Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives
Abstract
1. Introduction
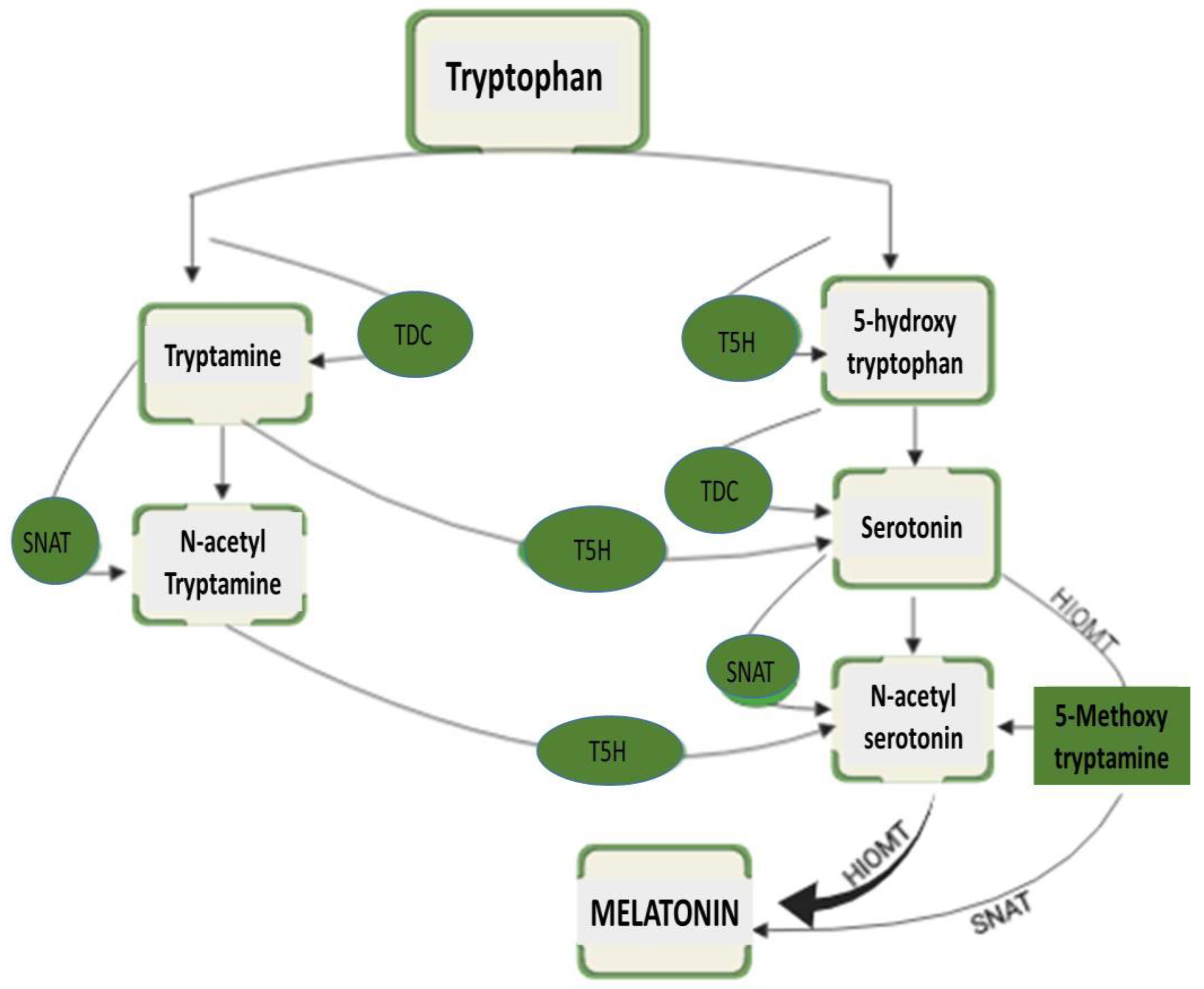
2. Production of Melatonin in Plants
3. Melatonin: Provoking Defense Mechanisms against Various Stresses in Plants
3.1. Salinity Stress
3.2. Cold Stress
3.3. Heat Stress
3.4. Drought and Ultraviolet Radiation Stress
3.5. Heavy Metal and Chemical Stress
3.6. Pathogen/Disease Resistance
3.7. Oxidative Stress
4. Melatonin: A Multifunctional Factor in Plants
4.1. Melatonin as Growth Promoter in Plants
4.1.1. In Vivo Effects of Melatonin on Growth Promotion
4.1.2. In Vitro Effect of Melatonin on Growth Promotion
4.2. Role of Melatonin in Crop Improvement
4.3. Role of Melatonin in Chlorophyll Preservation
4.4. Role of Melatonin in Photosynthetic Activity
4.5. Role of Melatonin in Increases in Biomass
- Increasing the rate of plant germination,
- Making plants resistant to environmental stresses,
- Gene manipulation of synthetic enzymes of melatonin, such as AANAT/ASMT.
4.6. Other Functions of Melatonin in Plants
5. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, X.; Xu, H.; Li, T. Research progress of melatonin biosynthesis and metabolism in higher plants. Plant Physiol. J. 2016, 52, 615–627. [Google Scholar]
- Macias, M.; Rodrigueez-Cabezas, M.; Reiter, R.; Osuna, A.; Acuña-Castrovejo, D. Presence and effects of melatonin in Trypanosoma cruzi. J. Pineal Res. 1999, 27, 86–94. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Pévet, P. Basic aspects of melatonin action. Sleep Med. Rev. 1998, 2, 175–190. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-x.; Manchester, L.C.; Simopoulos, A.P.; Maldonado, M.D.; Flores, L.J.; Terron, M.P. Melatonin in edible plants (phytomelatonin): Identification, concentrations, bioavailability and proposed functions. World Rev. Nutr. Diet. 2007, 97, 211–230. [Google Scholar]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Tan, D.-X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Balzer, I.; Hardeland, R. Melatonin in algae and higher plants-possible new roles as a phytohormone and antioxidant. Bot. Acta 1996, 109, 180–183. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Janas, K.M. Melatonin in plants. Acta Physiol. Plant. 2009, 31, 1. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Centre 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Somu, P.; Parida, U.K.; Mishra, G. Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae chaetomorpha linum extract against human colon cancer cell HCT-116. Biol. Trace Elem. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J.; DeMello, J. Amino Acids Higher Plants; CABI International: Boston, MA, USA, 2015; pp. 390–435. [Google Scholar]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Murata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (C ucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2014, 66, 681–694. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in A rabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.-P.; Scott, E.R.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, K.; Park, S.; Byeon, Y.; Back, K. Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 2013, 55, 7–13. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Tal, O.; Haim, A.; Harel, O.; Gerchman, Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 2011, 62, 1903–1910. [Google Scholar] [CrossRef]
- Tiryaki, I.; Keles, H. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J. Pineal Res. 2012, 52, 332–339. [Google Scholar] [CrossRef]
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Reiter, R.J.; He, C.; Liu, G.; Lei, Q.; Zuo, B.; Zheng, X.D.; Li, Q.; Kong, J. Changes in melatonin levels in transgenic ‘Micro-Tom’tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 2014, 56, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. Purslane: A plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 2005, 39, 331–332. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, J.; Xu, Y.; Wang, Y.; Hao, J.; Li, T. Production of transgenic Nicotiana sylvestris plants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. Vitro Cell. Dev. Biol. Plant 2012, 48, 275–282. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Role of melatonin to enhance phytoremediation capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Helton, P.; Reiter, R.J. Phytoremediative capacity of plants enriched with melatonin. Plant Signal. Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef]
- Behera, K.K. Phytoremediation, Transgenic Plants and Microbes. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2014; pp. 65–85. [Google Scholar]
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011, 6, 196–203. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Rasmussen, M.W.; Roux, M.; Petersen, M.; Mundy, J. MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 2012, 3, 169. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 2016, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Montazeri, M.; Pagheh, A.S.; Sharif, M.; Sarvi, S.; Hosseinzadeh, A.; Reiter, R.J.; Hadighi, R.; Joghataei, M.T.; Ghaznavi, H. The potential use of melatonin to treat protozoan parasitic infections: A review. Biomed. Pharmacother. 2018, 97, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hu, W.; Wang, Q.; Zeng, H.; Li, X.; Yan, Y.; Reiter, R.J.; He, C.; Shi, H. Identification, transcriptional and functional analysis of heat-shock protein 90s in banana (Musa acuminata L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J. Pineal Res. 2017, 62, e12367. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, W.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, A.; Li, X.; Kou, M.; Wang, W.; Chen, X.; Xu, T.; Zhu, M.; Ma, D.; Li, Z. Melatonin-stimulated triacylglycerol breakdown and energy turnover under salinity stress contributes to the maintenance of plasma membrane H+–ATPase activity and K+/Na+ homeostasis in sweet potato. Front. Plant Sci. 2018, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.; Chen, L.; Fu, J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, B.; Zhang, S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA 1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Xu, X. Effects of Exogenous Melatonin on Physiological Response of Cucumber Seedlings under High Temperature Stress. Master’s Thesis, Northwest A and F University, Xi’an, China, 2010. [Google Scholar]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, X.; Reiter, R.J.; Feng, S.; Wang, Y.; Liu, S.; Jin, L.; Li, Z.; Datla, R.; Ren, M. Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Abd El-Naby, S.K.M.; Abdelkhalek, A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci. Pol. Hortorum. Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Inhibition of ACC oxidase activity by melatonin and indole-3-acetic acid in etiolated lupin hypocotyls. In Advances in Plant Ethylene Research; Springer: Dordrecht, The Netherlands, 2007; pp. 101–103. [Google Scholar]
- Koyama, F.C.; Carvalho, T.L.; Alves, E.; da Silva, H.B.; De Azevedo, M.F.; Hemerly, A.S.; Garcia, C.R. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. J. Eukaryot. Microbiol. 2013, 60, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Kolář, J.; Macháčková, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Campbell, S.S.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). Vitro Cell. Dev. Biol. Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Melatonin: A potential regulator of plant growth and development? Vitro Cell. Dev. Biol. Plant 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Katekar, G.F. Auxins: On the nature of the receptor site and molecular requirements for auxin activity. Phytochemistry 1979, 18, 223–233. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, W.-B.; Reiter, R.J.; Wei, W.; Wang, B.-M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Cao, J.; O’Brien, R.; Murch, S.J.; Saxena, P.K. The mode of action of thidiazuron: Auxins, indoleamines, and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Rep. 2007, 26, 1481–1490. [Google Scholar] [CrossRef]
- Hardeland, R. New actions of melatonin and their relevance to biometeorology. Int. J. Biometeorol. 1997, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more–occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Reiter, R.J. Phytomelatonin: A review. J. Exp. Bot. 2008, 60, 57–69. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Asif, M.; Pervez, A.; Ahmad, R. Role of Melatonin and Plant-Growth-Promoting Rhizobacteria in the Growth and Development of Plants. CLEAN Soil Air Water 2019, 47, 1800459. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Zhang, Y.; Li, B.; Liang, W.; Wang, C. Control efficiency of exogenous melatonin against post-harvest apple grey mold and its influence on the activity of defensive enzymes. Plant Physiol. J 2017, 53, 1753–1760. [Google Scholar]
- Xin, D.; Si, J.; Kou, L. Postharvest exogenous melatonin enhances quality and delays the senescence of cucumber. Acta Hortic. Sin. 2017, 44, 891–901. [Google Scholar]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Aminaka, R.; Yoshioka, M.; Khatoon, M.; Komayama, K.; Takenaka, D.; Yamashita, A.; Nijo, N.; Inagawa, K.; Morita, N. Quality control of photosystem II: Impact of light and heat stresses. Photosynth. Res. 2008, 98, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, K.; Park, S.; Kim, Y.S.; Back, K. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J. Pineal Res. 2010, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2011, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Ortíz-Castro, R.; Méndez-Bravo, A.; Macías-Rodríguez, L.; López-Bucio, J. Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 490–508. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Arnao, M. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agric. Food Chem. 2008, 56, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef]
- Reiter, R.J.; Richardson, B.A.; Johnson, L.Y.; Ferguson, B.N.; Dinh, D.T. Pineal melatonin rhythm: Reduction in aging Syrian hamsters. Science 1980, 210, 1372–1373. [Google Scholar] [CrossRef]
- Wolf, K.; Kolář, J.; Witters, E.; van Dongen, W.; van Onckelen, H.; Macháčková, I. Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J. Plant Physiol. 2001, 158, 1491–1493. [Google Scholar] [CrossRef]
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol. Plant. 2003, 118, 605–612. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. New insights into the role of melatonin in plants and animals. Chem. Biol. Interact 2019, 299, 163–167. [Google Scholar] [CrossRef]

| Concentration of Melatonin | Type of Stress | Plant Species | Effect on Plants | References |
|---|---|---|---|---|
| 1 µM | Salinity | Cucumis sativus | Increased growth and germination rate; 5-fold increase in activities of antioxidant enzymes like catalase, superoxide dismutase. | [26] |
| 0.1 µM | Lupinus albus | Salinity stress diminished, which proved significant in maintaining plant’s photosynthetic ability. | [25] | |
| 50–100 µM | Soybean | Increased salt and drought stress tolerance and enhanced seed germination. | [27] | |
| 50 pg g−1 | Bermuda grass | Abiotic stress tolerance and upregulation and downregulation observed. | [28] | |
| 1 mM | Barley | Chlorophyll content increased 2-fold. | [70] | |
| 50–150 µM | Cucumber | Improved growth, photosynthetic ability and reduce oxidative damage by scavenging H2O2 or enhancing antioxidant enzyme activity. | [71] | |
| 500 mM | Vicia faba L. | Enhanced growth parameters, total phenolic and carbohydrate content, photosynthetic pigments, indole acetic acid and relative water content. | [72] | |
| 1 µM | Brassica napus L. | Not only enhanced root growth inhibition due to NaCl stress but also promoted seedling root growth. | [73] | |
| 0.5 µM | Ipomoea batatas L. | Stimulated triacylglycerol breakdown, fatty acid β-oxidation and energy turnover under salinity stress, thus improved K+/Na+ hemostasis and plasma membrane H+–ATPase activity. | [74] | |
| 0.1 µM | Malus hupehensis Rehd. | Upregulated expression of ion-channel genes MdNHX1 and MdAKT1 in leaves, alleviated [23] growth inhibition and photosynthetic capacity and reduced oxidative damage by directly scavenging H2O2. | [23] | |
| 100 µM | Avena sativa | Improved morphological growth, photosynthetic ability and reduced antioxidant stress by upregulating ROS-scavenging enzymes. | [75] | |
| 10–30 µM | Cold | Arabidopsis thaliana | Increased length of root, fresh weight and height of plant. | [30] |
| 100 µM | Bermudagrass | Improved photosystem II performance and accumulation of metabolites. Also antioxidant activity increased. | [76] | |
| 1 µM | Wheat seedlings | Enhanced activity of enzymes glutathione reductase, superoxide dismutase and ascorbate peroxide, which eventually increased plant growth by overcoming oxidative damage. | [34] | |
| 1 µM | Tomato | Promoted photosynthetic carbon fixation, antioxidant potential, accumulation of metabolites, expressions of cold-responsive genes in cold-stressed tomato. Further, reduced cold-induced damage to plant. | [77] | |
| 100 µM | Camellia sinensis L. | Mitigated cold-induced reductions in photosynthetic capacity by decreasing oxidative stress due to enhanced antioxidant potential and redox homeostasis. | [32] | |
| 5 µM | Pea seed | Germination rate increased to 73.2% as compared to 53.7%. | [78] | |
| 100 µM | Rice | Alleviated growth inhibition, accumulation of reactive oxygen species and cell death due to cold stress. Physiological, biochemical and photosynthetic abilities increased, also antioxidant enzyme activity enhanced. | [79] | |
| 1 mM | Wheat seedlings | Increased leaf surface area, exerting strong mitigating effect on relative water content and high antioxidant activities. Further alleviated reduction in pigment content and improved physiological morphology. | [31] | |
| 0.5 ng mL−1 | Heat | Ulva spp. | Tolerance against heat stress. | [37] |
| 0.3–6 µM | Phacelia tanacetifolia | Reverted the adverse impacts of low and high temperature on thermo-sensitive and photosensitive plants. | [38] | |
| 1000 µM | Arabidopsis thaliana | 60% increase in germination rate, which reached up to 92.8% due to antioxidant ability of melatonin. | [39] | |
| 100 µM | Tomato seedlings | Alleviated damage to antioxidant defense system and enhanced plant growth. It further increased cellular membrane stability and metabolic gene expression. | [40] | |
| 100 µM | Triticum aestivum L. | Enhanced antioxidant enzyme activities, thus modulating their defense mechanism, and stabilized photosynthetic machinery by increasing chlorophyll content. Also regulated transcription of stress-responsive genes. | [41] | |
| 10 µM | Tomato | Promoted cellular protein protection by decreasing insoluble and ubiquitinated protein level and enhancing heat shock proteins and autography to refold denatured proteins. | [36] | |
| 50 pg g−1 | Bermuda grass | 16-fold overexpression in zinc finger, heat shock transcription factors, CBF/DREB genes and target genes as compared to control. | [28] | |
| 20 µM | Arabidopsis thaliana | 50% increase in survival rate as compared to control under heat stress. | [80] | |
| 25–100 µM | Cucumber seedlings | Net photosynthetic rate increased and intercellular CO2 concentration decreased. | [81] | |
| 1–100 µM | Heavy metal | Red cabbage | Diminished toxicity of copper ions during seedling and germination growth. | [54] |
| 17 ng g−1 | Green algae | Relieved cadmium-induced stress. | [37] | |
| 0.1 µM | Watermelon seedlings | Enhanced plant growth, chlorophyll content and photosynthetic assimilation. Further, it lowered vanadium concentration in roots and leaves by reducing their transport from root to shoot, thus making plant tolerant to stress. | [51] | |
| 100 µM | Wheat plant | Improved plant growth, pigments and regulated uptake of essential elements. cPTIO combined with melatonin treatment enhanced oxidative stress and reduced antioxidant enzymes. | [82] | |
| 0.45–0.51 ng/g | Tomato plant | HsfA1a induced Cd tolerance by activating COMT1 gene transcription and melatonin induction upregulated expression of HSPs. | [83] | |
| 0.1 mM | Maize plant | Melatonin induced endogenous NO to mitigate Pb toxicity from maize plants, thus increasing their tolerance against stress. It further induced the antioxidant defense system of plants. | [52] | |
| 100 µM | Cucumber | Alleviated oxidative stress by promoting activity and transcripts of oxidative enzymes. It altered iron uptake and enhanced photosynthesis and biosynthesis of secondary metabolites. | [67] | |
| 5 µM | Pea plant | Enhanced tolerance to copper contamination and increased survival rate. | [57] | |
| 50 µM | Alfalfa and Arabidopsis seedlings | Decreased cadmium accumulation via modulating heavy metal transporter capacity of melatonin. Further, it re-established redox homeostasis via miR398. | [84] | |
| 10 µM | Drought | Medicago sativa | Enhanced plant tolerant phenotype, chlorophyll fluorescence and stomatal conductance. Further, it made the plant tolerant to drought by regulating nitro-oxidative and osmoprotective homeostasis. | [85] |
| 1 mM | Maize | A photoprotective and anti-oxidative agent which combined with melatonin resulted in maximum efficiency of photosystem II photochemistry, thus exerting defensive role against drought in maize. | [46] | |
| 100 µM | Apple | Enhanced nutrient uptake by increasing gene expression and also increased uptake, utilization and accumulation of 15N. Also positively affected growth and physiological parameters of Malus. | [86] | |
| 500 µM | Wheat seedlings | Enhanced drought tolerance due to increased antioxidant capacity, GSH and AsA formation by increasing related gene regulation. Further, stimulated epidermis cell enlargement and decreased membrane damage by maintaining grana lamella in chloroplast. | [87] | |
| 100 mM | Moringa oleifera L. | Increased growth parameter, yield quantity and quality by improving phenolic content, photosynthetic pigments and antioxidant enzyme system. | [16] | |
| 100 µM | Maize | Reduced reactive oxygen species burst and increased photosynthetic activity, thus allowing plant to tolerate drought stress while maintaining its growth. | [88] | |
| 0.1 mM | Pathogen/Disease resistant | Apple leaves | Increased resistance of Marssonina apple blotch-fungal disease against Diplocarpon Mali by modulating activities of antioxidant and defense enzymes, pathogenesis and hydrogen peroxide levels. | [89] |
| 100 µM | Banana | Melatonin upregulated nine MaHSP90s transcripts, which eventually made it resistant to Fusarium oxysporum cubens. | [66] | |
| 1 mM | Cucurbits and watermelon | Served as immune inducer and boosted plant immunity and suppressed pathogen growth. Further increased resistance against Podosphaera xanthii and Phytophthora capsici. Altered gene expression involved in pathogen-associated molecular pattern and effector-triggered immunity defenses, thus increased tolerance against stress. | [90] | |
| 5 mM | Potato | Inhibited mycelial growth, cell ultrastructure changes and reduced Phytopthora infestans stress tolerance. It further altered expressions of genes associated with stress tolerance and fungicide resistance, thus preventing potato late blight. | [91] | |
| 10 µM | Arabidopsis thaliana | Acted as defense signaling molecule in plants against pathogen. | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability 2021, 13, 294. https://doi.org/10.3390/su13010294
Nawaz K, Chaudhary R, Sarwar A, Ahmad B, Gul A, Hano C, Abbasi BH, Anjum S. Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability. 2021; 13(1):294. https://doi.org/10.3390/su13010294
Chicago/Turabian StyleNawaz, Khadija, Rimsha Chaudhary, Ayesha Sarwar, Bushra Ahmad, Asma Gul, Christophe Hano, Bilal Haider Abbasi, and Sumaira Anjum. 2021. "Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives" Sustainability 13, no. 1: 294. https://doi.org/10.3390/su13010294
APA StyleNawaz, K., Chaudhary, R., Sarwar, A., Ahmad, B., Gul, A., Hano, C., Abbasi, B. H., & Anjum, S. (2021). Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability, 13(1), 294. https://doi.org/10.3390/su13010294







