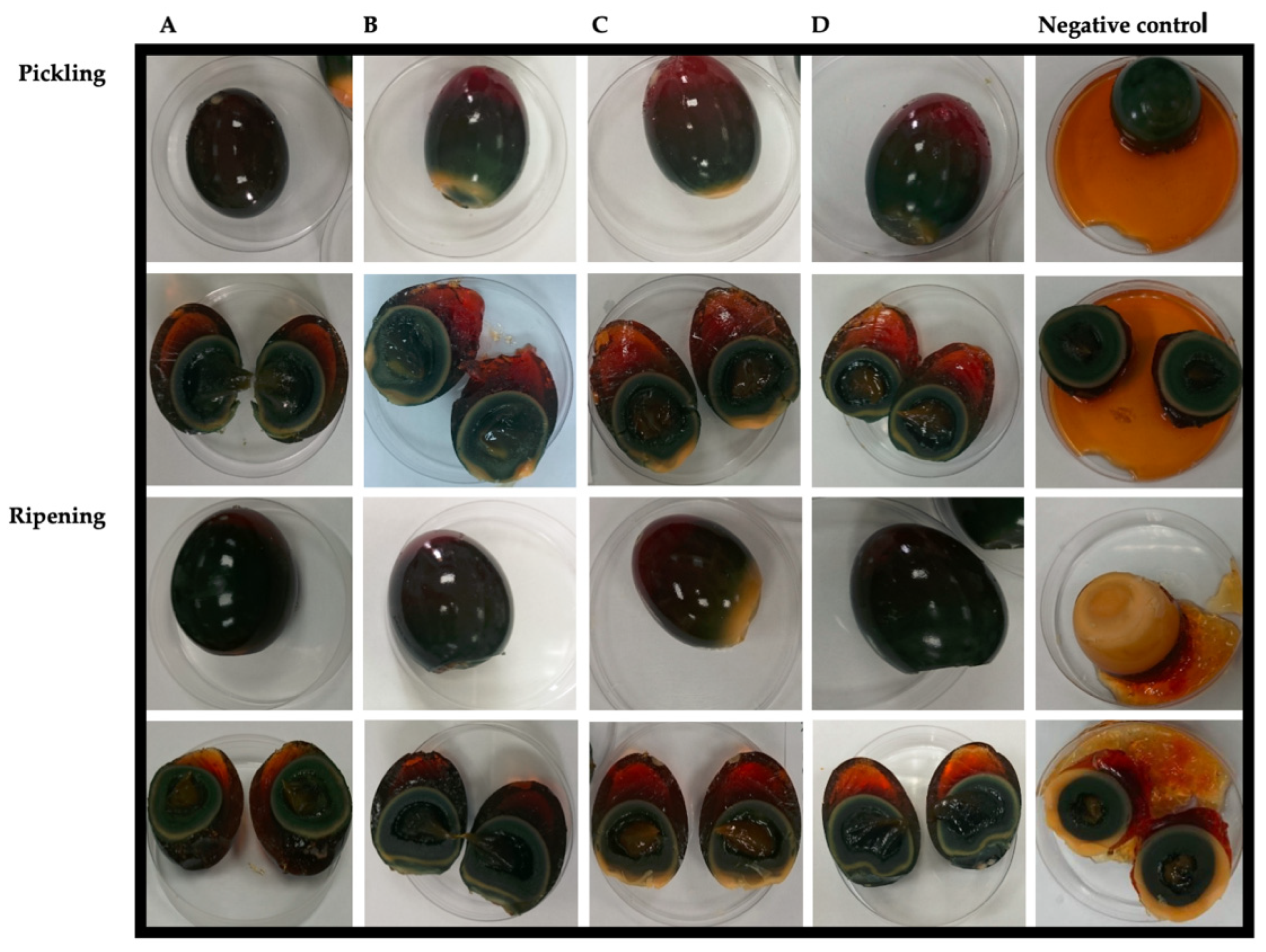
2.3. Pickling and Ripening Process
2.4. Hardness Ratio of Yolk
2.5. Texture Analysis
2.6. Color Characteristics
2.7. Sensory Evaluation
2.8. Mineral Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Confirmation of the Availability of the Pidan Ripening Process
3.2. The Hardening Percentages and Texture Characteristics
3.3. Color Characteristics of Pidan
3.4. Sensory Evaluation of Pidan
3.5. Mineral Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, Y.; Zhao, Y. Chapter 40—Inorganic Elements in Preserved Egg. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 427–434. [Google Scholar]
- Cai, J.; Sweeney, A.M. The Proof Is in the Pidan: Generalizing Proteins as Patchy Particles. ACS Cent. Sci. 2018, 4, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kaewmanee, T.; Benjakul, S.; Baharin, B.S. Comparative Study on the Nutritional Value of Pidan and Salted Duck Egg. Korean J. Food Sci. Anim. Resour. 2014, 34, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fung, D.Y.C. Alkaline-Fermented Foods: A Review with Emphasis on Pidan Fermentation. Crit. Rev. Microbiol. 1996, 22, 101–138. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, Y.; Xu, M.; Chen, Z.; Wang, S.; Tu, Y. Effects of Copper Ions on the Characteristics of Egg White Gel Induced by Strong Alkali. Poult. Sci. 2017, 96, 4116–4123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, C.; Luo, W.; Li, J.; Tu, Y.; Zhao, Y. Effects of Packaging Methods on the Quality of Heavy Metals–Free Preserved Duck Eggs During Storage. Poult. Sci. 2021, 100, 101051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tu, Y.; Xu, M.; Li, J.; Du, H. Physicochemical and Nutritional Characteristics of Preserved Duck Egg White. Poult. Sci. 2014, 93, 3130–3137. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Tu, Y.; Zhao, Y.; Luo, X.; Wang, J.; Wang, M. Changes in Gel Characteristics of Egg White under Strong Alkali Treatment. Food Hydrocoll. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Chang, H.-M.; Tsai, C.-F.; Li, C.-F. Changes of Amino Acid Composition and Lysinoalanine Formation in Alkali-Pickled Duck Eggs. J. Agric. Food Chem. 1999, 47, 1495–1500. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S. Duck egg albumen: Physicochemical and functional properties as affected by storage and processing. J. Food Sci. Technol. 2019, 56, 1104–1115. [Google Scholar] [CrossRef]
- Weijers, M.; van de Velde, F.; Stijnman, A.; van de Pijpekamp, A.; Visschers, R.W. Structure and rheological properties of acid-induced egg white protein gels. Food Hydrocoll. 2006, 20, 146–159. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, Y.; Li, J.; Xu, M.; Yang, Y.; Nie, X.; Yao, Y.; Du, H. Effects of alkaline concentration, temperature, and additives on the strength of alkaline-induced egg white gel. Poult. Sci. 2014, 93, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.-Y.; Chen, Y.-C.; Chen, Y.-P.; Shiu, J.-S.; Wang, S.-Y. Development of a heatable duck egg white translucent jelly: An evaluation of its physicochemical properties and thermal stability. Poult. Sci. 2021, 100, 101373. [Google Scholar] [CrossRef] [PubMed]
- Ganasen, P.; Benjakul, S. Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. LWT-Food Sci. Technol. 2010, 43, 77–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, X.; Li, J.; Xu, M.; Tu, Y. Effect of basic alkali-pickling conditions on the production of lysinoalanine in preserved eggs. Poult. Sci. 2015, 94, 2272–2279. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, D.; Shao, Y.; Xiong, C.; Li, J.; Tu, Y. Changes in physico-chemical properties, microstructures, molecular forces and gastric digestive properties of preserved egg white during pickling with the regulation of different metal compounds. Food Hydrocoll. 2019, 98, 105281. [Google Scholar] [CrossRef]
- Ge, L.; Liu, H. Engineering Grey Nanosystem as Activatable Ratio-colorimetric Probe for Detection of Lead Ions in Preserved Egg. Anal. Sci. 2020, 36, 1407–1413. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, X.; Aheto, J.H.; Wang, C.; Ernest, B.; Tian, X.; He, P.; Chang, X.; Wang, C. Application of volatile and spectral profiling together with multimode data fusion strategy for the discrimination of preserved eggs. Food Chem. 2020, 343, 128515. [Google Scholar] [CrossRef]
- Yang, B.; Ren, J.; Wang, M.; Luo, H.; Cao, Y. Concentrations and Chemical Fractions of Cu, Zn, Cd, and Pb at Ten Metallurgical Sites in China. Environ. Ence Pollut. Res. 2019, 26, 3603–3611. [Google Scholar] [CrossRef]
- Xu, L.; Yan, S.-M.; Cai, C.-B.; Yu, X.-P. Nondestructive Discrimination of Lead (Pb) in Preserved Eggs (Pidan) by Near-Infrared Spectroscopy and Chemometrics. J. Spectrosc. 2014, 2014, 253143. [Google Scholar] [CrossRef] [Green Version]
- Ganasen, P.; Benjakul, S. Effect of three cations on the stability and microstructure of protein aggregate from duck egg white under alkaline condition. Food Sci. Technol. Int. 2011, 17, 343–349. [Google Scholar] [CrossRef]
- Aendo, P.; Thongyuan, S.; Songserm, T.; Tulayakul, P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci. Total Environ. 2019, 689, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Aendo, P.; Netvichian, R.; Tippayalak, S.; Sanguankiat, A.; Khuntamoon, T.; Songserm, T.; Tulayakul, P. Health Risk Contamination of Heavy Metals in Yolk and Albumen of Duck Eggs Collected in Central and Western Thailand. Biol. Trace Elem. Res. 2017, 184, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, X.; Li, J.; Xu, M.; Tu, Y. Formation of lysinoalanine in egg white under alkali treatment. Poult. Sci. 2016, 95, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Chen, C.; Qiu, N.; Keast, R. Modulation of gut microbiota in rats fed whole egg diets by processing duck egg to preserved egg. J. Biosci. Bioeng. 2020, 130, 54–62. [Google Scholar] [CrossRef]
- Abín, R.; Laca, A.; Laca, A.; Díaz, M. Environmental assesment of intensive egg production: A Spanish case study. J. Clean. Prod. 2018, 179, 160–168. [Google Scholar] [CrossRef]
- Yegin, S.; Saha, B.; Kennedy, G.J.; Leathers, T.D. Valorization of egg shell as a detoxifying and buffering agent for efficient polymalic acid production by Aureobasidium pullulans NRRL Y-2311-1 from barley straw hydrolysate. Bioresour. Technol. 2019, 278, 130–137. [Google Scholar] [CrossRef]
- Ohshima, Y.; Takada, D.; Namai, S.; Sawai, J.; Kikuchi, M.; Hotta, M. Antimicrobial Characteristics of Heated Eggshell Powder. Biocontrol Sci. 2015, 20, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Patgiri, B.J.; Singh, T.R.; Fanasiya, K.M.; Bedarkar, P.; Prajapati, P.K. Analytical profile of Kukkutanda Tvak Bhasma (incinerated hen egg shells) prepared by two different methods. AYU 2017, 38, 158–164. [Google Scholar] [CrossRef]
- Cho, M.G.; Bae, S.M.; Jeong, J.Y. Egg Shell and Oyster Shell Powder as Alternatives for Synthetic Phosphate: Effects on the Quality of Cooked Ground Pork Products. Food Sci. Anim. Resour. 2017, 37, 571. [Google Scholar] [CrossRef] [Green Version]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Chong, B.W.; Othman, R.; Ramadhansyah, P.J.; Doh, S.I.; Li, X. Properties of Concrete with Eggshell Powder: A Review. Phys. Chem. Earth Parts A/B/C 2020, 120, 102951. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M. Egg shell waste as heterogeneous nanocatalyst for biodiesel production: Optimized by response surface methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hanein, T.; Simoni, M.; Woo, C.L.; Provis, J.L.; Kinoshita, H. Decarbonisation of Calcium Carbonate at Atmospheric Temperatures and Pressures, with Simultaneous Co2 Capture, through Production of Sodium Carbonate. Energy Environ. Sci. 2021, 14, 6595–6604. [Google Scholar] [CrossRef]
- Drake, M.A.; Delahunty, C.M. Chapter 20—Sensory Character of Cheese and Its Evaluation. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 517–545. [Google Scholar]
- Kelleher, S.D.; Saunders, W.S.; Fielding, W.R. Process for Isolating a Protein Composition and a Fat Composition from Mechanically Deboned Poultry. 2017. Available online: https://patents.justia.com/patent/20120171345 (accessed on 27 February 2022).
- Poitevin, E.; Nicolas, M.; Graveleau, L.; Richoz, J.; Andrey, D.; Monard, F.; Collaborators. Improvement of Aoac Official Method 984.27 for the Determination of Nine Nutritional Elements in Food Products by Inductively Coupled Plasma-Atomic Emission Spectroscopy after Microwave Digestion: Single-Laboratory Validation and Ring Trial. J. Aoac Int. 2009, 92, 1484–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jiang, A.; Chen, M.; Ockerman, H.W.; Chen, J. Effect of Different Alkali Treatments on the Chemical Composition, Physical Properties, and Microstructure of Pidan White. J. Food Sci. Technol. 2015, 52, 2264–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.-Y.; Lin, C.-M.; Patel, A.K.; Dong, C.; Shih, M.-K.; Hsieh, C.-W.; Hung, Y.-L.; Huang, P.-H. Development of novel green methods for preparation of lead-free preserved pidan (duck egg). J. Food Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Xu, M.; Yao, Y.; Wu, N.; Du, H.; Tu, Y. Alkali induced gelation behavior of low-density lipoprotein and high-density lipoprotein isolated from duck eggs. Food Chem. 2019, 311, 125952. [Google Scholar] [CrossRef]
- Ji, L.; Liu, H.; Cao, C.; Liu, P.; Wang, H.; Wang, H. Chemical and structural changes in preserved white egg during pickled by vacuum technology. Food Sci. Technol. Int. 2013, 19, 123–131. [Google Scholar] [CrossRef]
- Tu, Y.-G.; Zhao, Y.; Xu, M.-S.; Li, X.; Du, H.-Y. Simultaneous Determination of 20 Inorganic Elements in Preserved Egg Prepared with Different Metal Ions by ICP-AES. Food Anal. Methods 2012, 6, 667–676. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Pedrosa, L.F.; Barros, A.N.; Leite-Lais, L. Nutritional risk of vitamin D, vitamin C, zinc, and selenium deficiency on risk and clinical outcomes of COVID-19: A narrative review. Clin. Nutr. ESPEN 2021, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanidou, E.; Vlachakis, D.; Tsarouhas, K.; Panidis, D.; Tsitsimpikou, C.; Darviri, C.; Kouretas, D.; Bacopoulou, F. The prognostic role of micronutrient status and supplements in COVID-19 outcomes: A systematic review. Food Chem. Toxicol. 2022, 162, 112901. [Google Scholar] [CrossRef] [PubMed]
| Yolk Hardening Rate (%) | Hardness (g) | Elasticity | Cohesiveness | Gel Strength | Chewiness (mJ) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Pickling | Ripening | Pickling | Ripening | Pickling | Ripening | Pickling | Ripening | Pickling | Ripening | Pickling | Ripening |
| A | 72.34 ± 7.39 abA | 81.59 ± 2.26 bcB | 63.50 ± 6.98 ab | 91.20 ± 9.62 c | 0.78 ± 0.02 b | 0.76 ± 0.07 abc | 0.93 ± 0.03 ab | 0.93 ± 0.03 ab | 58.88 ± 7.26 b | 84.90 ± 9.44 d | 3.11 ± 0.31 c | 3.97 ± 0.40 d |
| B | 73.07 ± 4.72 abA | 81.33 ± 9.31 bcB | 59.67 ± 13.53 ab | 92.33 ± 11.79 c | 0.61 ± 0.21 a | 0.67 ± 0.02 a | 0.85 ± 0.14 a | 0.87 ± 0.05 a | 49.13 ± 2.37 ab | 80.67 ± 11.32 d | 2.39 ± 0.18 a | 3.98 ± 0.74 cd |
| C | 76.34 ± 11.33 abA | 78.78 ± 2.02 bcB | 57.00 ± 1.80 ab | 78.30 ± 3.47 b | 0.82 ± 0.03 b | 0.78 ± 0.06 bcd | 0.94 ± 0.01 ab | 0.93 ± 0.04 ab | 53.83 ± 2.05 ab | 72.68 ± 3.90 c | 2.65 ± 0.14 b | 3.31 ± 0.25 cd |
| D | 69.7 ± 2.75 abA | 80.47 ± 8.92 bcB | 52.10 ± 4.55 a | 83.13 ± 7.41 bc | 0.80 ± 0.02 b | 0.73 ± 0.09 ab | 0.94 ± 0.03 ab | 0.90 ± 0.12 ab | 48.88 ± 4.53 a | 74.38 ± 10.22 cd | 2.46 ± 0.26 a | 3.53 ± 0.40 cd |
| Negative control | 89.03 ± 0.0 cA | 86.53 ± 4.91 bB | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Positive control # | 65–80 | 70–85 | 45–65 | 50–70 | 0.70–0.85 | 0.75–0.88 | 0.90–0.95 | 0.93–0.97 | 40–60 | 45–65 | 1.90–2.80 | 2.00–2.85 |
| Group | Wh | L | a | b | Appearances | Mouthfeel | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Color of Albumins | Soft-Boiled of Yolk | Flavor | Taste | Elasticity of Egg White | Texture of Egg Yolk | Overall | |||||
| A | 77.11 ab | 18.99 ± 1.53 ab | 2.40 ± 0.46 a | 1.34 ± 0.15 b | 6.97 a | 6.47 a | 6.44 a | 6.35 a | 6.62 a | 6.65 a | 6.56 a |
| B | 76.64 b | 23.62 ± 0.91 b | 6.21 ± 0.76 d | 1.17 ± 0.35 b | 6.37 b | 6.00 b | 4.53 c | 4.77 c | 5.23 b | 5.19 b | 4.67 b |
| C | 80.45 a | 19.60 ± 1.38 ab | 3.66 ± 0.44 b | 0.67 ± 0.05 a | 6.82 ab | 6.59 a | 5.94 ab | 5.68 b | 6.35 a | 6.26 a | 6.38 a |
| D | 78.17 ab | 21.97 ± 1.69 ab | 4.44 ± 1.06 bc | 1.11 ± 0.35 ab | 4.70 c | 4.91 c | 4.98 c | 4.91 c | 5.23 b | 4.72 c | 4.86 b |
| Positive control * | 81.05 a | 18.99 ± 1.53 ab | 2.40 ± 0.46 a | 1.34 ± 0.15 b | 6.15 b | 6.03 b | 5.56 b | 5.91 ab | 6.59 a | 6.32 a | 6.29 a |
| Group | Sampling Area | Calcium | Magnesium | Zinc |
|---|---|---|---|---|
| A | albumen | 6 b | N.D. | 0.3 b |
| yolk | 133 a | N.D. | 2.21 a | |
| C | albumen | 4 b | 26.3 a | 0.3 b |
| yolk | 133 a | 3.48 b | 2.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-M.; Hou, C.-Y.; Shih, M.-K.; Hsieh, C.-W.; Hung, Y.-L.; Huang, P.-H. Use of Incinerated Eggshells to Produce Pidan. Sustainability 2022, 14, 6797. https://doi.org/10.3390/su14116797
Lin C-M, Hou C-Y, Shih M-K, Hsieh C-W, Hung Y-L, Huang P-H. Use of Incinerated Eggshells to Produce Pidan. Sustainability. 2022; 14(11):6797. https://doi.org/10.3390/su14116797
Chicago/Turabian StyleLin, Chia-Min, Chih-Yao Hou, Ming-Kuei Shih, Chang-Wei Hsieh, Yu-Lin Hung, and Ping-Hsiu Huang. 2022. "Use of Incinerated Eggshells to Produce Pidan" Sustainability 14, no. 11: 6797. https://doi.org/10.3390/su14116797
APA StyleLin, C.-M., Hou, C.-Y., Shih, M.-K., Hsieh, C.-W., Hung, Y.-L., & Huang, P.-H. (2022). Use of Incinerated Eggshells to Produce Pidan. Sustainability, 14(11), 6797. https://doi.org/10.3390/su14116797











