1. Introduction
Mechanical characteristics of lithium-ion battery cells are of major importance when designing applications with maximized energy density and lifetime [
1,
2]. With the inherent volume variations during the use of lithium-ion batteries, the integration of cells into modules, packs, and systems needs to account for geometric variations and the induced mechanical stresses changing with state-of-charge and state-of-health [
3,
4]. In this context, an inappropriate design will lead to premature ageing of components on all levels. In extreme cases, a mismatch of volume requirements and device integration can lead to catastrophic failure [
5].
In the past, chemical and electrochemical ageing effects of lithium-ion batteries have been the major focus in the international research community. Topics such as side reactions, the solid electrolyte interphase (SEI) growth, loss of lithium inventory, and separator clogging have been investigated in great detail [
6,
7,
8,
9]. The influence of mechanical ageing has often been ignored and is now attracting attention with the aim of the highest energy densities and the introduction of alloying materials with high volume variations during charging and discharging [
3]. Moreover, complex system integration in electric vehicles requires detailed understanding and quantitative prediction of geometric variations as a function of state-of-charge and state-of-health.
Mechanical ageing might be one of the reasons for early and unexpected cell death, especially considering that graphite can expand up to 7–12% [
3,
10,
11], Lithium Nickel Cobalt Aluminium Oxide (NCA) up to 5% [
3], Nickel Manganese Cobalt (NMC) 1–2% [
3,
12,
13], and silicon up to 280% [
14] within the given voltage limits. Thus, the question to be clarified is how serious is the influence of mechanics on cell ageing in commercially relevant cells with respect to its used case?
The volume change can have various causes: The first is lithium migration, in which electrode materials change in volume as a result of lithium intercalation and deintercalation into their crystal structures [
14,
15,
16,
17,
18]. In addition, the gas formation can occur due to side reactions [
6,
19,
20]. Furthermore, it is known that thick layers of electrolyte decomposition products are formed as an almost impermeable covering layer [
6,
21,
22]. Alternatively, lithium plating can also occur, resulting in swelling behavior [
6,
23,
24].
It has already been shown by other measurement methods, such as thickness gauge [
3,
25,
26,
27], pressure [
3], digital image correlation, [
28] and multi-scale investigation [
11], that a volume change takes place, which is strongly material dependent and measures between 3 and 10% for a full cell [
3,
11,
25,
26,
27,
28] between charge and discharge.
For our study, we investigated an ageing matrix consisting of 51 Samsung 35E battery cells. Twelve different ageing conditions were applied and a minimum of three batteries per ageing point were tested. At three ageing points, the C-rate was additionally varied with two batteries each. Before the data of all batteries can be evaluated in detail, the measuring method must be verified in a first step. Because long time frames of the study, in combination with expected effects in the lower micrometer range, it desires a careful evaluation of the method. In this context, the current study evaluates the application of strain gauges as a valuable tool to monitor geometric changes in cylindrical cells with high precision. Firstly, the validity of the strain gauge is evaluated by investigating the signal drifts as a function of temperature and time. Secondly, the results from one battery are presented as an illustrative example. Finally, a post-mortem investigation was performed. The data of the strain gauge, in combination with electrochemical characterization, gives information regarding the lithium-ion battery and their degradation as well as parameters for battery system designs.
2. Materials and Methods
2.1. Investigated Lithium-Ion Battery
A commercially available lithium-ion battery, Samsung SDI INR18650 35E, was selected as the test object to check the functionality of the strain gauge. Verification measurements and ageing measurements were carried out on this lithium-ion battery.
The battery’s specifications are given in
Table 1. The investigated battery was a high-energy lithium-ion battery with a usable voltage range of 2.65–4.20 V according to the datasheet of the producer [
29].
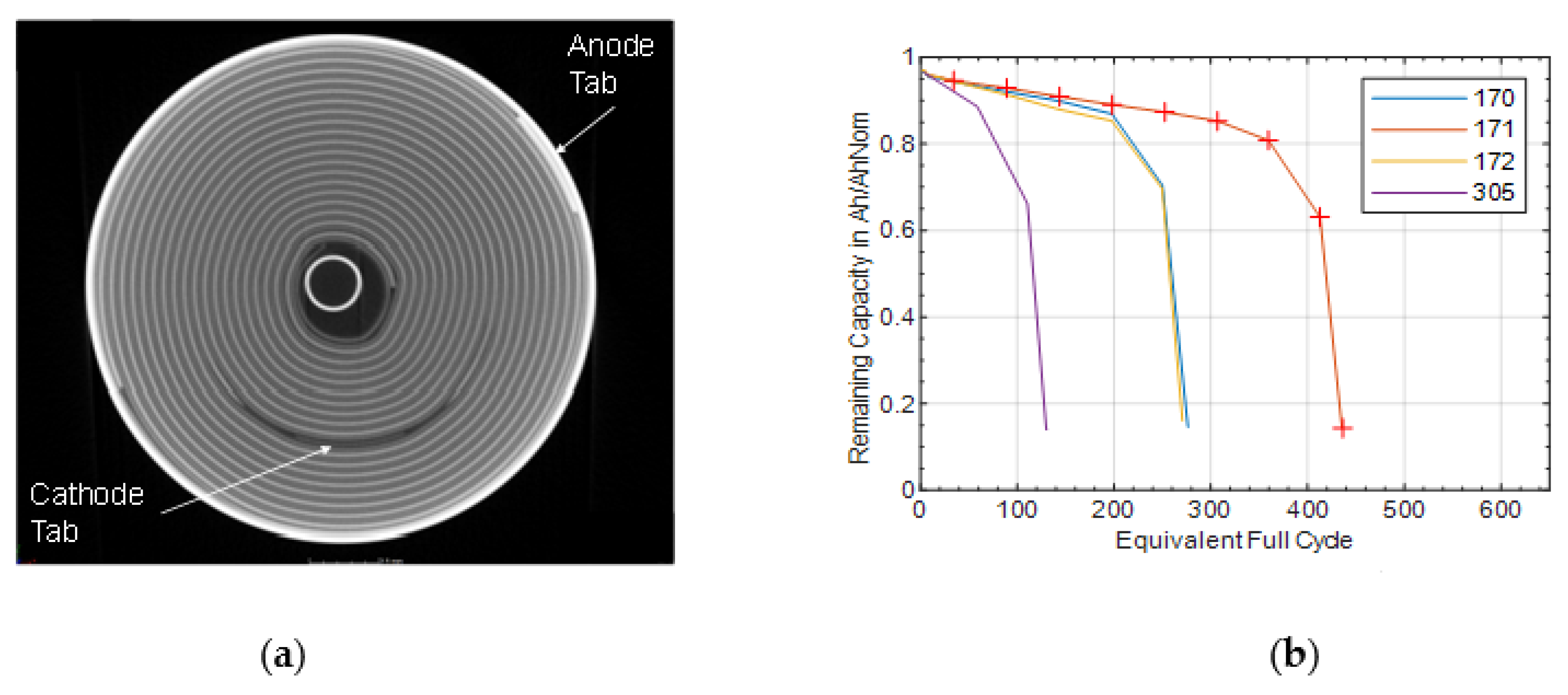
Computer tomography (CT) measurements were performed in order to obtain more information about the geometry and construction details of the investigated battery cell. A Werth TomoScope HV Compact (Werth Messtechnik GmbH, Gießen, Germany) was used with a microfocus transmission tube with up to 225 kV. All shown images have a resolution of 38 μm/voxel. The visualization software myVGL 3.2.5 (Volume Graphics GmbH, Heidelberg, Germany) was used for analyzing the data.
One Samsung 35E lithium-ion battery was opened under an argon atmosphere. A remaining voltage of 2.60 V was measured directly before opening. Spatially distributed discs of the double-coated electrodes, with a diameter of 20 mm, were taken. As preparation, each sample was washed with Dimethylcarbonat (Dimethylcarbonat Msynth®plus, Merck KGaA, Darmstadt, Germany) before it was dissolved in aqua regia. The solution was filled with distilled water until a 100 ml solution was obtained. Induced coupled plasma-optical emission spectrometer (ICP-OES) was conducted on this solution. The electrode compositions of anode and cathode were measured with Varian 725 induced coupled plasma-optical emission spectrometer (Agilent, Santa Clare, USA). The electrodes and the separator were evaluated by their surface morphology and color using a Keyence VK-9710 laser microscope (KEYENCE DEUTSCHLAND GmbH, Neu-Isenburg, Germany).
Electrical Tests
For examination and ageing of the lithium-ion battery, the cell test system Digatron MCFT 20-05-50ME with the precision of the current measurement of 0.2% (Digatron Power Electronics GmbH, Aachen, Germany) was used. The temperature sensor Dallas Semiconductor DS18B20 (Maxim Integrated, San Jose, USA) with a precision of ±1 °C was located on the lithium-ion battery case close to the strain gauge.
The lithium-ion battery test was conducted in a temperature chamber Binder MK 720 (BINDER GmbH, Tuttlingen, Germany) with a temperature precision of ±2 °C. The experiment was conducted at a constant ambient temperature of 25 °C. However, the lithium-ion battery temperature may have changed due to self-heating depending on the current.
The battery was electrically aged by charge-discharge cycling. The ageing cycles were performed at 25 °C and a mean state of charge (SOC) of 50%. A cycle depth of 50% was chosen. The lithium-ion battery was charged with 0.5 C to the upper charging voltage limit and discharged Ah-based to avoid voltage drift.
In order to determine the ageing of the lithium-ion battery, check-ups were performed at 25 °C. Check-ups were done every 50th equivalent full cycle and included capacity determination, pulse resistance, and quasi-open-circuit voltage (quasi-OCV) characteristics. During the check-up, the lithium-ion battery was first discharged down to 2.65 V, followed by a pause of 10 min. Then the battery was charged with 0.5 C (1.7 A) up to 4.20 V, followed by constant-voltage charging up to I < 0.02C (maximum 180 min). Afterwards, the lithium-ion battery was kept at rest for 30 min before the capacity was determined with a discharge of 0.5C until the voltage of 2.65 V was reached, followed by a pause of 10 min. Next, the lithium-ion battery was charged, followed by a pause of 30 min.
The quasi-OCV was measured with a current of 0.1C. For this, the lithium-ion battery was discharged down to 2.65 V and charged up to 4.20 V with a current of 0.1C, followed by a pause of 30 min.
2.2. Strain Gauges
The strain gauge 1-LY11-6/120A from HBM Germany (Hottinger Baldwin Messtechnik GmbH, Darmstadt, Germany) was attached to the lithium-ion battery housing with superglue Z70 (Hottinger Baldwin Messtechnik GmbH, Darmstadt, Germany) according to the enclosed manufacturer’s instructions. It was assumed that the height (z-direction) of the battery does not change, as there is enough buffer in the housing in this direction. Therefore, it was assumed that only the diameter will change. In order to measure the change in diameter without the influence of edge effects, the strain gauge was placed in the middle of the housing height in the circumferential direction.
The area and the measuring grid area of the strain gauge were 6 × 13 mm² and 6 × 2.7 mm², respectively. The resistance of the strain gauge was the electrical resistance between the two connecting cables. The change in volume of the measuring body caused a strain of the measuring grid. Due to the strain of the measuring grid, the resistance of the measuring grid changed [
30]. This resistance change induced a signal which was amplified by a measuring amplifier Q.bloxx A116 120/350 and converted into a strain
ε in µm/m by Gantner Q.station 101DT (Gantner Instruments Test & Measurement GmbH, Darmstadt, Germany) according to Equation (1).
The signal is the measured signal of the strain gauge. The k factor is the characteristic value of a strain gauge. The value here is k = 2.04. The bridge factor F indicates how many active strain gauges are present in the Wheatstone bridge. For a quarter bridge, the manufacturer indicated a value of one.
The resulting diameter change of the cylindrical lithium-ion battery is calculated as follows: It is assumed that the elongation of the lithium-ion battery in the circumferential direction is constant over the height of the casing. The change in diameter can be inferred from the elongation of the circumference using Equation (2).
Δ
d is the diameter change of the lithium-ion battery.
U0 in mm represents the circumference at the start of the test.
U0 is calculated using Equation (3).
ε is the measured strain in µm/m and
d0 is the diameter at the start of the test. In our case, this corresponds to 18.5 mm.
4. Discussion
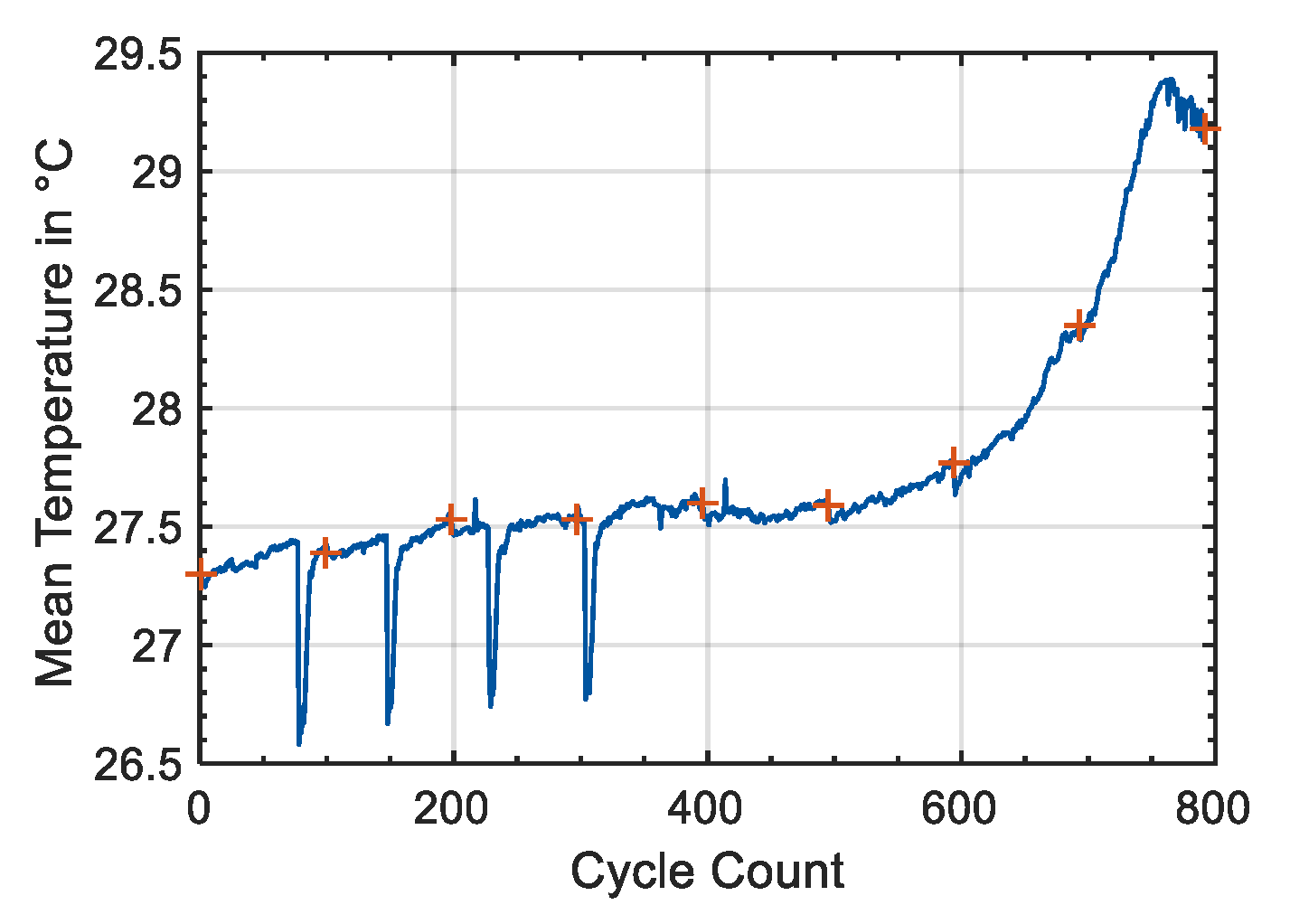
As could be shown in the results section, a strain gauge is a very stable and reliable measuring tool. During cycling of the Samsung 35E, the maximum permissible limit of the sensor, 50,000 µm/m [
30], was not exceeded until the end-of-life of the battery. Herein, a drift of ≤30 µm/m can be assumed at a cycle number of more than 100,000 equivalent full cycles. The lithium-ion battery considered here fell below the end of life capacity of 60% as given by the producer [
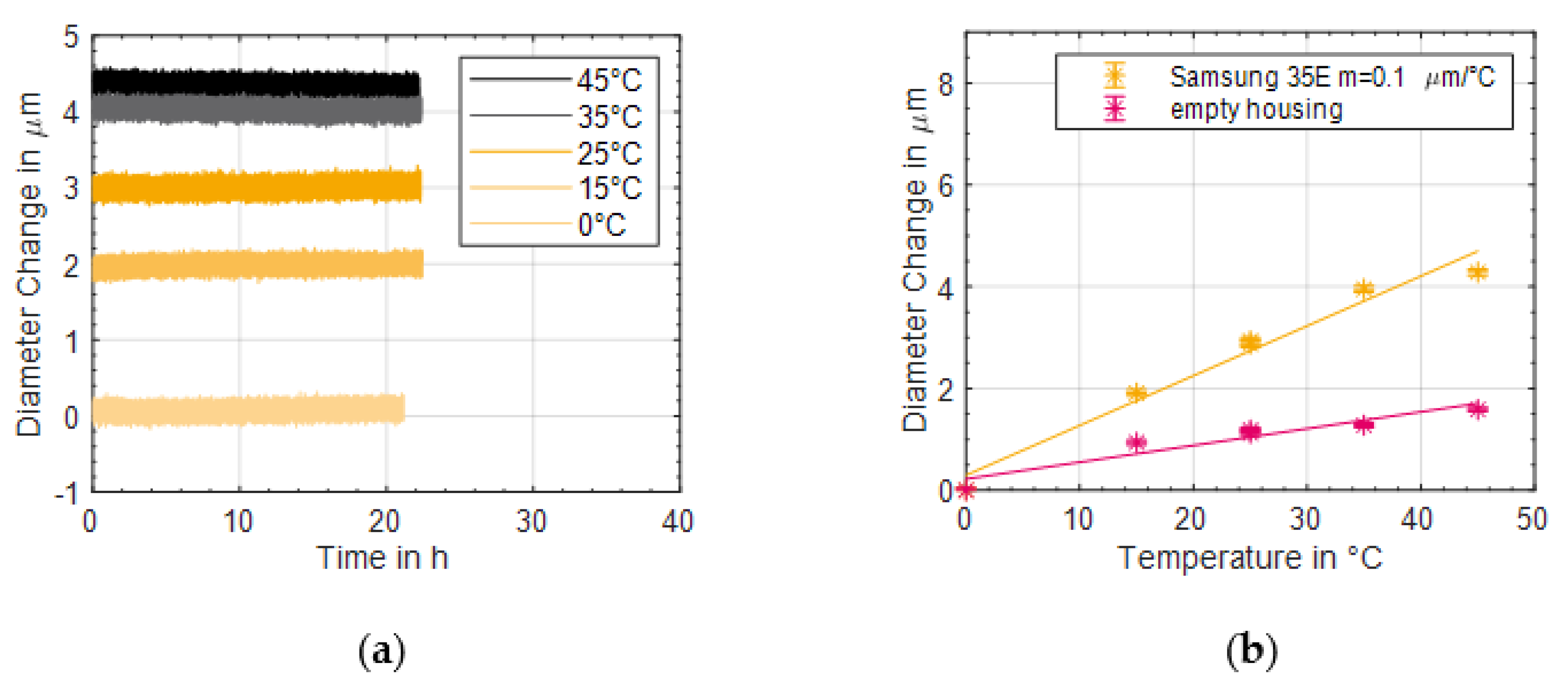
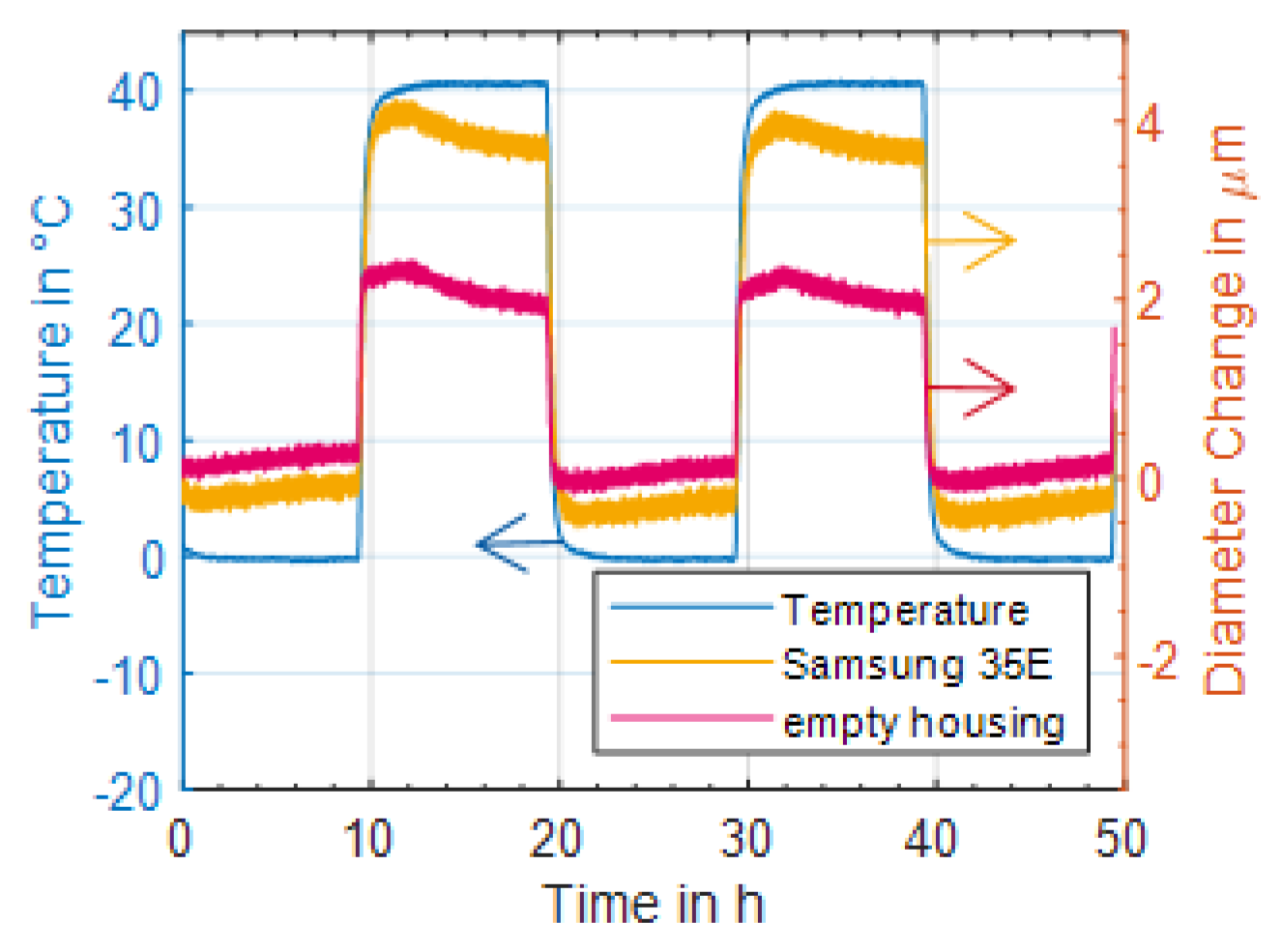
29] already after 420 equivalent full cycles. Therefore, it can be assumed that the drift of the strain gauge was low enough for a precise time series measurement. This finding was confirmed with the investigations of the temperature influence, see
Figure 2. Here, the diameter change of the Samsung 35E was measured at different temperatures for more than 20 h and the temperature influence was determined. It turned out that the measurement yields stable results at all temperatures. In addition, a linear temperature influence of the battery and strain gauge of 0.1 µm/°C could be demonstrated, which renders the temperature impact on measured results neglectable. Furthermore, it could be shown, that the expansion of the jelly roll due to temperature change had only a minor impact. Thus, the influence of temperature can be neglected. Since the lithium-ion battery did not heat up to more than 30 °C during ageing, the temperature influence can be limited to a maximum of 0.5 µm (
Figure 10). This can be considered as minor compared to the measured diameter changes of more than 10 µm during charge and discharge.
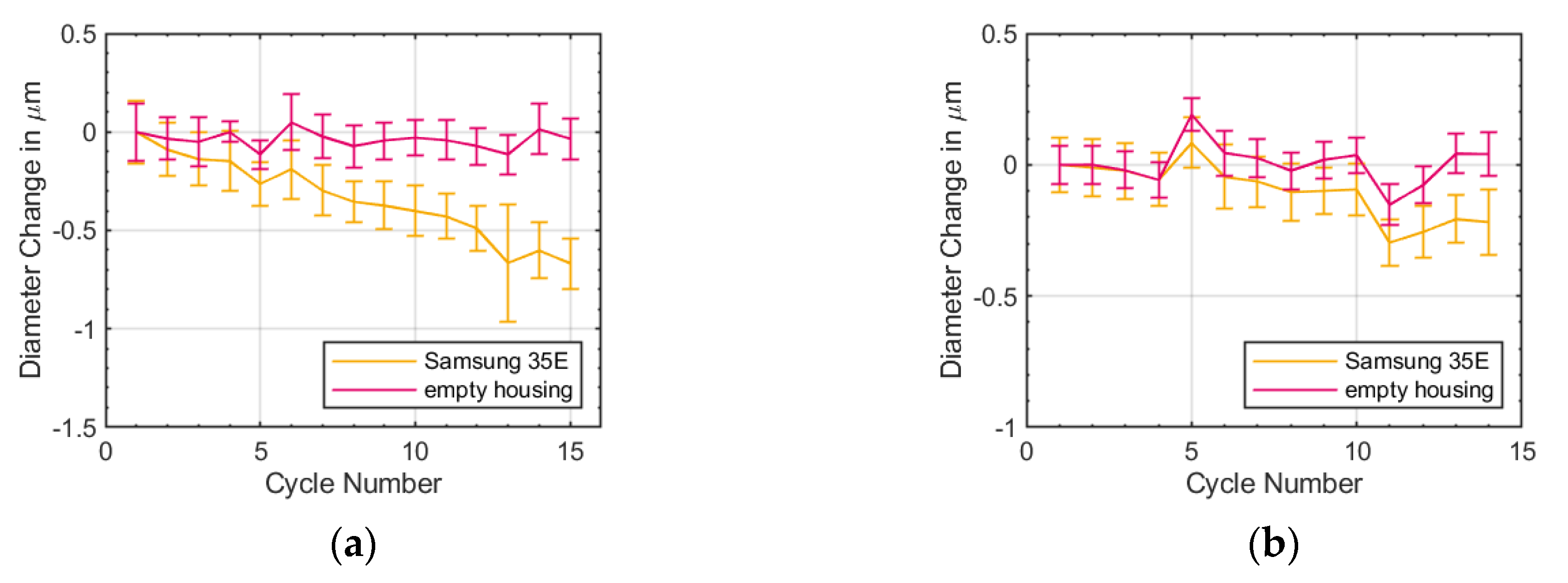
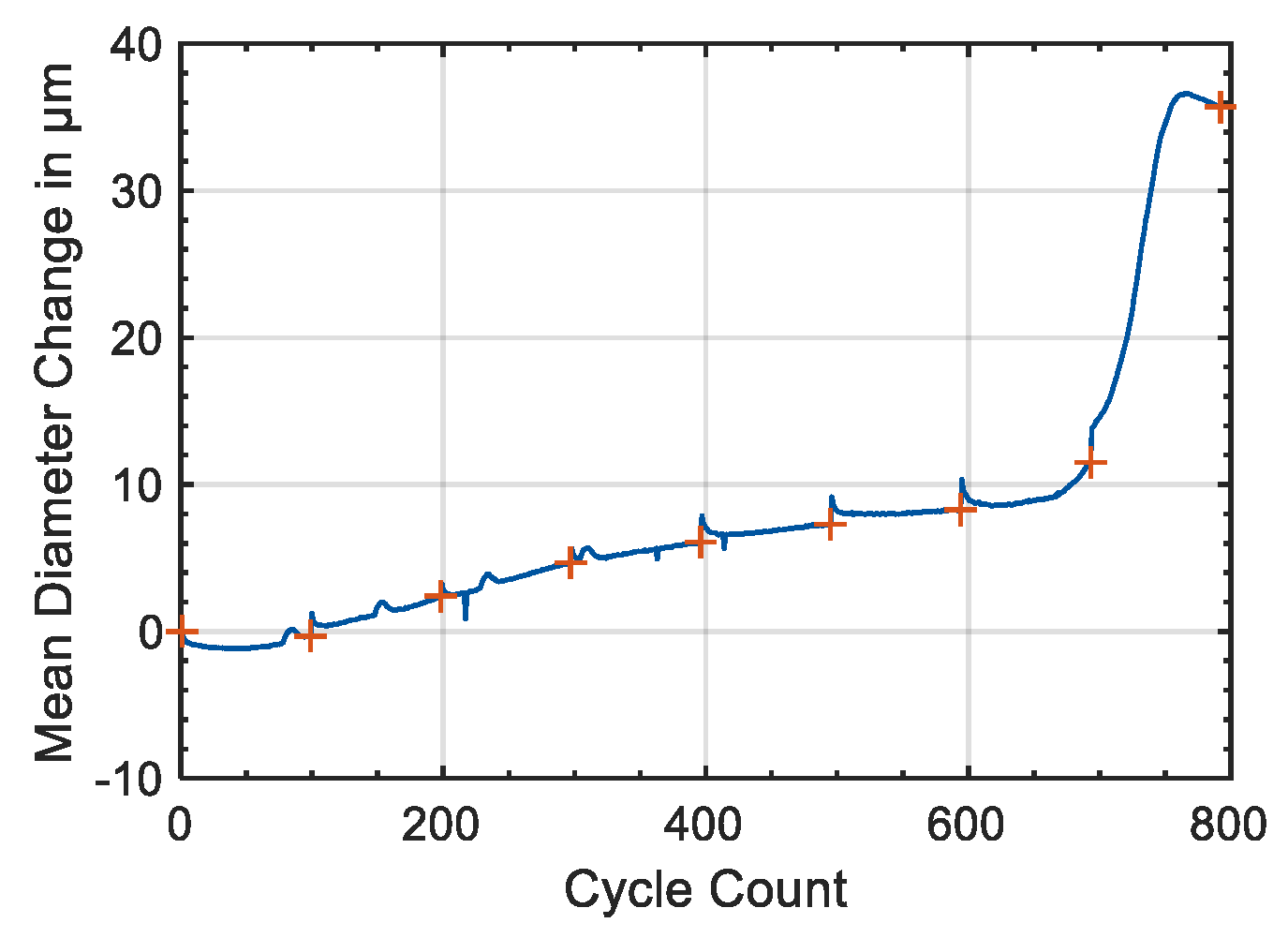
As shown in
Figure 4 the mean diameter change of the Samsung 35E was decreasing with temperature cycle number at 0 and 40 °C. The reason for this decreasing might be a reduction of the mechanical stress within the jelly roll or a homogenization of the electrodes. This behavior requires further investigation. The decrease in the diameter change showed that the irreversible diameter change from
Figure 7 is caused by effect within the battery and that the diameter of the battery was increasing over ageing.
The diameter change of the discharge process was around 10.7 µm with a standard deviation of 4.4 µm for an as-received lithium-ion battery. Rather large variations in battery production and ageing characteristics could be the reason for the large deviation in diameter change [
31]. Further results illuminated this aspect and will be reported elsewhere.
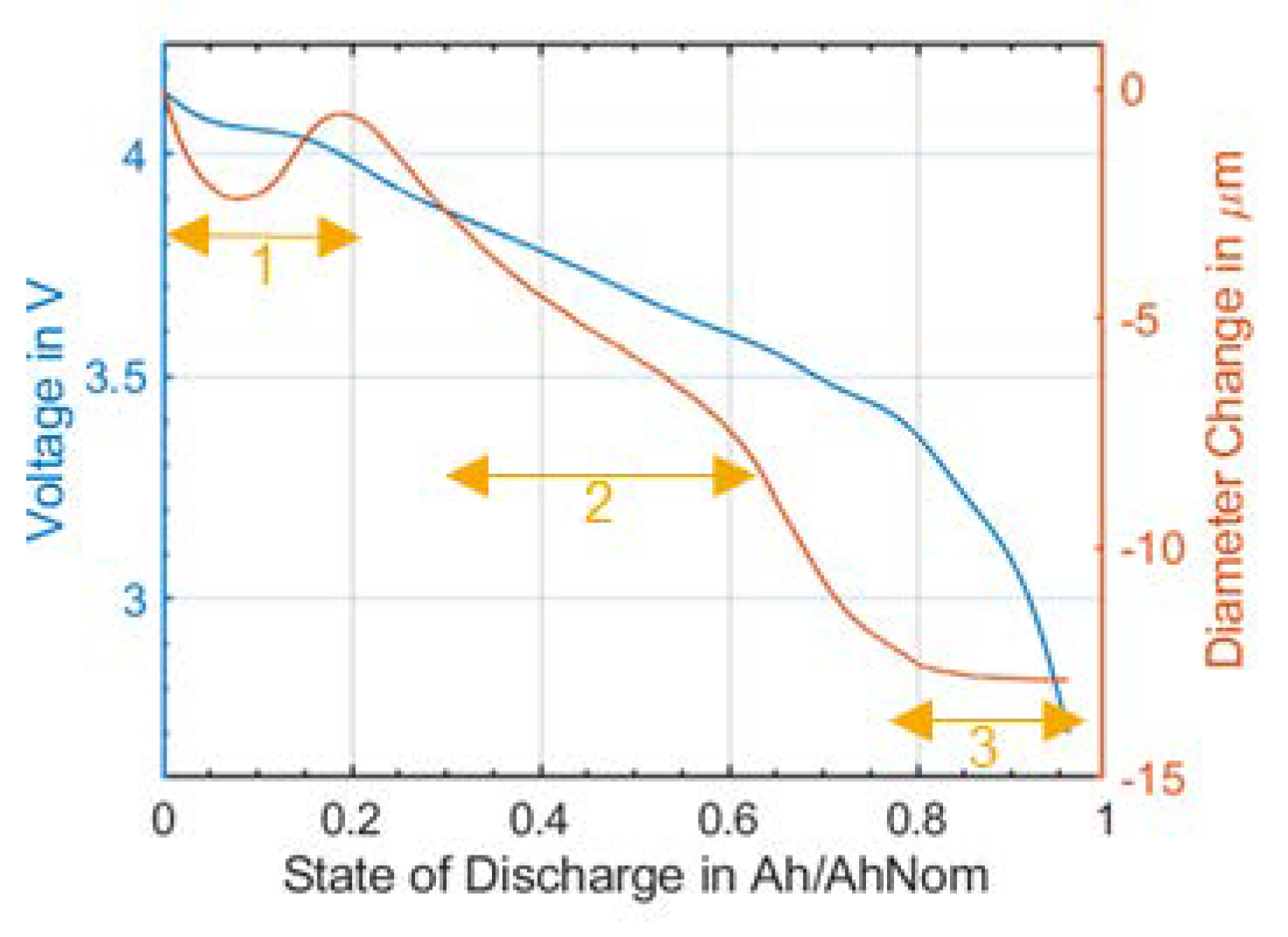
If one considers the diameter change during discharging (
Figure 5), a characteristic trend can be seen: It can be divided into three areas. A minimum at high SOC, an area with a constant gradient in medium SOC region, and a flattening slope towards the end of discharge. The impact of the electrode material of this effect is still under investigation, but relating our results with the literature suggests that the effect at low SOC might be caused by the anode [
3]. Furthermore, there was little volume change in the region of medium SOC because of the stage transition of graphite (stage transition: 2L→2 [
32]), which shows a plateau type of characteristic when relating to the state of charge and volume [
3].
Due to the differences in diameter change that have been observed between charge and discharge, it can be assumed that intercalation and de-intercalation induce asymmetric volume changes at anode and cathode (
Figure 6).
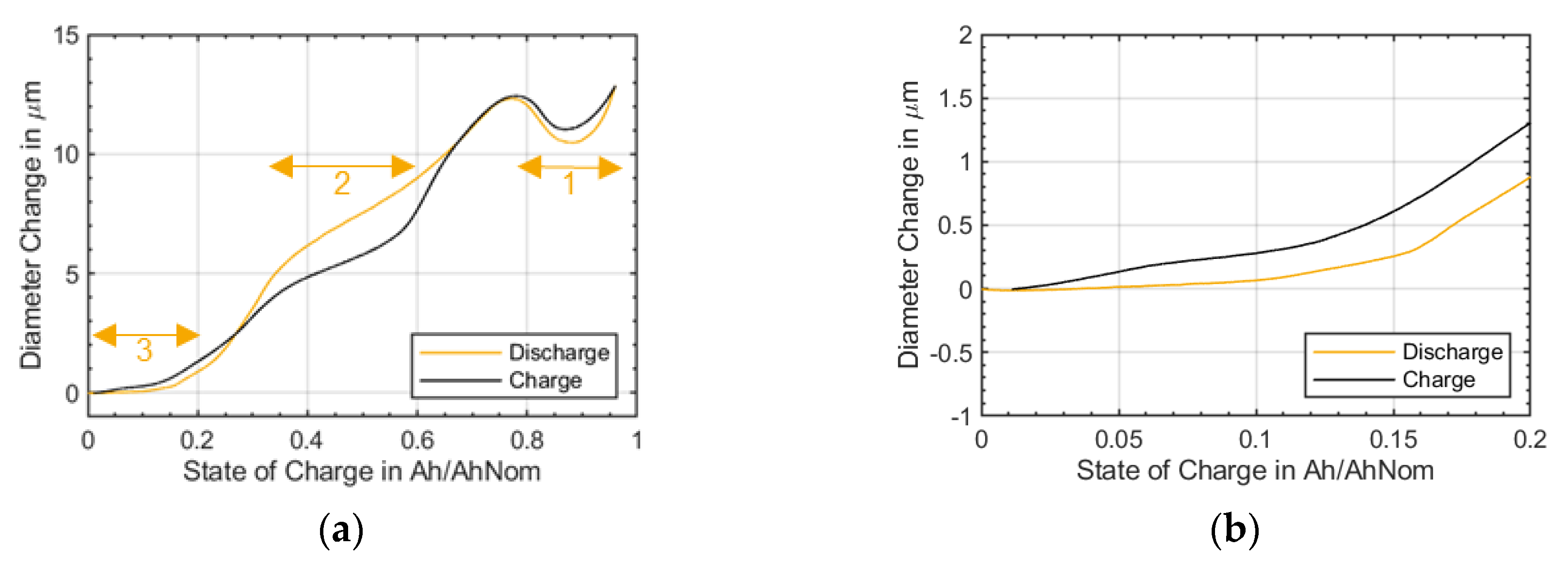
As can be seen in
Figure 7, the shape of the curves remained nearly the same until a remaining capacity of 80%. Then, the diameter changes receded and the characteristic shape of the curve at the beginning of charge and end of charge started to disappear. In addition, the area with a constant gradient in medium SOC range was no longer clearly visible. This might be explained by a loss of active material or active lithium which can cause less active volume overall that is used for charging and discharging or due to the fact that the electrodes have shifted to each other so that not all areas of the electrodes are used anymore. Pore clogging may lead to an increase in the reversible diameter change of the battery. In this case, the pores would no longer be available as void space for accommodating the increase in the volume of the particle during intercalation, so the diameter change of the active material was translating directly into diameter change of the cylindrical lithium-ion battery as monitored by the strain gauges.
Figure 4 shows that the irreversible diameter change stems from the battery and not from the strain gauge characteristic. Hence, the reasons for the irreversible diameter change could be an increase of the SEI, the non-uniform formation of a covering layer or lithium plating, or gassing [
19,
20,
21,
22,
23,
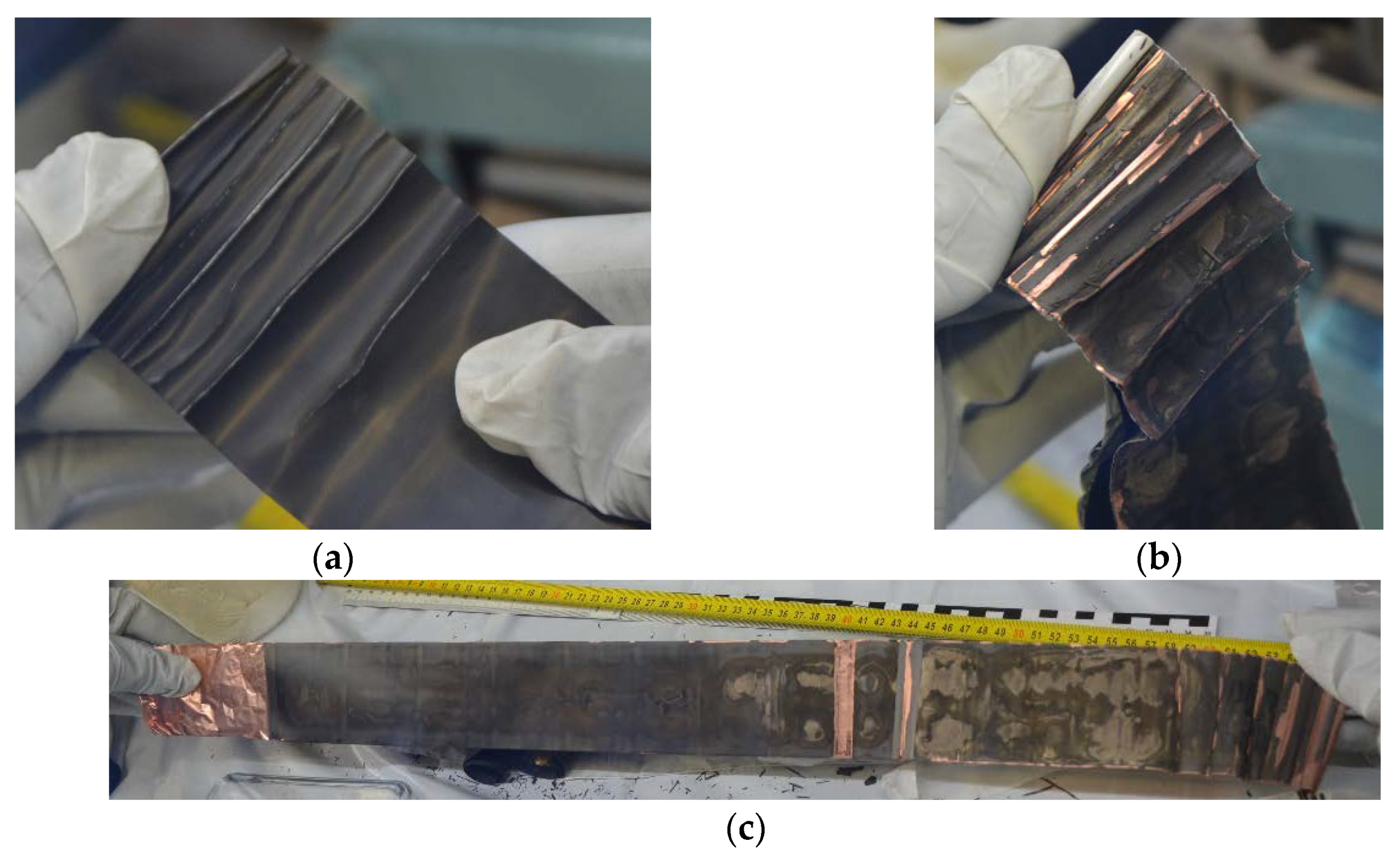
24]. The post-mortem results showed that a covering layer was formed over aging (
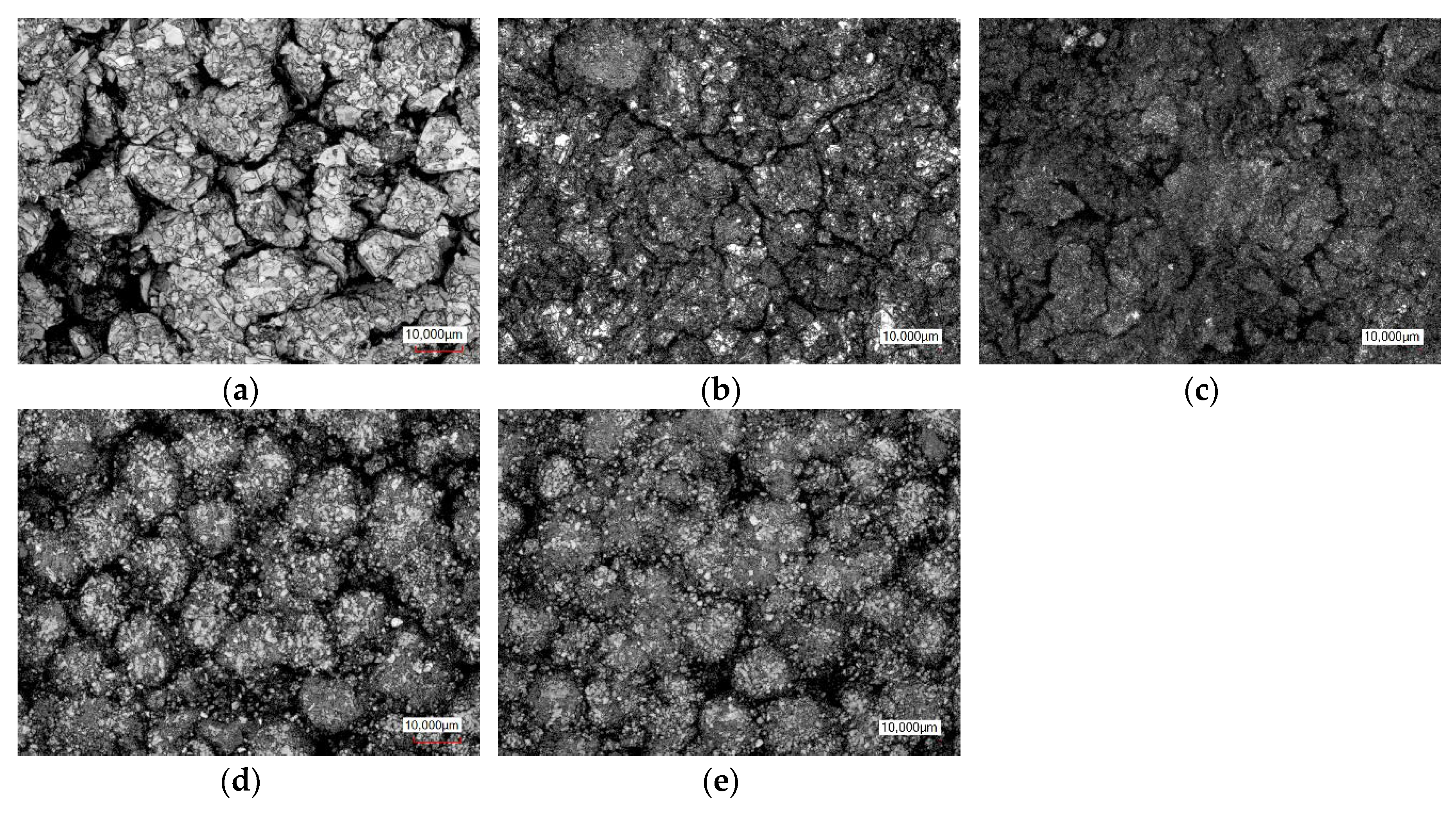
Figure 12 and
Figure 13). The exact separation between SEI growths, plating or covering layer formation is unfortunately not possible. In addition, a thickening of the anode could also be observed, which fits in with the formation of the covering layer. The thickness of the anode changed from 174 µm (347) to 211 µm (171). The thickness of the cathode stayed constant with 156 µm.
The s-shaped course of the diameter change described in
Figure 8 indicated two different effects that led to the diameter change variation. The s-shape course was completed from a remaining capacity of 80%. Afterwards, the change in diameter increased linearly. Which effects played a role here is to be clarified further. The s-shaped course existed up to a remaining capacity of 80%, which correlated well with the already shown roll over the capacity (
Figure 1b).
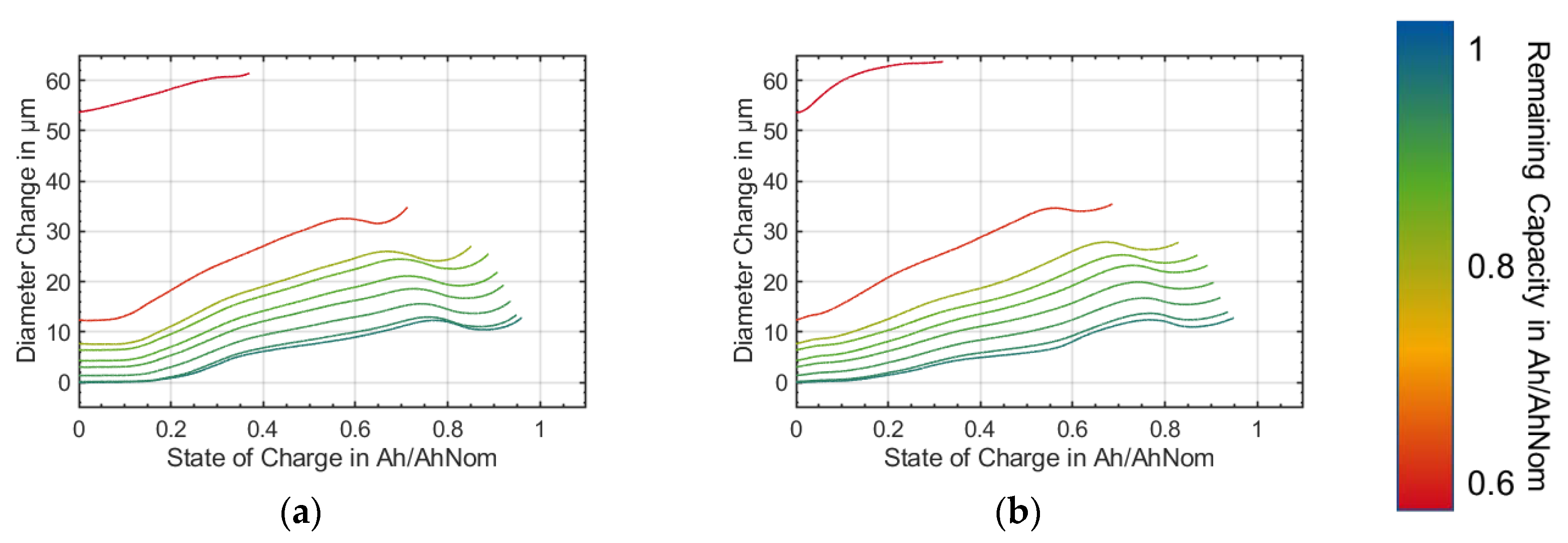
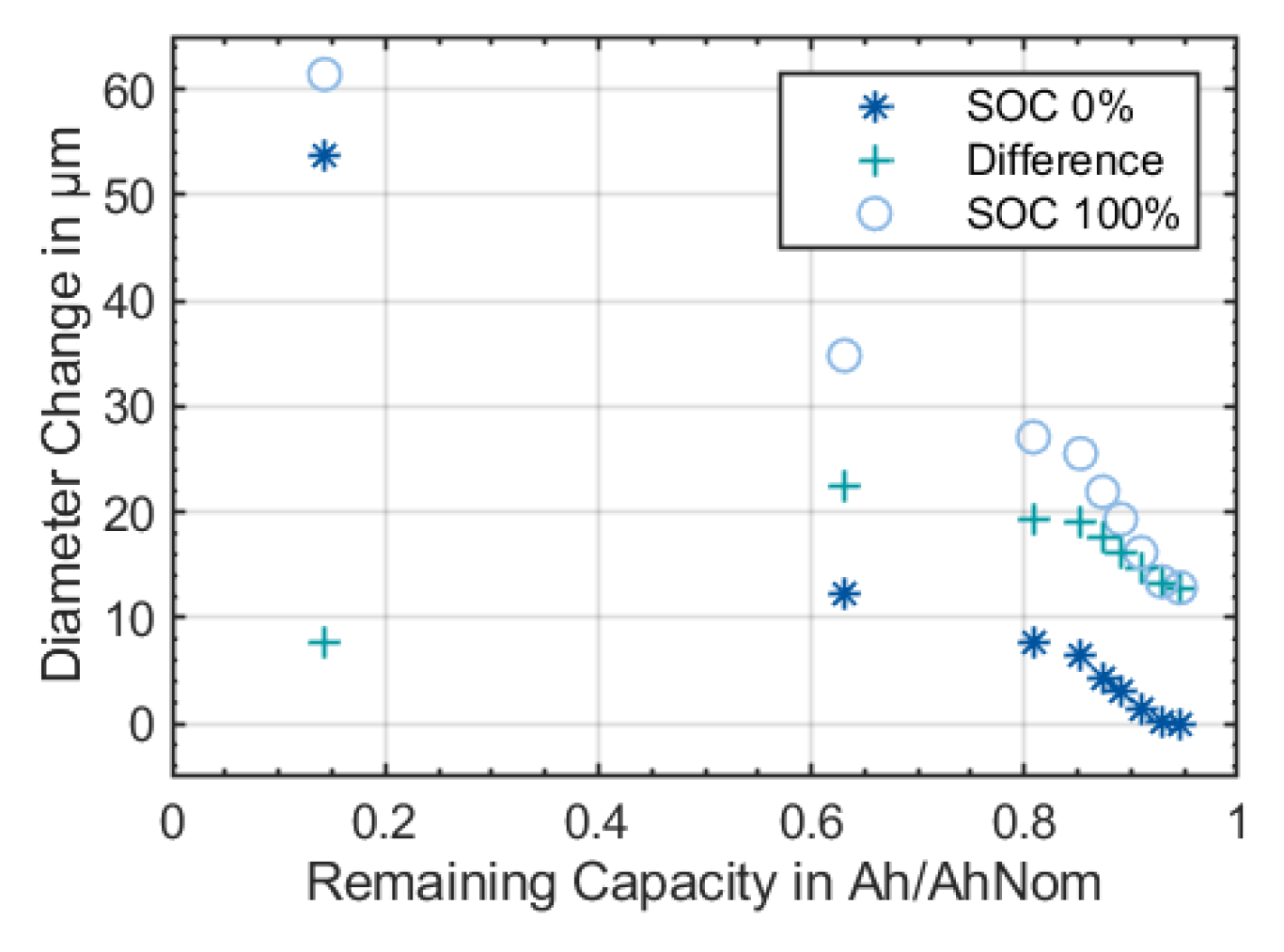
Furthermore, the irreversible diameter change accelerated significantly at lower states-of-health (
Figure 7 and
Figure 8). After check-up seven at around 420 equivalent full cycles, the lithium-ion battery suddenly lost more capacity from cycle to cycle. This was accompanied by a sudden increase in the diameter, see
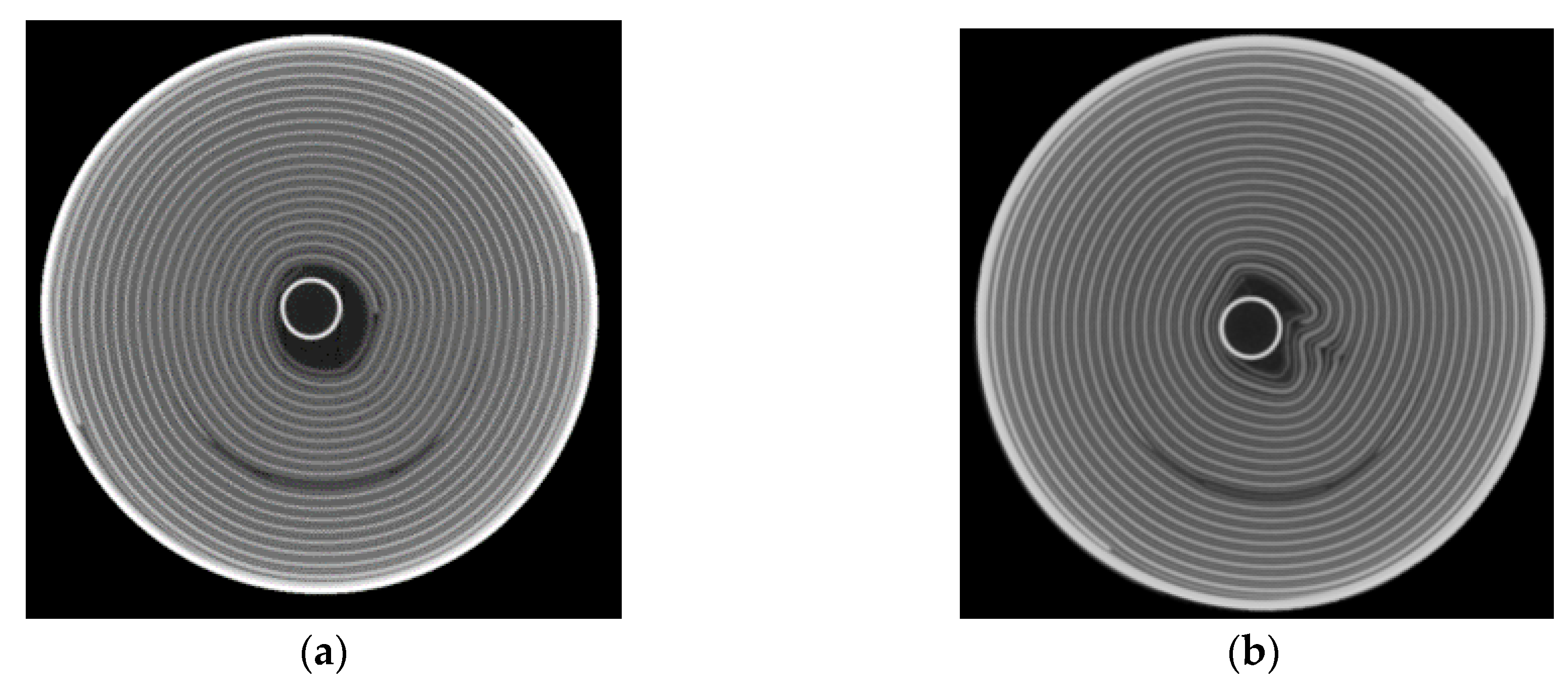
Figure 9. It can be assumed that due to the expansion of the anode, the pressure in the housing was increased to such an extent that the winding was deformed to reduce the pressure (
Figure 11). However, the pressure increased both during the charging and discharging cycles as well as during ageing due to the ageing effects described.
At this point, it is not possible to say whether the jelly roll deformation is the reason for the sudden cell death. However, the deformation of the jelly roll could be a reason for the increasing diameter change.
Overall, there was a direct correlation between the diameter change of the lithium-ion battery and the capacity. The precise interplay between mechanistic, intercalation driven, and chemical effects deserves further investigation. The precise interplay between mechanistic, intercalation driven, and chemical effects deserves further investigation. Nevertheless, Dahn et al. [
33] have shown that macroscopic deposition layers were an indicator of capacity roll-over, which was in agreement with our results.
The results can be summed up as follows: It is important to consider the volume expansion when designing and ageing lithium-ion batteries. Therefore, the strain gauges is a stable and reproducible method to analyze the volume expansion and its impact on capacity and cell death.
5. Conclusions
Electrochemical failure mechanisms like SEI growth, formation of a covering layer, plating, or gas formation induce mechanical stress and therefore a volume expansion. All factors increasing the probability for sudden death of the battery cell. A straightforward and reliable method for measuring the volume expansion and their influence on the state-of-health of the lithium-ion battery is the strain gauge. As we demonstrated, it yielded stable and reproducible results, when monitoring geometric variations on the micrometer scale.
The diameter change of the lithium-ion battery described a characteristic trend and is SOC and load direction-dependent. For as-received batteries of type Samsung 35E, a mean diameter change of 10.7 µm during a full discharge process was measured. Different phenomena seemed to play a role in the ageing of the battery so that the change in diameter changed as a function of the ageing of the battery. Two effects can be observed over ageing using strain gauges: First, an increase of irreversible diameter change, and second, an increase of reversible diameter change. Both reversible and irreversible expansion increased with ageing. Overall, two different ageing effects led to the diameter change variation. CT measurements showed deformation of the jelly roll and post-mortem analysis showed the formation of a covering layer and the increase in the thickness of the anode.
Finally, direct correlation between the diameter change and the capacity loss of this lithium-ion battery can be identified. Therefore, the strain gauge is a tool for predicting the sudden cell death and might even serve as a diagnostic tool for the state-health, including safety aspects.