New Monoterpenoid Indole Alkaloids from Tabernaemontana crassa Inhibit β-Amyloid42 Production and Phospho-Tau (Thr217)
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the New Isolates
2.2. Biological Activity of the New Isolates
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. General Spectra for Structural Characterization
3.5. Cytotoxic Activity
3.6. Cell Culture and Treatment
3.7. Western Blot Analysis
3.8. Enzyme Linked Immunosorbent Assay (ELISA) for Aβ42, pTau217, pTau396 and pTau181
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| NFTs | Neurofibrillary tangles |
| Aβ | Amyloid beta |
| APP | Amyloid precursor protein |
| BACE1 | β-site APP-cleaving enzyme 1 |
| CDK5 | Cyclin-dependent kinase 5 |
| FBS | Fetal bovine serum |
| MIAs | Monoterpenoid indole alkaloids |
| TDDFT | Time-dependent density functional theory |
| ELISA | Enzyme-linked immunosorbent assay |
| NCSTN | Nicastrin |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 2 |
| GSK3β | Glycogen synthase kinases-3β |
| pCDK5 | Phospho-cyclin-dependent kinase 5 (Tyr 15) |
| pTau217 | Phospho-tau (Thr217) |
| pTau181 | Phospho-tau (Thr181) |
| pTau396 | Phospho-tau (Ser396) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| TBST | Tris-buffered saline |
References
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the national institute on aging-Alzheimer’s association research framework. JAMA Neurol. 2019, 76, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Strooper, B.D.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar]
- Durairajan, S.S.K.; Selvarasu, K.; Bera, M.R.; Rajaram, K.; Iyaswamy, A.; Li, M. Alzheimer’s disease and other tauopathies: Exploring efficacy of medicinal plant-derived compounds in alleviating tau-mediated neurodegeneration. Curr. Mol. Pharmacol. 2022, 15, 361–379. [Google Scholar] [CrossRef]
- Selkoe, D.J. Treatments for Alzheimer’s disease emerge. Science 2021, 373, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Vincent, B. Cdk5-p25 as a key element linking amyloid and tau pathologies in Alzheimer’s disease: Mechanisms and possible therapeutic interventions. Life Sci. 2022, 308, 120986. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kritskiy, O.; Watson, L.A.; Barker, S.J.; Dey, D.; Raja, W.K.; Lin, Y.T.; Ko, T.; Cho, S.; Penney, J.; et al. Inhibition of p25/Cdk5 attenuates tauopathy in mouse and iPSC models of frontotemporal dementia. J. Neurosci. 2017, 37, 9917–9924. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, J.W.; Guo, L.L.; Xiong, F.; Ran, X.Q.; Guo, Y.R.; Yao, Y.G.; Hao, X.J.; Luo, R.C.; Zhang, Y. Monoterpenoid indole alkaloid dimers from Kopsia arborea inhibit cyclin-dependent kinase 5 and tau phosphorylation. Phytochemistry 2022, 203, 113392. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Luo, R.C.; Ye, M.S.; Tang, H.Y.; Ma, Y.L.; Chen, Y.N.; Wang, X.M.; Lu, Q.Y.; Liu, S.; Li, X.N.; et al. Harpertrioate A, an A,B,D-seco-limonoid with promising biological activity against Alzheimer’s disease from twigs of Harrisonia perforata (Blanco) Merr. Org. Lett. 2021, 23, 262–267. [Google Scholar] [CrossRef]
- Zaima, K.; Hirata, T.; Hosoya, T.; Hirasawa, Y.; Koyama, K.; Rahman, A.; Kusumawati, I.; Zaini, N.C.; Shiro, M.; Morita, H. Biscarpamontamines A and B, an Aspidosperma−iboga bisindole alkaloid and an aspidosperma−aspidosperma bisindole alkaloid, from Tabernaemontana sphaerocarpa. J. Nat. Prod. 2009, 72, 1686–1690. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Yuwen, H.S.; Guo, L.L.; Liu, J.W.; Hao, X.J. Alkaloids from Tabernaemontana divaricata combined with fluconazole to overcome fluconazole resistance in Candida albicans. Bioorg. Chem. 2021, 107, 104515. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Y.X.; Goto, M.; Guo, L.L.; Li, X.N.; Morris-Natschke, S.L.; Lee, K.H.; Hao, X.J. Taburnaemines A–I, cytotoxic vobasinyl-iboga-type bisindole alkaloids from Tabernaemontana corymbosa. J. Nat. Prod. 2018, 81, 562–571. [Google Scholar] [CrossRef]
- Luca, V.D.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.N.; Lin, L.P.; Ding, J.; Yue, J.M. Indole alkaloids from three species of the Ervatamia genus: E. officinalis, E. divaricata, and E. divaricata Gouyahua. J. Nat. Prod. 2007, 70, 54–59. [Google Scholar] [CrossRef]
- Bhadane, B.S.; Patil, M.P.; Maheshwari, V.L.; Patil, R.H. Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytother. Res. 2018, 32, 1181–1210. [Google Scholar] [CrossRef]
- Packard, R.B. An ethnography of the religious imagination in Africa by James W. Fernandez. J. Interdiscip. Hist. 1984, 14, 902–904. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Zhang, Y.; Guo, L.L.; Wang, Y.H.; Goto, M.; Morris-Natschke, S.L.; Lee, K.H.; Hao, X.J. Tabercorymines A and B, two vobasinyl–ibogan-type bisindole alkaloids from Tabernaemontana corymbosa. Org. Lett. 2017, 19, 4964–4967. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; He, H.P.; Di, Y.T.; Li, S.F.; Cheng, Y.Y.; Yang, W.; Li, Y.; Yu, J.P.; Zhang, Y.; Hao, X.J. Indole alkaloids from Ervatamia chinensis. Phytochemistry 2012, 74, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, X.; Yuan, Y.X.; Guo, L.L.; Hao, X.J. Cytotoxic monoterpenoid indole alkaloids from Tabernaemontana corymbosa as potent autophagy inhibitors by the attenuation of lysosomal acidification. J. Nat. Prod. 2020, 83, 1432–1439. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.L.; Yang, G.M.; Guo, F.; Di, Y.T.; Li, S.L.; Chen, D.Z.; Hao, X.J. New vobasinyl-ibogan type bisindole alkaloids from Tabernaemontana corymbosa. Fitoterapia 2015, 100, 150–155. [Google Scholar] [CrossRef]
- Okuyama, E.; Gao, L.H.; Yamazaki, M. Analgesic components from Bornean medicinal plants, Tabernaemontana pauciflora BLUME and Tabernaemontana pandacaqui POIR. Chem. Pharm. Bull. 1992, 40, 2075–2079. [Google Scholar] [CrossRef]
- Goldblatt, A.; Hootele, C.; Pecher, J. The alkaloids of voacanga thouarsii var. obtuse. Phytochemistry 1970, 9, 1293–1298. [Google Scholar] [CrossRef]
- Figueiredo, E.R.; Vieira, I.J.C.; Souza, J.J.; Braz-Filho, R.; Mathias, L.; Kanashiro, M.M.; Côrtes, F.H. Isolation, identification and antileukemic activity of the monoterpene indole alkaloids from Tabernaemontana salzmannii (A. DC.), Apocynaceae. Rev. Bras. Farmacogn. 2010, 20, 675–681. [Google Scholar] [CrossRef]
- Sharma, P.; Cordell, G.A. Heyneanine hydroxyindolenine, a new indole alkaloid from Ervatamia coronaria var. plena. J. Nat. Prod. 1988, 51, 528–531. [Google Scholar] [CrossRef]
- Lo, M.W.; Matsumoto, K.; Iwai, M.; Tashima, K.; Kitajima, M.; Horie, S.; Takayama, H. Inhibitory effect of iboga-type indole alkaloids on capsaicin-induced contraction in isolated mouse rectum. J. Nat. Med. 2011, 65, 157–165. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Cordell, G.; Farnsworth, N.R. Anticancer indole alkaloids of Ervatamia heyneana. Phytochemistry 1980, 19, 1213–1218. [Google Scholar] [CrossRef]
- Knox, J.R.; Slobbe, J. Three novel alkaloids from Ervatamia orientalis. Tetrahedron. Lett. 1971, 24, 2149–2151. [Google Scholar] [CrossRef]
- Sheludko, Y.; Gerasimenko, I.; Kolshorn, H.; Stöckigt, J. New alkaloids of the sarpagine group from Rauvolfia serpentina hairy root culture. J. Nat. Prod. 2002, 65, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, H.S.; Yuan, Y.X.; Hao, X.J.; He, H.P.; Zhang, Y. Two new monoterpenoid indole alkaloids from Tabernaemontana divaricata. Nat. Prod. Res. 2018, 33, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Thomas, N.F.; Abdullah, Z.; Kam, T.S. Seco-tabersonine alkaloids from Tabernaemontana corymbosa. Phytochemistry 2009, 70, 424–429. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, H.; Yuan, C.M.; Zhang, Y.; Cao, M.M.; Zhang, H.Y.; Feng, Y.; Ding, X.; Zhou, Q.; Zhao, Q.; et al. Cytotoxic indole alkaloids from the fruits of Melodinus cochinchinensis. Phytochemistry 2015, 116, 367–373. [Google Scholar] [CrossRef]
- Xiang, Q.; Bi, R.; Xu, M.; Zhang, D.F.; Tan, L.W.; Zhang, C.; Fang, Y.R.; Yao, Y.G. Rare genetic variants of the Transthyretin gene are associated with Alzheimer’s disease in Han Chinese. Mol. Neurobiol. 2017, 54, 5192–5200. [Google Scholar] [CrossRef]
- Luo, R.C.; Su, L.Y.; Li, G.Y.; Yang, J.; Liu, Q.J.; Yang, L.X.; Zhang, D.F.; Zhou, H.J.; Xu, M.; Fan, Y.; et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 2020, 16, 52–69. [Google Scholar] [CrossRef]
- Luo, R.C.; Fan, Y.; Yang, J.; Ye, M.S.; Zhang, D.F.; Guo, K.; Li, X.; Bi, R.; Xu, M.; Yang, L.X.; et al. A novel missense variant in ACAA1 contributes to early-onset Alzheimer’s disease, impairs lysosomal function, and facilitates amyloid-β pathology and cognitive decline. Sig. Transduct. Target. Ther. 2021, 6, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Rubins, H.B.; Robins, S.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med. 1999, 341, 410–418. [Google Scholar] [CrossRef]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; et al. A Helsinki heart study: Primary-prevention trial with Gemfibrozil in middle-aged men with dyslipidemia. N. Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar] [CrossRef]
- Corbett, G.T.; Gonzalez, F.J.; Pahan, K. Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP. Proc. Natl. Acad. Sci. USA 2015, 112, 8445–8450. [Google Scholar] [CrossRef]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold. Spring. Harbor. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Dunys, J.; Valverde, A.; Checler, F. Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer’s disease? J. Biol. Chem. 2018, 293, 15419–15428. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Jansonius, B.; Delaidelli, A.; Somasekharan, S.P.; Bhanshali, F.; Vandal, M.; Negri, G.L.; Moerman, D.; MacKenzie, I.; Calon, F.; et al. eEF2K inhibition blocks Aβ42 neurotoxicity by promoting an NRF2 antioxidant response. Acta Neuropathol. 2017, 133, 101–119. [Google Scholar] [CrossRef]
- Kumar, S.K.; LaPlant, B.; Chng, W.J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A.K. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Anelidze, S.; Stomrud, J.E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.Y.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683–1695. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Janelidze, S.; Palmqvist, S.; Cullen, N.; Svenningsson, A.L.; Strandberg, O.; Mengel, D.; Walsh, D.M.; Stomrud, E.; Dage, J.L.; et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 2020, 143, 3234–3241. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.H.; Serrano, G.E.; Leuzy, A.; et al. Discriminative accuracy of plasma phospho-tau217 for alzheimer disease vs other neurodegenerative disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Li, C.Z.; Götz, J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 2017, 16, 863–883. [Google Scholar] [CrossRef]
- Mazanetz, M.P.; Fischer, P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug. Discov. 2007, 6, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Zukerberg, L.R.; Patrick, G.N.; Nikolic, M.; Humbert, S.; Wu, C.L.; Lanier, L.M.; Gertler, F.B.; Vidal, M.; Van Etten, R.A.; Tsai, L.H. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 2000, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Cheng, C.; Uchida, Y.; Nakajima, O.; Ohshima, T.; Yagi, T.; Taniguchi, M.; Nakayama, T.; Kishida, R.; Kudo, Y.; et al. Fyn and Cdk5 mediate Semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002, 35, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, T.Y.; Juang, J.L. Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell. Death Differ. 2007, 14, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Su, L.Y.; Luo, R.C.; Liu, Q.; Su, J.R.; Yang, L.X.; Ding, Y.Q.; Xu, L.; Yao, Y.G. Atg5- and Atg7-dependent autophagy in dopaminergic neurons regulates cellular and behavioral responses to morphine. Autophagy 2017, 13, 1496–1511. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.C.; Huang, A.S.; Wu, A.J.; Iyaswamy, A.; Ho, O.K.; Kong, A.H.; Sreenivasmurthy, S.G.; Zhu, Z.; Su, C.; Liu, J.; et al. Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies. J. Biomed. Sci. 2022, 29, 85. [Google Scholar] [CrossRef]
- Sreenivasmurthy, S.G.; Iyaswamy, A.; Krishnamoorthi, S.; Reddi, R.N.; Kammala, A.K.; Vasudevan, K.; Senapati, S.; Zhu, Z.; Su, C.F.; Liu, J.; et al. Bromo-protopine, a novel protopine derivative, alleviates tau pathology by activating chaperone-mediated autophagy for Alzheimer’s disease therapy. Front. Mol. Biosci. 2022, 9, 1030534. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. Corner flapping: A simple and fast algorithm for exhaustive generation of ring conformations. J. Am. Chem. Soc. 1989, 111, 8950–8951. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 1993, 2, 187–198. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Spec Dis, Version 1.60; University of Würzburg: Würzburg, Germany, 2012. [Google Scholar]
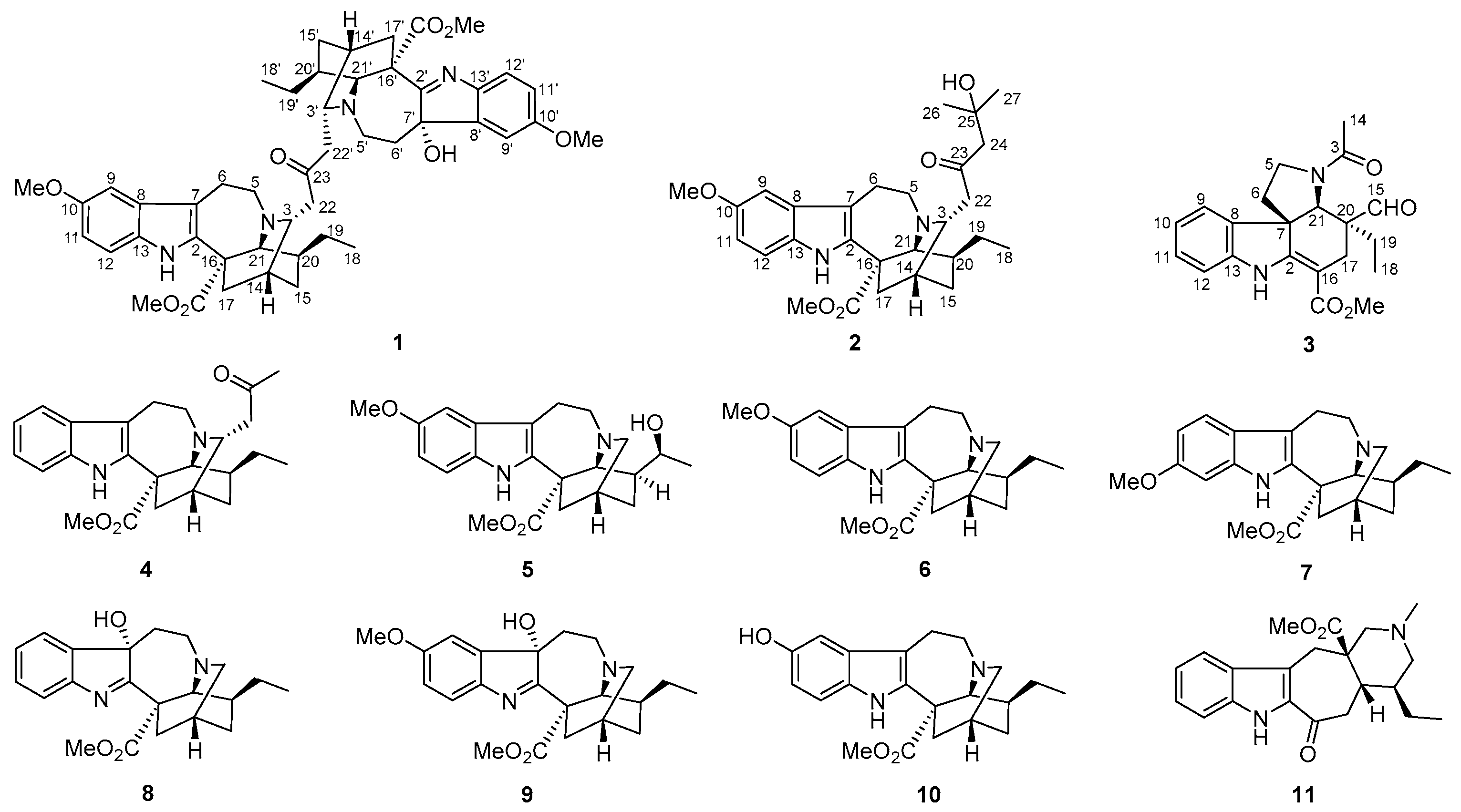

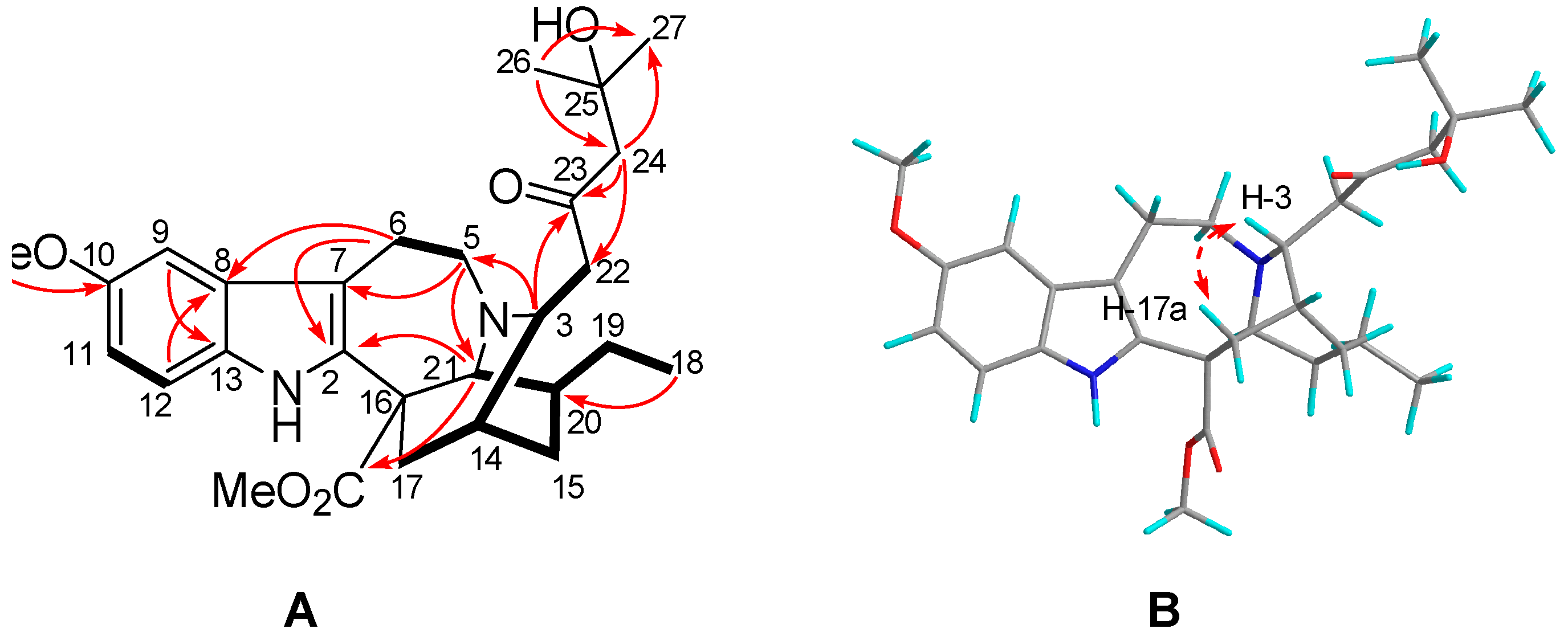

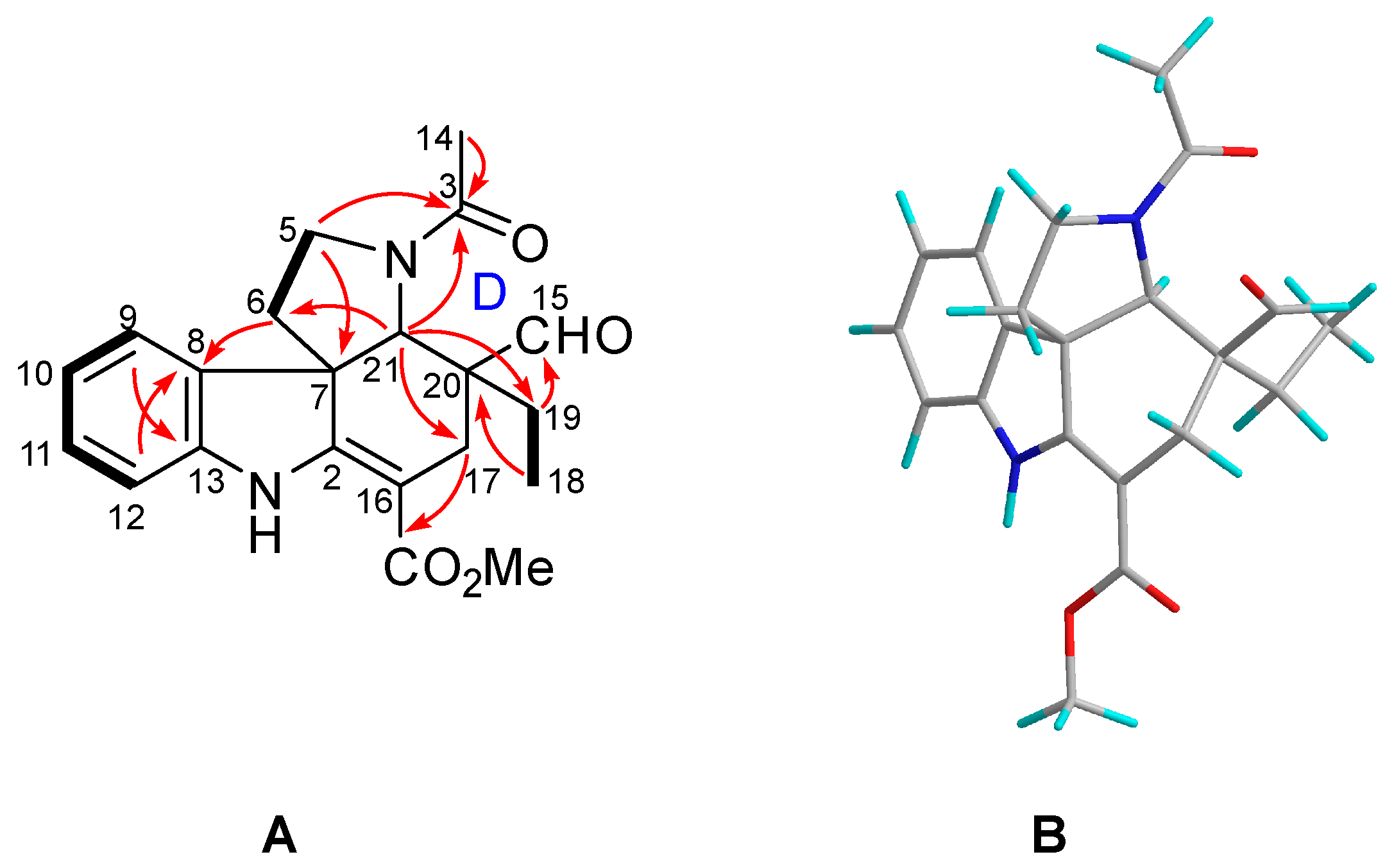


| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 2 | 139.2 | 2′ | 188.5 | ||
| 3 | 3.28 (m) | 56.4 | 3′ | 3.23 (dd, 8.0, 4.0) | 52.5 |
| 5a | 3.16 (t, 6.0) | 52.3 | 5′a | 3.00 (ddd, 15.0, 4.0, 2.0) | 47.3 |
| 5b | 3.22 (ddd, 6.0, 4.0, 1.5) b | 5′b | 3.34 (ddd, 14.0, 11.0, 4.0) | ||
| 6a | 3.10 (ddd, 18.5, 7.5, 3.5) | 22.6 | 6′a | 1.83 (m) 1.83 (m) | 35.1 |
| 6b | 2.89 (ddd, 18.5, 7.5, 3.5) | 6′b | |||
| 7 | 110.2 | 7′ | 88.6 | ||
| 8 | 129.9 | 8′ | 145.6 | ||
| 9 | 6.93 (d, 2.5) | 101.0 | 9′ | 6.86 (d, 2.5) | 108.9 |
| 10 | 154.8 | 10′ | 159.9 | ||
| 11 | 6.68 (dd, 8.5, 2.5) | 112.1 | 11′ | 6.81 (dd, 8.5, 2.5) | 114.2 |
| 12 | 7.16 (d, 8.5) | 112.2 | 12′ | 7.22 (d, 8.5) | 121.7 |
| 13 | 132.2 | 13′ | 146.2 | ||
| 14 | 1.61 (m) b | 31.5 | 14′ | 1.60 (m) b | 31.8 |
| 15a | 1.20 (m) | 27.6 | 15′a | 1.14 (m) | 27.8 |
| 15b | 1.54 (m) b | 15′b | 1.46 (m) | ||
| 16 | 55.7 b | 16′ | 55.7 b | ||
| 17a | 1.85 (ddd, 16.0, 5.0, 2.5) | 38.2 | 17′a | 2.35 (ddd, 14.0, 5.0, 3.0) | 37.9 b |
| 17b | 2.71 (m) b | 17′b | 2.76 (dd, 14.0, 2.0) | ||
| 18 | 0.82 (t, 7.5) b | 12.0 b | 18′ | 0.82 (t, 7.5) b | 12.0 b |
| 19a | 1.34 (m) 1.53 (m) b | 27.5 | 19′a | 1.30 (m) 1.45 (m) | 27.3 |
| 19b | 19′b | ||||
| 20 | 1.25 (m) b | 38.9 | 20′ | 1.26 (m) | 37.9 b |
| 21 | 3.53 (br s) | 59.0 | 21′ | 3.96 (br s) | 58.6 |
| 22a | 2.51 (dd, 16.0, 2.0) | 46.9 | 22′a | 2.50 (br d, 16.0) | 47.0 |
| 22b | 2.72 (m) b | 22′b | 2.68 (m) b | ||
| 23 | 210.4 | ||||
| NH | 9.26 (s) | OH-7′ | 4.53 (s) | ||
| OMe-10 | 3.78 (s) | 55.9 | OMe-10′ | 3.79 (s) | 56.0 |
| CO2Me | 175.2 | CO2Me’ | 173.7 | ||
| 3.63 (s) | 52.6 | 3.58 (s) | 52.9 |
| No. | 2 a | 3 b | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 139.3 | 161.1 | ||
| 3 | 3.37 (dd, 8.5, 4.0) | 56.3 | 171.0 | |
| 5a | 3.20 (dd, 8.0, 6.0) 3.23 (dd, 8.0, 5.0) | 52.4 | 3.77 (ddd, 12.0, 9.0, 1.2) | 46.5 |
| 5b | 3.90 (ddd, 12.0, 9.0, 7.8) | |||
| 6a | 2.93 (dt, 15.0, 6.0) | 22.7 | 1.95 (m) | 38.2 |
| 6b | 3.14 (ddd, 15.0, 8.0, 5.0) | 2.55 (ddd, 9.0, 7.8, 1.2) c | ||
| 7 | 110.2 | 56.0 | ||
| 8 | 123.0 | 136.7 | ||
| 9 | 6.95 (d, 2.5) | 101.0 | 7.10 (d, 7.2) | 122.4 |
| 10 | 154.8 | 6.87 (td, 7.2, 1.2) | 121.9 | |
| 11 | 6.70 (dd, 8.5, 2.5) | 112.1 | 7.19 (td, 7.2, 1.2) | 129.4 |
| 12 | 7.19 (d, 8.5) | 112.3 | 7.09 (d, 7.2) | 110.9 |
| 13 | 132.2 | 144.7 | ||
| 14 | 1.71 (m) | 31.6 | 2.11 (s) | 22.7 |
| 15a | 1.28 (m)c | 27.6 | 204.5 | |
| 15b | 1.53 (m) | |||
| 16 | 55.8 | 89.9 | ||
| 17a | 1.90 (ddd, 13.5, 5.0, 2.0) | 38.3 | 2.53 (br d, 16.2) c 2.67 (br d, 16.2) | 23.6 |
| 17b | 2.75 (dd, 14.0, 2.0) | |||
| 18 | 0.88 (t, 7.5) | 12.0 | 0.73 (t, 7.2) | 9.0 |
| 19a | 1.41 (m) | 27.7 | 1.29 (m) | 27.9 |
| 19b | 1.59 (dt, 13.0, 7.5) | 1.81 (m) | ||
| 20 | 1.28 (m) c | 38.9 | 55.6 | |
| 21 | 3.57 (br s) | 59.0 | 4.05 (br s) | 69.5 |
| 22a | 2.64 (dd,17.0, 8.0) | 48.1 | ||
| 22b | 2.80 (br d, 17.0) | |||
| 23 | 211.9 | |||
| 24a | 2.58 (br d, 6.0) 3.78 (br d, 6.0) c | 55.6 | ||
| 24b | ||||
| 25 | 69.9 | |||
| 26 | 1.16 (s) | 29.9 | ||
| 27 | 1.15 (s) | 29.8 | ||
| OMe-10 | 3.78 (s) | 55.9 | ||
| CO2Me | 175.2 | 168.1 | ||
| 3.64 (s) | 52.7 | 3.71 (s) | 51.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Han, L.-L.; Huang, K.-P.; Ma, Y.-H.; Guo, L.-L.; Guo, Y.; Ran, X.; Yao, Y.-G.; Hao, X.-J.; Luo, R.; et al. New Monoterpenoid Indole Alkaloids from Tabernaemontana crassa Inhibit β-Amyloid42 Production and Phospho-Tau (Thr217). Int. J. Mol. Sci. 2023, 24, 1487. https://doi.org/10.3390/ijms24021487
Li S, Han L-L, Huang K-P, Ma Y-H, Guo L-L, Guo Y, Ran X, Yao Y-G, Hao X-J, Luo R, et al. New Monoterpenoid Indole Alkaloids from Tabernaemontana crassa Inhibit β-Amyloid42 Production and Phospho-Tau (Thr217). International Journal of Molecular Sciences. 2023; 24(2):1487. https://doi.org/10.3390/ijms24021487
Chicago/Turabian StyleLi, Sheng, Ling-Ling Han, Ke-Pu Huang, Ye-Han Ma, Ling-Li Guo, Yarong Guo, Xiaoqian Ran, Yong-Gang Yao, Xiao-Jiang Hao, Rongcan Luo, and et al. 2023. "New Monoterpenoid Indole Alkaloids from Tabernaemontana crassa Inhibit β-Amyloid42 Production and Phospho-Tau (Thr217)" International Journal of Molecular Sciences 24, no. 2: 1487. https://doi.org/10.3390/ijms24021487
APA StyleLi, S., Han, L.-L., Huang, K.-P., Ma, Y.-H., Guo, L.-L., Guo, Y., Ran, X., Yao, Y.-G., Hao, X.-J., Luo, R., & Zhang, Y. (2023). New Monoterpenoid Indole Alkaloids from Tabernaemontana crassa Inhibit β-Amyloid42 Production and Phospho-Tau (Thr217). International Journal of Molecular Sciences, 24(2), 1487. https://doi.org/10.3390/ijms24021487






