Abstract
Cancer stem cells (CSCs) possess a significant ability to renew themselves, which gives them a strong capacity to form tumors and expand to encompass additional body areas. In addition, they possess inherent resistance to chemotherapy and radiation therapies used to treat many forms of cancer. Scientists have focused on investigating the signaling pathways that are highly linked to the ability of CSCs to renew themselves and maintain their stem cell properties. The pathways encompassed are Notch, Wnt/β-catenin, hedgehog, STAT3, NF-κB, PI-3K/Akt/mTOR, sirtuin, ALDH, MDM2, and ROS. Recent studies indicate that directing efforts towards CSC cells is essential in eradicating the overall cancer cell population and reducing the likelihood of tumor metastasis. As our comprehension of the mechanisms that stimulate CSC activity, growth, and resistance to chemotherapy advances, the discovery of therapeutic drugs specifically targeting CSCs, such as small-molecule compounds, holds the potential to revolutionize cancer therapy. This review article examines and analyzes the novel anti-CSC compounds that have demonstrated effective and selective targeting of pathways associated with the renewal and stemness of CSCs. We also discussed their special drug metabolism and absorption mechanisms. CSCs have been the subject of much study in cancer biology. As a possible treatment for malignancies, small-molecule drugs that target CSCs are gaining more and more attention. This article provides a comprehensive review of the current state of key small-molecule compounds, summarizes their recent developments, and anticipates the future discovery of even more potent and targeted compounds, opening up new avenues for cancer treatment.
1. Introduction
Stem cells (SCs) that are found in niches possess the ability to undergo self-renewal and specialize into many cell types within adult somatic tissues. Cancer stem cells (CSCs) are a distinct group of cells having stem cell characteristics that are present within malignancies. They have a vital function in initiating and facilitating the growth and advancement of cancer. This is achieved by disrupting various signaling pathways, leading to cellular and tumor-specific molecular diversity. As a result, CSCs are regarded as the highest point in the hierarchical model of tumorigenesis, progression, metastasis, and resistance to drugs [1,2,3]. Several malignancies have been found to exhibit an enhanced ability to initiate tumor growth, partially replicate the diversity of cells and molecules, and overall display greater resistance to traditional anticancer treatments compared to the responses of other tumor cells. Previous research has discovered CSCs that have a significant impact on the start, development, dissemination, and resistance to specific therapies of solid tumors [4,5,6,7].
SCs and CSCs share functional and phenotypic similarities, including the capacity for self-renewal and differentiation. Nevertheless, there exist notable distinctions in the biological activities of SCs and CSCs, mostly ascribed to a severe disturbance in the capacity of CSCs to renew themselves without assistance. Contrary to SCs, which undergo differentiation and ultimately produce specialized offspring, CSCs generate offspring that display unregulated proliferation and do not undergo terminal differentiation. CSCs acquire a malignant phenotype due to variations in their cell-cycle characteristics, division, replicative potential, molecular pathway activation and inactivation, and DNA damage control. At present, the clinical difficulties that persist are tumor recurrence, metastasis [7,8]. Since CSCs have distinct metabolic properties, a new approach is the targeting of CSCs [9,10]. CSCs exhibit resistance to traditional cancer treatments and typically possess a high capacity for tumor formation and metastasis [11]. CSCs also exhibit stemness acquired through the process of epithelial-to-mesenchymal transition (EMT), which refers to their capacity for self-renewal and differentiation [12,13]. The concept of stemness plays a crucial role in driving the development and progression of cancer. It allows CSCs to undergo self-renewal, invade surrounding tissues, spread to distant sites, and regenerate tumors [11,14,15].
All cells exhibit the same tumorigenic activity when seen through the lens of the tumor clonal evolution model [16]. CSCs, on the other hand, are the only cells that exhibit self-renewal capacity, tumor-initiating capability, and pluripotency in the tumor CSC model demonstrated in Figure 1 [9,17]. This may explain why early tumor shrinkage for evaluating the effects of therapies is usually poorly predictive of the patient outcome and overall survival [18,19,20]. Despite the fact that standard chemotherapy may be able to eliminate the majority of cancer cells that are not stem cells, CSCs are more resistant to chemotherapy and have the potential to cause tumor relapse [12,21]. Non-stem cancer cells can acquire cancer stemness when they undergo dedifferentiation during traditional cancer therapy through the connections between EMT and differentiation status [22,23,24].
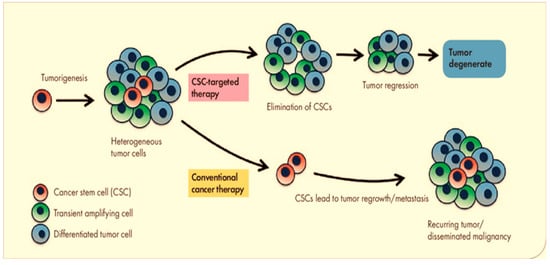
Figure 1.
In the tumor CSC model, only CSCs have tumor-initiating capability.
Increasing research suggests that specifically focusing on CSCs is essential for eradicating the entire population of cancer cells. As our comprehension of the mechanisms that drive CSC activity, progression, and chemoresistance improves, the creation of therapeutic drugs or treatment methods specifically targeting CSCs may result in significant advancements in malignancy therapy [21,25,26]. For the purpose of our investigation, we have focused our attention on the small-molecule compounds that target certain pathways that are closely connected with the renewal and stemness of CSCs.
2. Small-Molecular Compounds of Cancer Stem Cells Related to Specific Signaling Pathways
2.1. Small-Molecule Compounds Targeting Notch Signaling Pathway
Notch signaling performs the role of receptors for ligands that are bound to membranes. Notch1 through Notch4 are the four Notch genes that it possesses, and it possesses five ligands: Delta-like 1 (DLL1), DLL3, DLL4, and Jagged1, Jagged2 [27,28]. Notch receptors have an intracellular domain and an extracellular domain, which have several epidermal growth factor (EGF) domains [29]. The Notch proteins may impact many types of cell activities, including apoptosis, angiogenesis, differentiation, and proliferation [30,31]. Therefore, Notch influences morphogenesis and organogenesis [32]. Notch receptor activation is induced by Jagged or Delta ligands in neighboring cells.
The fundamental roles of Notch signaling are implicated in developmental processes, during the regulation of embryonic development and adult tissue homeostasis; during the differentiation, maintenance, and cellular homeostasis of SCs [33,34]. There is a correlation between CSCs and Notch receptors and/or ligands in a number of different types of tumors [27,31,35,36,37]. Notch activity and epigenetic modulation of the Notch regulators have been described in breast CSCs (BCSCs), which are cells that originate from breast cancer [38,39,40,41]. The activation of Notch1 improves survival of CD24(+) CD29(high) progenitor cells via a cyclin D1-dependent route, hinders self-renewal of mammary SCs, and makes their transformation easier [42]. The HER2 has Notch-RBP-Jκ binding sites in its promoter [43] and is triggered in both types of breast stem cells [39,44] and BCSCs by Notch1 signaling. The presence of the erythropoietin receptor (EpoR) was observed on the surface of BCSCs, and it was shown that erythropoietin (Epo) interacts with Notch1 to sustain the ability of BCSCs to self-renew [45]. The activation of Notch results in the creation of dimers of Hes and/or Hey proteins, which inhibit the transcription of several genes by capturing transcriptional activators or associating with co-repressors [32,46,47]. In recent years, BCSCs were demonstrated to be correlated with several oncogenes, such as cyclo-oxygenase (COX)-2 [48], HER2 [49,50], MYC[51], and Akt [52], in addition to transcription factors like STAT3 [53], HIF-1 [54], and NF-κB [55]. As Notch signaling interacts with these oncogenes and/or transcriptional factors [31], through these signals it may have an effect on BCSCs and the progression of breast cancer. Table 1 displays specific small chemical compounds that target cancer stem cells across various pathways, including the Notch pathway.

Table 1.
Targeted small-molecule compounds of cancer stem cells.
Epigallocatechin-3-gallate (EGCG) (1, Figure 2), which is the primary polyphenol found in green tea, effectively prevents the self-renewal ability of head-neck squamous carcinoma (HNSC) CSCs. The primary site of absorption for EGCG is the intestine, and the metabolism of EGCG by gut microbes is crucial before it can be absorbed [119]. EGCG, as a means of suppressing HNSC CSC characteristics, reduced the transcriptional activity of Notch, hence inhibiting Notch signaling [56]. Stem-like cells found in brain tumors appear to be specifically susceptible to GSI-18 (2, Figure 2), which inhibits the Notch pathway [59]. The vitamin D compounds (3, Figure 2) also decreased the expression of Notch signaling molecules, such as Notch1, Notch2, Notch3, JAG1, JAG2, HES1, and NFκB, which play a role in maintaining BCSCs. Vitamin D has the potential to be utilized as an agent for preventing triple-negative breast cancer (TNBC) by controlling CSCs and promoting differentiation [60]. Niclosamide (4, Figure 2) is a well-established antihelminthic medication that disrupts the functioning of mitochondria in intestinal parasites. Studies have shown that 4 has the potential to be an effective anticancer drug [120]. However, its limited ability to dissolve in water must be addressed before additional preclinical and clinical research can be performed. 4 was discovered to be a CSC inhibitor using a dye exclusion technique. It suppressed the activity of Notch, Wnt, and SHH stem pathways, hindered the development of spheroids, and triggered programmed cell death in the side population sphere of breast cancer. Niclosamide’s therapeutic properties were also validated through animal experiments [61].
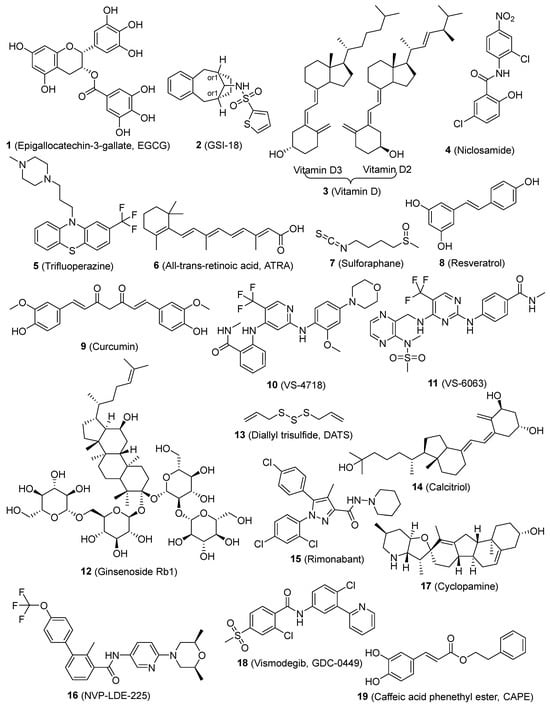
Figure 2.
Chemical structures of targeted small-molecule compounds of cancer stem cells.
2.2. Small-Molecule Compounds Targeting Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway is crucial in controlling various biological processes, including cell proliferation, angiogenesis, polarity, asymmetric cell division, tissue homeostasis, and cancer development [121,122,123]. The Wnt/β-catenin signaling system has the ability to stimulate the formation of pluripotent stem (iPS) cells, hence enhancing the process of reprogramming somatic cells. This highlights the significance of this pathway in promoting the renewal and multipotency of stem cells [124,125,126]. Abnormal Wnt/β-catenin signaling has been widely documented in several types of cancers [127,128,129,130]. The importance of β-catenin in maintaining the characteristics of epidermal CSCs was recognized early on. Removing this gene leads to the elimination of epidermal CSCs [131]. Several publications have demonstrated that intestinal SCs can undergo a transformation into CSCs due to abnormal Wnt/β-catenin signaling. This suggests that SCs are the cells responsible for initiating tumors in the intestine and highlights the significance of Wnt/β-catenin in facilitating the transition from SCs to CSCs during the development of intestinal cancer [132,133,134]. Mammary SCs were also identified as target cells in the process of Wnt-induced mammary gland tumorigenesis [135,136,137]. Wnt signaling can impact distinct pools of mammary SCs, giving rise to different types of tumors [138]. A comprehensive review has been conducted on the small-molecule compounds of Wnt [139]. Here, we discuss a few Wnt inhibitors that were reported to target CSCs.
Trifluoperazine (5, Figure 2) hinders the production of CSC tumor spheroids and reduces the expression of the CSC markers (CD44/CD133). 5 hinders the Wnt/β-catenin signaling pathway in lung cancer sphere that is resistant to gefitinib. The coadministration of 5 with either gefitinib or cisplatin effectively circumvents drug resistance in lung CSCs. 5 suppresses the growth of tumors and increases the inhibitory effect of gefitinib in animal models of lung cancer metastasis and orthotopic CSCs [62]. All-trans retinoic acid (ATRA) (6, Figure 2) is commonly used to treat a range of disorders characterized by excessive cell growth and inflammation. Nevertheless, the therapeutic effectiveness of the substance is at risk because of its limited solubility and durability [140]. The latter was overcome by incorporating it into a solid matrix of lipidic nanoparticles (SLNs). ATRA repressed the expression of the stem cell markers Oct4, Sox2, Nestin, and CD44 and hindered the growth of HNSC, thyroid, and breast CSCs both in laboratory conditions and in living organisms. The potential anti-tumor effects of ATRA may be linked to the suppression of Wnt/β-catenin signaling [63,64,65]. Sulforaphane (SFN) (7, Figure 2), a prominent member of the isothiocyanates, is found in abundance in cruciferous plants and is renowned for its remarkable anti-cancer properties [141]. SFN suppresses breast CSCs and reduces the activity of the Wnt/β-catenin pathway, which is responsible for the self-renewal of these cells. SFN has the potential to act as a chemopreventive drug for breast CSCs and should be further evaluated in clinical settings [66]. Resveratrol (8, Figure 2), a naturally occurring polyphenolic molecule, hinders the growth of breast CSCs and triggers a process called autophagy by suppressing the Wnt/β-catenin signaling pathway [67]. A natural substance called curcumin (9, Figure 2) blocks the migration of breast CSCs by intensifying the negative feedback loop between E-cadherin and β-catenin [70]. Additionally, 9 demonstrated its interventional effect on lung CSCs through the suppression of sonic hedgehog and Wnt/β-catenin pathways [71]. EGCG (1) efficiently decreases the activity of lung CSCs by suppressing the formation of tumorspheres, reducing the expression of markers specific to lung CSCs, inhibiting cell growth, and promoting apoptosis. EGCG suppresses Wnt/β-catenin activation, while the increase in Wnt/β-catenin counteracts the inhibitory impact of EGCG on lung CSCs [57]. VS-4718 and VS-6063 (10 and 11, Figure 2) selectively target CSCs by inhibiting the activity of focal adhesion kinase (FAK). A fascinating interaction between FAK and the Wnt/β-catenin pathway has been discovered, in which FAK inhibition prevents the activation of β-catenin by decreasing the phosphorylation of tyrosine 654 on β-catenin. The selective targeting of CSCs by FAK inhibitors offers a logical basis for the therapeutic advancement of FAK inhibitors with the goal of enhancing long-lasting responses in cancer treatment [73]. The natural product ginsenoside Rb1 (12, Figure 2) demonstrates strong cytotoxic effects on CSCs obtained from patients with ovarian carcinoma. 12 further enhances the sensitivity of CSCs to therapeutically significant amounts of cisplatin and paclitaxel. These effects are associated with the Wnt/β-catenin signaling pathway. Furthermore, no signs of toxicity were seen when administering doses of 12 that resulted in an anti-CSC impact [74]. Diallyl trisulfide (DATS) (13, Figure 2), a naturally occurring organosulfur chemical found in garlic, effectively reduced breast CSCs by preventing the activation of the Wnt/β-catenin pathway [75]. Calcitriol (14, Figure 2) reduces the number of ovarian CSCs by blocking their Wnt signaling pathway, which hinders the growth of xenograft tumors [76]. Rimonabant (15, Figure 2) can effectively decrease the growth of both tumor-differentiated cells and colon CSCs by regulating Wnt activity. It also has the ability to manage their survival throughout extended periods of cultivation. 15 exhibits non-toxicity against healthy colonic epithelial cells, indicating its potential specificity towards cancer cells [77].
2.3. Small-Molecule Compounds Targeting Hedgehog Signaling
The hedgehog signaling pathway plays a crucial role in embryonic development, tissue regeneration, maintenance of adult tissue balance, and the formation of tumors [142,143,144,145]. Hedgehog family ligands, Desert hedgehog (Dhh), Indian hedgehog (Ihh), and Sonic hedgehog (Shh), can generate mature peptides after autoprocessing and lipid modification [146,147,148]. Tumor initiation and progression can occur as a result of abnormal activation of the hedgehog signaling pathway [145,149]. The hedgehog signaling pathway induces the expression of JAG2, a ligand of the Notch signaling pathway, during the development of cancer [150]. Hedgehog signaling pathway facilitates cellular evasion of apoptosis, disrupts cellular energy metabolism, modulates the process of EMT, aids in evading the immune system, sustains CSCs, and promotes metastasis.
The connection between Notch and hedgehog triggers the creation of Hes3 and Shh by activating several signaling pathways, such as Akt, STAT3, and mTOR. These pathways play a crucial role in promoting the survival of neural stem cells [151]. The presence of abnormal hedgehog signaling in human breast cancer can affect the capacity of BCSC to self-renew and differentiate [152].
Emerging data from laboratory experiments conducted in test tubes and living organisms indicates that the improper reactivation of the Shh signaling pathway controls genes that stimulate the growth of different types of human CSCs. NVP-LDE-225 (16, Figure 2) suppresses epithelial–mesenchymal transition (EMT) and development of human prostate CSCs in mice by blocking the Shh signaling system [78]. Curcumin (9) has been identified as a promising therapeutic drug and demonstrates cellular-level actions that contribute to its wide range of health benefits [153]. Multiple comprehensive studies have conclusively shown that it has the capacity to augment the therapeutic efficacy of diverse bioactive substances. The documented therapeutic benefits of this substance are numerous, mainly due to its antioxidant and anti-inflammatory qualities. However, its effectiveness is limited by low bioavailability caused by insufficient absorption, quick metabolism, and excretion. 9 suppressed the functions of bladder CSCs by blocking the SHH pathway. This suggests that 9 could be a promising chemopreventive therapy for treating bladder cancer [72]. Cyclopamine (17, Figure 2) induced a significant decrease in the growth of adherent glioma lines that predominantly express Gli1, a crucial target of the hedgehog pathway. However, this reduction was not observed in glioma lines that showed no signs of pathway activity. 17 effectively decreased or eradicated the population of stem-like cells in gliomas [79]. Vismodegib (GDC-0499) (18, Figure 2) suppressed the growth of pancreatic CSCs and triggered programmed cell death. The molecular mechanism of 18 involves inhibiting the actions of Smoothened in the SHH signaling pathway. Hence, 18 can be utilized for the treatment of pancreatic cancer by specifically targeting pancreatic CSCs [80].
2.4. Small-Molecule Compounds Targeting the NF-κB Signaling Pathway
The NF-κB transcription factors control the expression of several crucial genes involved in cell proliferation and survival, as well as innate and adaptive immunity. NF-κB played a role in promoting tumor inflammation [154] and the angiogenesis of tumors [155], which is triggered in a diverse range of cancers [154,156,157]. The NF-κB family comprises RelA (p65), RelB, c-Rel, p105/p50, and p100/p52. These molecules can form hetero- or homodimers and are a collection of evolutionarily conserved molecules. The most prevalent type, known as the canonical route, is the p65/p50 heterodimer [156,158]. NF-κB is triggered by various stimuli, including pro-inflammatory cytokines, interleukin-1β (IL-1β), bacteria and lipopolysaccharides (LPS), EGF, T- and B-cell mitogens, viral proteins, physical and chemical stresses, and double-stranded RNA [157,159]. NF-κB is also activated by cellular stresses, such as chemotherapeutic drugs and ionizing radiation [160]. The activation of NF-κB was found to be necessary in the neoplastic transformation induced by arsenite, which exhibits EMT and CSC-like characteristics in human keratinocytes [161], human ovarian CSC metastatic property [162], and human cervical CSC growth [82], while preventing differentiated glioma CSCs from developing a fully-fledged postmitotic phenotype [163]. In addition, studies also indicate that NF-κB regulates the self-renewal of breast CSCs in a model of HER2-dependent tumorigenesis [164].
Caffeic acid phenethyl ester (CAPE) (19, Figure 2), specifically caffeic acid phenethyl ester, hinders the proliferation of MDA-MB-231 (MDA-231) cells, suppresses the expression of the mdr gene, and inhibits NF-κB, EGFR, and VEGF. The administration of CAPE resulted in a dose-dependent suppression of self-renewal in breast CSCs and the generation of progenitor cells. The administration of CAPE results in considerable alterations in the features of breast CSCs. These changes include the suppression of self-renewal, the generation of progenitor cells, and the growth of clones in soft agar. Additionally, there is a notable decrease in the content of CD44, which is indicative of a reduced potential for malignancy [81]. Morusin (20, Figure 3) is a compound found in mulberry, specifically in the branch and root bark of several Morus species. It is a prenylated flavone and has a wide range of pharmacological effects. Nevertheless, there is a dearth of comprehensive research regarding its absorption and disposal [165]. 20 exhibits anti-cancer properties via reducing the activity of NF-κB, a protein that is increased in CSCs. 20 possesses the capacity to selectively target and eliminate CSCs and can hinder the growth and movement of human cervical cells by reducing the activity of NF-κB, hence inducing apoptosis [82]. The combination of disulfiram (21, Figure 3) and copper suppressed the growth of breast CSCs and increased the effectiveness of paclitaxel in breast cancer cell lines. This effect is likely due to the simultaneous activation of reactive oxygen species (ROS) and inhibition of NF-κB [83]. Eugenol (22, Figure 3) enhances the inhibitory effect of cisplatin on the NF-κB signaling pathway. Comparable outcomes were noted regarding the increase in cell division, suppression of the transformation from epithelial to mesenchymal cells, and indicators of cellular pluripotency in tumor xenografts. The findings offer compelling preclinical evidence for the combination of cisplatin and 22 as a therapeutic strategy for TNBC. This method specifically targets the resistant ALDH-positive cells and inhibits the NF-κB pathway [84].
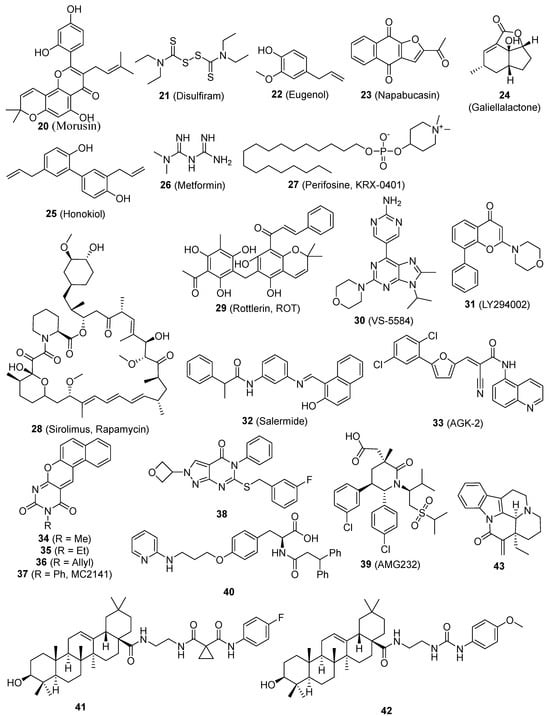
Figure 3.
Chemical structures of targeted small-molecule compounds of cancer stem cells.
2.5. Small-Molecule Compounds Targeting STAT3 Signaling Pathway
In both normal and cancer cells, the transcription factor STAT3 plays an essential role in a number of signal transduction pathways [166]. STAT3 is commonly activated across diverse cellular contexts [167]. STAT3 has a crucial role in governing both regular SCs and CSCs. P63, a constituent of the p53 protein family, exhibits both physical and functional associations with STAT3 and participates in its activities. A recent review thoroughly examined the functions of STAT3 in cancer and CSCs [168]. STAT3 is excessively active in numerous cancer types and has demonstrated to be a significant route in the propagation of cancer mediated by CSCs. Napabucasin (BBI608) (23, Figure 3) was taken in, processed into M1 as the only significant metabolite present in the bloodstream, and mainly eliminated through the feces. The administration of a single 240-mg dose orally was typically well tolerated [169]. 23 is a novel cancer stemness inhibitor now undergoing Phase III clinical trials. Clinical trials of 23 have demonstrated promising anti-tumor effects both as a standalone treatment and when used in combination with traditional therapies. Furthermore, 23 does not exhibit any notable pharmacokinetic interactions when used in combination therapy. 23 has the ability to effectively target CSCs, hence suppressing the spread of cancer metastasis and preventing the recurrence of the disease in patients with different forms of cancer [85,86,87]. 23 inhibits the activity of stem cells in cancer cells by specifically targeting STAT3 [8]. Curcumin (9) and EGCG (1) specifically blocked the process of STAT3 phosphorylation and maintained the link between STAT3 and NF-kB. They act as antitumor medicines that inhibit the growth of breast CSCs [58]. ALDH expression serves as an indicator for the presence of CSC-like cells in many human prostate cancer cell lines. Furthermore, ALDH+ cells demonstrate the presence of phosphorylated STAT3. Treatment with Galiellalactone (24, Figure 3) decreases the percentage of prostate cancer cells that express ALDH and triggers apoptosis in these ALDH-positive cells. The results emphasize the potential of targeting the STAT3 pathway in prostate cancer cells, particularly prostate CSC-like cells, as a feasible therapeutic approach. Additionally, 24 shows promise as a molecule for future development of drugs for prostate cancer [88]. Honokiol (HNK) (25, Figure 3) suppressed the movement of individual cells and eliminated the characteristics of stem cells in breast cancer cells. Mechanistic investigations demonstrated that HNK hindered the phosphorylation/activation of STAT3 in a manner that relied on LKB1, hence blocking its recruitment to the typical binding sites of the promoters, which include Nanog, Oct4, and Sox2. The molecular investigations of xenografts treated with HNK confirmed the mechanistic findings observed in vitro [89]. Metformin (26, Figure 3), the primary medication used to treat diabetes, hinders the process of cell transformation and specifically targets and eliminates CSCs in breast cancer cell lines. 26 selectively hinders the movement of NF-κB into the nucleus and the process of STAT3 phosphorylation in CSCs when compared to non-stem cancer cells within the same group. Metformin has the potential to impede metabolic stress and activate the inflammatory pathways linked to several types of cancer [90].
2.6. Small-Molecule Compounds Targeting PI-3K/Akt/mTOR Signaling Pathway
The phosphatidylinositol-3-kinase (PI-3K)/Akt/mTOR pathway plays a pivotal role in regulating cell proliferation, growth, motility, angiogenesis, and survival in tumor cells [170,171]. mTOR is a serine/threonine kinase that frequently acts as a downstream mediator of PI-3K/Akt signaling in tumor cells. Activation of PI-3K/Akt is highly associated with downstream targets, namely mTOR-mediated 4BEP-1 and S6K1 [172]. The MAPK pathway also controls mTOR in the activation of pro-angiogenic/inflammatory chemicals in tumor cells [173]. It is also possible for mTOR to phosphorylate Akt [174]. The abnormal activation of the mTOR pathway is believed to have significant implications for the proliferation of cancer cells and their resistance to anti-cancer drugs across various forms of cancer [175,176,177]. The PI-3K/Akt/mTOR pathway plays a crucial role in the process of EMT during the development of cancer [178]. The occurrence of several changes in cancer is mostly due to heightened activation of the PI3-K/Akt/mTOR pathway [171,179].
Multiple data suggest that the PI-3K/Akt/mTOR signaling pathway is significant in the biology of CSCs. Furthermore, CSCs exhibit greater sensitivity to blockage of this system using small compounds compared to regular SCs [180]. The CD133/PI-3K/Akt signaling axis was discovered to play a crucial role in the behavior of glioma CSCs [181]. Perifosine (KRX-0401) (27, Figure 3), an Akt inhibitor, was reported to reduce serial mammosphere formation and may be a novel agent as putative therapy for breast CSCs [52]. Sirolimus (Rapamycin) (28, Figure 3), by mTOR inhibition, effectively reduces EMT and CSC-like properties in colorectal cancer cells [91]. Rottlerin (ROT) (29, Figure 3), a commonly utilized inhibitor of protein kinase C-delta (PKC-δ), has been found to induce autophagy in human pancreatic CSCs via inhibiting the PI-3K/Akt/mTOR pathway [92]. The deregulation of the PI-3K pathway in numerous types of cancer has firmly established PI-3K as a universally recognized target for treatment. 29 triggered autophagy, which subsequently led to death in human pancreatic CSCs. This outcome was accomplished by suppressing the PI3K/Akt/mTOR pathway and stimulating the caspase cascade. The delivery of 29 led to a decrease in cell viability, which was influenced by the dosage and time of exposure, as well as the development of cytoplasmic vacuoles. 29 can initiate autophagy, a biological process that leads to the demise of pancreatic CSCs [92]. When comparing VS-5584 (30, Figure 3) to non-CSC in solid tumor cell populations, it is seen that 30 is considerably more potent, exhibiting a 30-fold enhancement in its capacity to inhibit the proliferation and survival of CSCs. 30 selectively decreases the numbers of CSC in several mice xenograft models of human cancer. Similarly, the ex vivo therapy with 30 specifically decreased the population of CSC in tumors that were surgically removed from patients with breast and ovarian cancer. Research on the mechanisms involved has demonstrated that effectively targeting CSC necessitates the suppression of many elements within the PI-3K-mTOR pathway. Merely silencing a single PI3K isoform or mTOR is insufficient to reproduce the effects of 30 [93]. LY294002 (31, Figure 3), which inhibits the PI-3K enzyme, decreases the growth, creation of spherical structures, and ability of colon CSCs to renew themselves. Additionally, it inhibits the activation of Akt via phosphorylation and the synthesis of cyclin D1 in colon CSCs. 31 effectively suppressed PI-3K activity in vivo, resulting in decreased tumor formation, enhanced identification of cleaved caspase 3, and elevated production of the inflammatory chemokine CXCL8 [94].
2.7. Small-Molecule Compounds Targeting Sirtuin Signaling Pathway
Sirtuins are crucial in regulating multiple metabolic processes and are classified as members of the histone deacetylase family III [182,183]. There are seven members called sirtuins (SIRT1-7) [184]. Individual sirtuin members may have different subcellular locations, different expression patterns, and distinct substrates [185]. Sirtuins regulate nonhistone proteins through lysine deacetylation [185]. Recent research has shown that sirtuins are the master regulators of a wide variety of cellular processes, including apoptosis, the cell cycle, extracellular matrix, differentiation, gene expression, DNA repair, proliferation, telomere activity, metabolism, and senescence [186,187]. Several cellular functions mentioned above play crucial roles in maintaining and regulating both normal SCs and CSCs. The recent proposal of inhibiting sirtuins has shown promise as an effective technique for combating cancer. Chemical modifications applied to sirtinol resulted in a collection of SIRT1/2 inhibitors, some of which are more effective than sirtinol itself. The primary objective of these inhibitors is to specifically target SIRT1. Salermide (32, Figure 3) had notable effectiveness against colorectal carcinoma CSCs during the testing process, but the SIRT2-selective inhibitor AGK-2 (33, Figure 3) had the most powerful effect on glioblastoma multiforme CSCs [95]. Benzodeazaoxaflavins (34, 35, and 36, Figure 3) are inhibitors of SIRT1/2. They have been found to have pro-apoptotic properties in human U937 leukemia cells. Additionally, when combined with the SIRT1-selective MC2141 (37, Figure 3), they have shown antiproliferative effects in CSCs from patients with colorectal carcinoma and glioblastoma multiforme. These CSCs are known to be highly tumorigenic, resistant to conventional cancer chemotherapy, and are responsible, at least in part, for cancer relapse or recurrence [96].
2.8. Small-Molecule Compounds Targeting ALDH Signaling Pathway
Aldehyde dehydrogenase 1 (ALDH1) is one of the most extensively studied markers used to select human CSCs. ALDH1 is composed of detoxifying enzymes that catalyze the oxidation of (retina)aldehydes to retinoids [188]. The human mammary cells that were chosen for their higher ALDH1 activity had the most extensive ability to differentiate into various lineages and had the best capability for development in a xenograft model. This suggests that the population of ALDH1 positive cells is enriched with mammary stem cells. Moreover, it was demonstrated that the ALDH1 positive population exhibited greater tumorigenic ability across successive passages, as opposed to the ALDH1 negative population [189]. The precise role of ALDH1 in (mammary) stem cells is not well understood; however, it is believed to be involved in cellular differentiation, primarily through the retinoid signaling pathway [190]. Flow cytometry with fluorescent substrates for ALDH1 has been the established method for assessing ALDH1 activity in live cells, considered the “gold standard” [189,191,192].
The specific isoform of ALDH1A that is responsible for the enzymatic activity is still a subject of debate; nonetheless, ALDH1A1 is often regarded as having the most significant impact. Consequently, extensive research has been conducted on the correlation between the expression of ALDH1A1 protein and clinicopathologic markers. In the majority of tumor types, including colorectal carcinoma [193], clear cell renal cell carcinoma [194], esophageal squamous cell carcinoma [195], gastric cancer [196], urothelial carcinomas of the urinary bladder [197], and squamous cell carcinoma of the head and neck [198], increased ALDH1A1 protein expression was correlated with tumor metastasis and an unfavorable prognosis. The prognostic significance of ALDH1A1 for breast cancer continues to be a subject of controversy, notwithstanding the existence of numerous independent investigations [189,199,200]. A recent meta-analysis identified ALDH1A1 as a biomarker for predicting tumor growth and poor survival in breast cancer patients [201]. Given the existence of high-quality commercial antibodies targeting ALDH1A1, it is crucial to utilize immunohistochemistry to analyze ALDH1A1 expression.
ALDH participates in aldehyde detoxification and retinoic acid synthesis, which contribute to tissue and cellular homeostasis [202,203]. ALDH performs its function in cell differentiation using retinoic acid. ALDH is present in progenitor cells, SCs, and CSCs, making it useful for isolating cell populations with CSC traits from various tumor types [204]. Specific inhibitors demonstrate a decrease in ALDH activity, resulting in reduced cell proliferation, invasion, drug sensitivity, and loss of stem cell characteristics [205,206]. Therefore, ALDH is a promising therapeutic target for treating CSCs [205]. Compound 38 (Figure 3) was discovered to be a unique inhibitor of ALDH1A, demonstrating selectivity over the similar ALDH2 class. The injection of 38 resulted in the depletion of the CD133+ putative CSC population. Additionally, it had a synergistic effect with cisplatin and obtained effective quantities when IP in vivo [97]. All-trans retinoic acid suppresses the stemness facilitated by ALDH-1 and hinders the development and expansion of ovarian tumors [207] and gastric carcinoma [208].
2.9. Small-Molecule Compounds Targeting MDM2
Murine double minute 2 (MDM2) plays a crucial role in suppressing the function of p53 by facilitating its ubiquitination and subsequent destruction [209,210,211]. p53 additionally enhances the expression of MDM2, leading to a feedback loop of negative self-regulation [212]. Recent research indicates that p53 plays a role in both non-malignant stem cells and CSCs, and it also has an impact on the microenvironment and CSC niche [213,214]. Consequently, therapeutic approaches have been devised to specifically target CSCs by restoring the normal activity of wild-type p53 (wtp53). These tactics involve the use of RING finger E3 ligases and CSC maintenance. Most inhibitors that target MDM2 are created and formulated to bind competitively to the deep hydrophobic p53-binding cleft of MDM2. This binding prevents the connection between MDM2 and p53, resulting in the activation of p53-mediated cell death and tumor suppression [215,216]. MDM2 overexpression can hinder the functionality of the p53 protein, which is commonly referred to as the “genome guardian.” Glioblastoma stem cells exhibit a high vulnerability to AMG232 (39, Figure 3). Out of all the stem cells, the ones with MDM2 gene amplification, specifically the 464T stem cells, were the most responsive to 39. The concentration of 39 required to inhibit 50% of the growth of these cells, known as the IC50, was 5.3 nM. 39 efficiently suppresses the activity of Nestin and ZEB1, which are factors associated with stemness [98]. A recent discovery revealed that MDM2-selective inhibitors stimulate significant expression of MDM4, its homologue. Compound 40 (Figure 3) exhibited a low nanomolar IC50 for both MDM2 and MDM4 targets. Significantly, 40 had the ability to suppress cell proliferation in a manner that depended on the concentration. It achieved an IC50 value of 356 ± 21 nM in neuroblastoma SHSY5Y cells, indicating its potency. Furthermore, it effectively hindered the growth of CSC [99].
2.10. Small-Molecule Compounds Targeting ROS Signaling
CSCs have reduced levels of intracellular ROS compared to non-CSCs [217,218]. Furthermore, the uncontrolled accumulation of ROS plays a role in the transformation of healthy hematopoietic stem cells into leukemic cells [219,220]. The presence of reduced levels of basal and radiation-induced ROS was seen in CD44+/CD24- breast CSCs, and this was found to be associated with their tumorigenic properties [217]. CD13, also known as Aminopeptidase N, is an enzyme that plays a role in the metabolic route of ROS [221,222], and was essential in the continued existence of CSCs [223]. Dong et al. detected a metabolic shift to glucose metabolism in basal-like breast cancer, accompanied by reduced levels of ROS [224]. They noted that the absence of fructose-1,6-biphosphatase (FBP1) triggers glycolysis, enhances glucose absorption, promotes the synthesis of tetrameric PKM2 macromolecules, and sustains ATP generation under low oxygen conditions. Disruption of FBP1 function also hinders the generation of ROS and the consumption of oxygen. This alteration in metabolism results in an enhanced CSC-like characteristic by enhancing the interaction between T-cell factor and β-catenin.
An imbalance in levels of ROS can result in the proliferation of cells with aberrant growth and the propensity to develop tumors. Hence, maintaining an equilibrium in the quantity of ROS is crucial for both the generation and breakdown processes, which play a significant role in tumor growth and the self-renewal of CSCs. The Glutathione peroxidases (GPx) family is a component of the cellular defense system against ROS and may have a role in regulating cellular oxidative stress and responses mediated by redox signaling. Certain members of the GPx family may also be involved in the self-renewal of CSCs [225,226,227]. Compounds 41 and 42 (Figure 3), two oleanolic acid derivatives, showed inhibition of different types of CSCs. After treatment with both compounds, ROS levels will increase significantly in cancer cells, which may eliminate CSCs. 41 and 42 show potential as anti-CSC drugs and can be further explored as a novel category of chemotherapeutic agents [100]. (-)-15-Methylene-eburnamonine (43, Figure 3) eradicates leukemia stem cells. The medicines also reduce the frequency of primary leukemia stem cells in vitro. The cytotoxic effects seem to be caused by the oxidative stress pathways [101].
2.11. Small-Molecule Compounds Targeting Other Signaling Pathway
The DNA methyltransferases (DNMTs) play a role in regulating gene expression through epigenetic mechanisms and are significant targets for cancer treatment. Compound 44 (Figure 4), the initial non-nucleoside DNMTi examined in a CSC line, suppressed cell proliferation in mouse medulloblastoma stem cells [102]. Triamino Glycosylated antitumor ether lipid (Triamino GAEL) (45, Figure 4) has more potency than salinomycin (56, Figure 4) against BT474 CSCs. Comprehending the amalgamation of elements that are vital for the increased ability of GAELs to kill cells is significant in order to explore new possibilities for creating powerful substances that can target drug-resistant cancer cells and CSCs [103]. Histone deacetylase (HDAC) inhibitors have been suggested as a potential treatment for counteracting CSCs in solid tumors. When examined in human osteosarcoma, rhabdomyosarcoma, and Ewing’s sarcoma stem cells. MC1742 and MC2625 (46 and 47, Figure 4), two HDAC inhibitors (HDACi), increased the levels of acetyl-H3 and acetyl-tubulin and inhibited the proliferation of CSC via inducing death. MC1742 induced osteogenic differentiation at non-toxic dosages [104]. Human tissue transglutaminase (hTG2) is a versatile enzyme with multiple functions. Several clinical disorders, such as CSC survival and metastatic characteristics, are linked to the excessive expression and dysregulation of hTG2 activities. The inhibitor VA4 (48, Figure 3), which has been optimized, effectively inhibits the invasion of epidermal CSCs with an EC50 value of 3.9 μM [105]. Compound 49 (Figure 4), the most powerful ATX inhibitor, effectively decreased the in vitro chemotherapeutic resistance of 4T1 breast cancer stem-like cells to paclitaxel and greatly reduced B16 melanoma metastasis in vivo [106]. The induction of apoptosis by MND (50, Figure 4) was as strong as salinomycin, and it effectively prevented migration, invasion, and the formation of cancer stem cell populations. Furthermore, MND displayed successful regression of tumors in mouse MCF-7 xenografts when administered orally. Investigations into the mechanisms showed that MND significantly inhibited the EGF-induced growth, movement, and tyrosine kinase (TK) signaling in breast cancer cells. Pertussis toxin has the ability to nullify the biological effects of MND [107]. Blocking the activity of lysine specific demethylase 1 (LSD1) has been demonstrated to trigger the maturation of leukemia stem cells in acute myeloid leukemia (AML). Compound 51 (Figure 4) has significant efficacy, as indicated by a Kd value of 32 nM and an EC50 value of 0.67 μM in a surrogate cellular biomarker experiment [108]. The DYRK family comprises kinases that are overexpressed in malignancy and regulate multiple cancer hallmarks. The most powerful suppressant, 52 (Figure 4) (with an IC50 of 50 nM or less), greatly reduced the viability, clonogenic survival, migration, and invasion of glioblastoma cells with stem cell-like characteristics. Target engagement was verified with genetic knockdown and cellular thermal shift tests. The thermal stability of DYRK1A in cells is enhanced following compound treatment, hence validating its binding in cells [109]. Resveratrol hinders the features of pancreatic CSC in both humans and KrasG12D transgenic mice by blocking the activity of proteins that maintain pluripotency and EMT [68]. Resveratrol hinders the features of pancreatic CSC in both humans and KrasG12D transgenic mice by blocking pluripotency maintenance factors and EMT. A separate investigation on cancer stem-like cells (CD24−/CD44+/ESA+) showed that resveratrol controls FAS expression, hinders lipogenesis, and triggers death in these cells. This represents a new and unique anti-tumor action of resveratrol [69]. Cabozantinib (53, Figure 4), a novel therapeutic candidate now undergoing phase II clinical trials, exerts its effects by targeting the surface marker of pancreatic CSC and the tyrosine kinase receptor c-Met. 53 caused a decrease in cancer stem cell markers and the pluripotency transcription factor SOX2, as well as triggered apoptosis in subclones that were treated with gemcitabine for a long period of time [110]. δ-Tocotrienol (VEDT) (54, Figure 4), a naturally occurring variant of vitamin E, specifically suppresses the viability, survival, self-renewal, and expression of Oct4 and Sox2 transcription factors in pancreatic ductal adenocarcinoma (PDAC) stem-like cells. VEDT effectively inhibited the proliferation and spread of gemcitabine-resistant PDAC stem-like cells in a model where these cells were transplanted into the appropriate anatomical location [111]. Doxycycline (55, Figure 4) has the ability to hinder the survival and reproduction of breast CSCs. Following the administration of 55, the levels of stem cell factors Oct4, Sox2, Nanog, and CD44 were notably reduced, indicating its potential effectiveness as a therapeutic agent against cancer [112]. Salinomycin (56, Figure 4) significantly decreases the percentage of CSCs by more than 100 times compared to paclitaxel. Administering 56 to mice suppresses the growth of mammary tumors in vivo and promotes enhanced epithelial differentiation of tumor cells. Furthermore, the administration of 56 leads to the suppression of breast CSC gene expression [113,114]. Ironomycin (AM5) (57, Figure 4), a synthetic derivative of salinomycin (56), has enhanced efficacy and specificity against breast CSCs both in laboratory settings and in living organisms. This is achieved by effectively collecting and isolating iron within lysosomes. These findings demonstrate the widespread occurrence of iron balance in breast CSCs, indicating that iron and iron-related processes could be prospective targets for combating these cells [115]. Panaxynol (58, Figure 4), a naturally occurring inhibitor of Hsp90, effectively suppressed the capacity of non-small cell lung cancer (NSCLC) CSCs to form spheres at extremely low doses in the nanomolar range. 58 exerted its effects on both the N-terminal and C-terminal regions of Hsp90 through a specific mechanism, while causing little deleterious effects [116]. The coadministration of a thioxodihydroquinazolinone derivative (59, Figure 4) and cisplatin exhibits the ability to eradicate cancer stem cell-like populations in ovarian cancer. Continued advancement of thioxodihydroquinazolinone compounds has the potential to result in a more potent therapy for metastatic ovarian cancer that is resistant to cisplatin [117]. WYC-209 (6, Figure 4), a man-made retinoid, effectively prevents the growth of malignant murine melanoma tumor-repopulating cells (TRCs, cells similar to CSCs) by 50% at a concentration of 0.19 µM, and this effect is directly proportional to the dosage. WYC-209 predominantly triggers apoptosis of TRCs through the caspase 3 pathway [118].
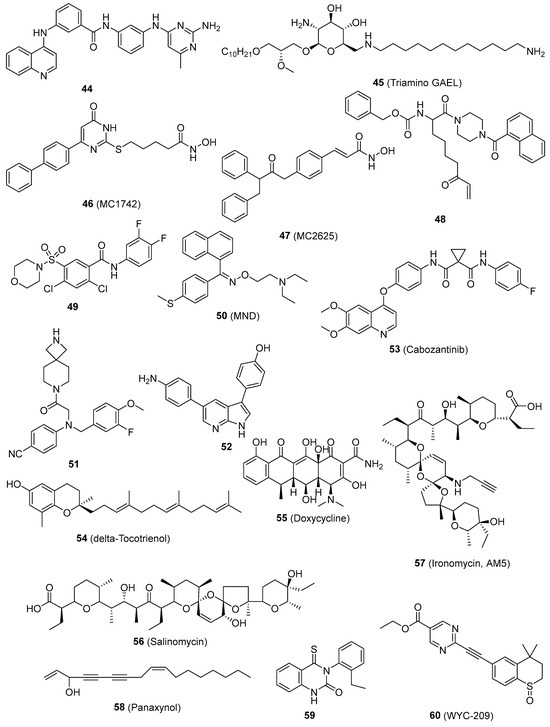
Figure 4.
Chemical structures of targeted small-molecule compounds of cancer stem cells.
3. Conclusions and Future Prospects
A detailed overview of the small-molecular inhibitors that selectively target several pathways directly linked to the regeneration and stemness of CSCs has been provided. The pathways include Notch, hedgehog, Wnt/β-catenin, NF-κB, STAT3, PI-3K/Akt/mTOR, sirtuin, ALDH, MDM2, ROS, and others. The hypothesis suggests that the occurrence of cancer is attributed to the disturbance of self-renewal mechanisms in stem cells. This suggests that the components of these pathways could be potential targets for therapeutic development. Recent studies indicate that it may be imperative to focus on CSC subpopulations in order to eradicate the whole cancer cell population. Small-molecule compounds are largely acquired for small-molecule discovery through high-throughput screening of compound libraries, natural products, medication repurposing, and structural optimization based on structure-activity connections. From a mechanistic standpoint, a significant majority of small-molecule drugs are categorized as competitive inhibitors, which largely impede their function by reducing the interaction between regulators and substrates. Small-molecule drugs exhibit excellent selectivity, resulting in minimal detrimental effects on normal cells. These molecular chemicals present a different method for treating cancer and give a fresh opportunity for tackling drug-resistant cancers that do not react to conventional therapies. Additionally, it can be utilized in conjunction with other anti-neoplastic medications to augment effectiveness, surmount resistance, and mitigate bad events. The exploration and advancement of small-molecule medicines that specifically target CSCs are slowly becoming recognized as highly effective ways for combating tumors. While numerous small-molecule drugs have demonstrated promising inhibitory actions, there are pressing challenges that require immediate attention. Some small-molecule drugs still need to be improved in terms of their intracellular action because of the selectivity of cell absorption. Some studies have focused solely on investigating the inhibitory effects of small compounds. However, there is still a need to optimize their ADME qualities, such as lipophilicity and solubility. Furthermore, because to the extensive range of subtyping stages and distinct characteristics observed at various stages of cancer and CSCs, it is imperative to provide additional clarification and subdivision regarding the precise suitability of small-molecule compounds. It is crucial to create compound libraries of excellent quality that come from a wide range of sources. The use of Artificial Intelligence and Machine Learning technologies, specifically the DEREPLICATOR+ and DP4-AI tools based on mass spectrometry and NMR, enables quick identification and clarification of chemical structures from intricate materials. Through ongoing study, these revolutionary therapeutic methods are expected to be implemented in clinical settings, potentially resulting in significant advancements in cancer therapy. Ultimately, the ability of small molecules to be used as drugs can be continuously improved, and the characteristics of compounds can be thoroughly examined from several angles, such as pharmacodynamics, pharmacokinetics, and toxicity. As our comprehension of CSC deepens, the discovery of therapeutic drugs specifically targeting CSCs may result in significant advancements in cancer therapies.
To summarize, there has been significant research on CSCs in the field of cancer biology. There is a rising interest in using small-molecule compounds that specifically target CSCs as potential therapies to combat tumors. This article presents a thorough summary of recent progress in important small-molecule compounds and predicts the development of even more effective and specific compounds in the future, thereby providing new possibilities for cancer treatment.
Author Contributions
Conceptualization, S.G. and S.Z.; investigation, S.G. and S.Z.; writing—original draft preparation, S.G. and S.Z.; writing—review and editing, M.L. and G.W.; funding acquisition, M.L. and G.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Institute of Minority Health and Health Disparities (NIMHD), U54MD007595 (G. Wang), and NIH/NIGMS SC1 GM144021 (ML).
Conflicts of Interest
The authors do not have any financial affiliations with organizations or entities that have a financial stake in the subject matter or materials covered in the work. The authors have stated that there are no conflicting interests.
Abbreviations
| ALDH1 | Aldehyde dehydrogenase 1 |
| ATRA | All-trans retinoic acid |
| BCSCs | Breast CSCs |
| CAPE | Caffeic acid phenethyl ester |
| COX | Cyclo-oxygenase |
| CSCs | Cancer stem cells |
| DATS | Diallyl trisulfide |
| Dhh | Desert hedgehog |
| DLL1 | Delta-like 1 |
| DNMTs | DNA methyltransferases |
| EGCG | Epigallocatechin-3-gallate |
| EGF | Epidermal growth factor |
| EMT | Epithelial–mesenchymal transition |
| Epo | Erythropoietin |
| EpoR | Erythropoietin receptor |
| FAK | Focal adhesion kinase |
| FBP1 | Fructose-1,6-biphosphatase |
| GPx | Glutathione peroxidases |
| HDAC | Histone deacetylase |
| HDACi | HDAC inhibitors |
| HNK | Honokiol |
| HNSC | Head-neck squamous carcinoma |
| hTG2 | Human tissue transglutaminase |
| Ihh | Indian hedgehog |
| LPA | Lysophosphatidic acid |
| LPS | Lipopolysaccharides |
| LSD1 | Lysine-specific demethylase 1 |
| MDM2 | Murine double minute 2 |
| NSCLC | Non-small cell lung cancer |
| PDAC | Pancreatic ductal adenocarcinomas |
| PKC-δ | Protein kinase C-delta |
| PI3K | Phosphatidylinositol-3-kinase |
| ROS | Reactive oxygen species |
| ROT | Rottlerin |
| SCs | Stem cells |
| Shh | Sonic hedgehog |
| SFN | Sulforaphane |
| SLNs | Solid matrix of lipidic nanoparticles |
| TK | Tyrosine kinase |
| TNBC | Triple-negative breast cancer |
References
- Pang, L.Y.; Argyle, D.J. Using naturally occurring tumours in dogs and cats to study telomerase and cancer stem cell biology. Biochim. Biophys. Acta 2009, 1792, 380–391. [Google Scholar] [CrossRef]
- Kakarala, M.; Wicha, M.S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. 2008, 26, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F. Self-renewal and solid-tumor stem cells. Biol. Blood Marrow Transpl. 2005, 11 (Suppl. S2), 14–16. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Warrier, N.M.; Kelkar, N.; Johnson, C.T.; Govindarajan, T.; Prabhu, V.; Kumar, P. Understanding cancer stem cells and plasticity: Towards better therapeutics. Eur. J. Cell Biol. 2023, 102, 151321. [Google Scholar] [CrossRef] [PubMed]
- Nairuz, T.; Mahmud, Z.; Manik, R.K.; Kabir, Y. Cancer stem cells: An insight into the development of metastatic tumors and therapy resistance. Stem Cell Rev. Rep. 2023, 19, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Lucchetti, D.; Farina, M.; Corbi, M.; Cufino, V.; Cittadini, A.; Sgambato, A. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J. Gastroenterol. WJG 2014, 20, 923–942. [Google Scholar] [CrossRef]
- Chakravarti, B.; Akhtar Siddiqui, J.; Anthony Sinha, R.; Raza, S. Targeting autophagy and lipid metabolism in cancer stem cells. Biochem. Pharmacol. 2023, 212, 115550. [Google Scholar] [CrossRef] [PubMed]
- Botchkina, G.; Ojima, I. Prostate and Colon Cancer Stem Cells as a Target for Anti-Cancer Drug Development; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105. [Google Scholar] [CrossRef] [PubMed]
- Verstappe, J.; Berx, G. A role for partial epithelial-to-mesenchymal transition in enabling stemness in homeostasis and cancer. Semin. Cancer Biol. 2023, 90, 15–28. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Larsimont, J.-C.; Liagre, M.; Muñoz-Couselo, E.; Lapouge, G.; Brisebarre, A.; Dubois, C.; Suppa, M.; Sukumaran, V.; del Marmol, V.; et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 2018, 562, 434–438. [Google Scholar] [CrossRef]
- Eid, R.A.; Alaa Edeen, M.; Shedid, E.M.; Kamal, A.S.S.; Warda, M.M.; Mamdouh, F.; Khedr, S.A.; Soltan, M.A.; Jeon, H.W.; Zaki, M.S.A.; et al. Targeting Cancer Stem Cells as the Key Driver of Carcinogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2023, 24, 1786. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, N.D.; Weinberg, R.A.; Chaffer, C.L. Cell Plasticity and Heterogeneity in Cancer. Clin. Chem. 2013, 59, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.A.; Sahranavard, S.; Salehi, A.; Bagheri, V. Selectively targeting cancer stem cells: Current and novel therapeutic strategies and approaches in the effective eradication of cancer. IUBMB Life 2021, 73, 1045–1059. [Google Scholar] [CrossRef]
- Coart, E.; Saad, E.D.; Shi, Q.; Sommeijer, D.W.; Zalcberg, J.R.; Maughan, T.; Goldberg, R.M.; Schmoll, H.-J.; Punt, C.J.A.; Cutsem, E.V.; et al. Trial-level association between response-based endpoints (RBEs) and progression-free (PFS)/overall survival (OS) in first-line therapy for metastatic colorectal cancer (mCRC) in the ARCAD database. J. Clin. Oncol. 2015, 33 (Suppl. S3), 666. [Google Scholar] [CrossRef]
- Zabor, E.C.; Heller, G.; Schwartz, L.H.; Chapman, P.B. Correlating Surrogate Endpoints with Overall Survival at the Individual Patient Level in BRAFV600E-Mutated Metastatic Melanoma Patients Treated with Vemurafenib. Clin. Cancer Res. 2016, 22, 1341–1347. [Google Scholar] [CrossRef][Green Version]
- Miyoshi, N.; Haraguchi, N.; Mizushima, T.; Ishii, H.; Yamamoto, H.; Mori, M. Targeting cancer stem cells in refractory cancer. Regen. Ther. 2021, 17, 13–19. [Google Scholar] [CrossRef]
- Kim, M.; Bakyt, L.; Akhmetkaliyev, A.; Toktarkhanova, D.; Bulanin, D. Re-Sensitizing Cancer Stem Cells to Conventional Chemotherapy Agents. Int. J. Mol. Sci. 2023, 24, 2122. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Dekmezian, C.; Pajonk, F. Radiation-Induced Reprogramming of Breast Cancer Cells. Stem Cells 2012, 30, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Malfettone, A.; Soukupova, J. New Insights into the Crossroads between EMT and Stemness in the Context of Cancer. J. Clin. Med. 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Unternaehrer, J.J. Epithelial-mesenchymal Transition and Cancer Stem Cells: At the Crossroads of Differentiation and Dedifferentiation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2019, 248, 10–20. [Google Scholar] [CrossRef]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Bhowmik, A.; Biswas, S.; Sarkar, R.; Ghosh, R.; Pakhira, S.; Mondal, M.; Sen, S.; Saha, P.; Hajra, S. Therapeutic Effectiveness of Anticancer Agents Targeting Different Signaling Molecules Involved in Asymmetric Division of Cancer Stem Cell. Stem Cell Rev. Rep. 2023, 19, 1283–1306. [Google Scholar] [CrossRef] [PubMed]
- Zamfirescu, A.M.; Yatsenko, A.S.; Shcherbata, H.R. Notch signaling sculpts the stem cell niche. Front. Cell Dev. Biol. 2022, 10, 1027222. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, G.; Guo, S. Regulation of angiogenesis via Notch signaling in breast cancer and cancer stem cells. Biochim. Biophys. Acta 2013, 1836, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Kovall, R.A. More complicated than it looks: Assembly of Notch pathway transcription complexes. Oncogene 2008, 27, 5099–5109. [Google Scholar] [CrossRef] [PubMed]
- Danovi, S.A. Angiogenesis: Turning it down a Notch. Nature Reviews Cancer Nat. Rev. Cancer 2008, 8, 572–573. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Gonzalez-Perez, R.R. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim. Biophys. Acta 2011, 1815, 197–213. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Perdigoto, C.N.; Bardin, A.J. Sending the right signal: Notch and stem cells. Biochim. Biophys. Acta 2013, 1830, 2307–2322. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Notch signaling in stem cell systems. Stem Cells 2006, 24, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sullenger, B.A.; Rich, J.N. Notch signaling in cancer stem cells. Adv. Exp. Med. Biol. 2012, 727, 174–185. [Google Scholar]
- Pannuti, A.; Foreman, K.; Rizzo, P.; Osipo, C.; Golde, T.; Osborne, B.; Miele, L. Targeting Notch to target cancer stem cells. Clin. Cancer Res. 2010, 16, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Zhdanovskaya, N.; Firrincieli, M.; Lazzari, S.; Pace, E.; Scribani Rossi, P.; Felli, M.P.; Talora, C.; Screpanti, I.; Palermo, R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers 2021, 13, 5106. [Google Scholar] [CrossRef] [PubMed]
- Harrison, H.; Farnie, G.; Howell, S.J.; Rock, R.E.; Stylianou, S.; Brennan, K.R.; Bundred, N.J.; Clarke, R.B. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010, 70, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Jackson, K.W.; McNicholas, E.; Kawamura, M.J.; Abdallah, W.M.; Wicha, M.S. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004, 6, R605–R615. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Storci, G.; Giovannini, C.; Pandolfi, S.; Pianetti, S.; Taffurelli, M.; Santini, D.; Ceccarelli, C.; Chieco, P.; Bonafe, M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells 2007, 25, 807–815. [Google Scholar] [CrossRef]
- Yousefi, H.; Bahramy, A.; Zafari, N.; Delavar, M.R.; Nguyen, K.; Haghi, A.; Kandelouei, T.; Vittori, C.; Jazireian, P.; Maleki, S.; et al. Notch signaling pathway: A comprehensive prognostic and gene expression profile analysis in breast cancer. BMC Cancer 2022, 22, 1282. [Google Scholar] [CrossRef]
- Ling, H.; Sylvestre, J.R.; Jolicoeur, P. Notch1-induced mammary tumor development is cyclin D1-dependent and correlates with expansion of pre-malignant multipotent duct-limited progenitors. Oncogene 2010, 29, 4543–4554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Fischer, W.H.; Gill, G.N. Regulation of the ERBB-2 promoter by RBPJκ and NOTCH. J. Biol. Chem. 1997, 272, 14110–14114. [Google Scholar] [CrossRef] [PubMed]
- Politi, K.; Feirt, N.; Kitajewski, J. Notch in mammary gland development and breast cancer. Semin. Cancer Biol. 2004, 14, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.M.; Kim, K.; Vlashi, E.; McBride, W.H.; Pajonk, F. Effects of recombinant erythropoietin on breast cancer-initiating cells. Neoplasia 2007, 9, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis. Blood 2006, 107, 2223–2233. [Google Scholar] [CrossRef]
- Radtke, F.; Raj, K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat. Rev. Cancer 2003, 3, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Xin, X.; Liu, L.; Tutunea-Fatan, E.; Rodriguez-Torres, M.; Vincent, K.; Postovit, L.M.; Hess, D.; Lala, P.K. COX-2 Induces Breast Cancer Stem Cells via EP4/PI3K/AKT/NOTCH/WNT Axis. Stem Cells 2016, 34, 2290–2305. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Paulson, A.; Iovino, F.; Wicha, M.S. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008, 27, 6120–6130. [Google Scholar] [CrossRef]
- Korkaya, H.; Wicha, M.S. HER-2, notch, and breast cancer stem cells: Targeting an axis of evil. Clin. Cancer Res. 2009, 15, 1845–1847. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Tang, L.; Chen, Q.; Guan, N.; Xu, K.; Guan, X. MYC dysfunction modulates stemness and tumorigenesis in breast cancer. Int. J. Biol. Sci. 2021, 17, 178–187. [Google Scholar] [CrossRef]
- Korkaya, H.; Paulson, A.; Charafe-Jauffret, E.; Ginestier, C.; Brown, M.; Dutcher, J.; Clouthier, S.G.; Wicha, M.S. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009, 7, e1000121. [Google Scholar] [CrossRef]
- Zhou, J.; Wulfkuhle, J.; Zhang, H.; Gu, P.; Yang, Y.; Deng, J.; Margolick, J.B.; Liotta, L.A.; Petricoin, E., 3rd; Zhang, Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 2007, 104, 16158–16163. [Google Scholar] [CrossRef]
- Balamurugan, K.; Mendoza-Villanueva, D.; Sharan, S.; Summers, G.H.; Dobrolecki, L.E.; Lewis, M.T.; Sterneck, E. C/EBPδ links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene 2019, 38, 3765–3780. [Google Scholar] [CrossRef]
- Pratt, M.A.; Tibbo, E.; Robertson, S.J.; Jansson, D.; Hurst, K.; Perez-Iratxeta, C.; Lau, R.; Niu, M.Y. The canonical NF-κB pathway is required for formation of luminal mammary neoplasias and is activated in the mammary progenitor population. Oncogene 2009, 28, 2710–2722. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, H.J.; Kang, H.J.; Kwon, H.W.; Lim, Y.C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur. J. Cancer 2013, 49, 3210–3218. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Chung, S.S.; Vadgama, J.V. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Down-regulation of STAT3–NFκB Signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Fan, X.; Matsui, W.; Khaki, L.; Stearns, D.; Chun, J.; Li, Y.-M.; Eberhart, C.G. Notch Pathway Inhibition Depletes Stem-like Cells and Blocks Engraftment in Embryonal Brain Tumors. Cancer Res. 2006, 66, 7445–7452. [Google Scholar] [CrossRef]
- Shan, N.L.; Wahler, J.; Lee, H.J.; Bak, M.J.; Gupta, S.D.; Maehr, H.; Suh, N. Vitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2017, 173, 122–129. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chao, T.-K.; Chang, C.-C.; Yo, Y.-T.; Yu, M.-H.; Lai, H.-C. Drug Screening Identifies Niclosamide as an Inhibitor of Breast Cancer Stem-Like Cells. PLoS ONE 2013, 8, e74538. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Wu, A.T.H.; Chang, P.M.H.; Chen, K.-Y.; Yang, C.-N.; Yang, S.-C.; Ho, C.-C.; Chen, C.-C.; Kuo, Y.-L.; Lee, P.-Y.; et al. Trifluoperazine, an Antipsychotic Agent, Inhibits Cancer Stem Cell Growth and Overcomes Drug Resistance of Lung Cancer. Am. J. Respir. Crit. Care Med. 2012, 186, 1180–1188. [Google Scholar] [CrossRef]
- Lim, Y.C.; Kang, H.J.; Kim, Y.S.; Choi, E.C. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/β-catenin pathway. Eur. J. Cancer 2012, 48, 3310–3318. [Google Scholar] [CrossRef]
- Mei, D.; Lv, B.; Chen, B.; Xiao, S.; Jiang, J.; Xie, Y.; Jiang, L. All-trans retinoic acid suppresses malignant characteristics of CD133-positive thyroid cancer stem cells and induces apoptosis. PLoS ONE 2017, 12, e0182835. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Goswami, C.P.; Badve, S.; Sledge Jr, G.W.; Nakshatri, H. Identification of FDA-approved Drugs Targeting Breast Cancer Stem Cells Along With Biomarkers of Sensitivity. Sci. Rep. 2013, 3, 2530. [Google Scholar] [CrossRef]
- Quillard, T.; Devalliere, J.; Coupel, S.; Charreau, B. Inflammation dysregulates Notch signaling in endothelial cells: Implication of Notch2 and Notch4 to endothelial dysfunction. Biochem. Pharmacol. 2010, 80, 2032–2041. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol Inhibits Breast Cancer Stem-Like Cells and Induces Autophagy via Suppressing Wnt/β-Catenin Signaling Pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef]
- Shankar, S.; Nall, D.; Tang, S.-N.; Meeker, D.; Passarini, J.; Sharma, J.; Srivastava, R.K. Resveratrol Inhibits Pancreatic Cancer Stem Cell Characteristics in Human and KrasG12D Transgenic Mice by Inhibiting Pluripotency Maintaining Factors and Epithelial-Mesenchymal Transition. PLoS ONE 2011, 6, e16530. [Google Scholar] [CrossRef]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K.; Liu, W.; Kobayashi, A.; Xing, F.; Fukuda, K.; Hirota, S.; Sugai, T.; et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Yang, X.; Chen, Y.; Jiang, Y.; Wang, S.-J.; Li, Y.; Wang, X.-Q.; Meng, Y.; Zhu, M.-M.; Ma, X.; et al. Curcumin Suppresses Lung Cancer Stem Cells via Inhibiting Wnt/β-catenin and Sonic Hedgehog Pathways. Phytother. Res. 2017, 31, 680–688. [Google Scholar] [CrossRef]
- Wang, D.; Kong, X.; Li, Y.; Qian, W.; Ma, J.; Wang, D.; Yu, D.; Zhong, C. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem. Biophys. Res. Commun. 2017, 493, 521–527. [Google Scholar] [CrossRef]
- Kolev, V.N.; Tam, W.F.; Wright, Q.G.; McDermott, S.P.; Vidal, C.M.; Shapiro, I.M.; Xu, Q.; Wicha, M.S.; Pachter, J.A.; Weaver, D.T. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 2017, 8, 51733–51747. [Google Scholar] [CrossRef]
- Deng, S.; Wong, C.K.C.; Lai, H.-C.; Wong, A.S.T. Ginsenoside-Rb1 targets chemotherapy-resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. Oncotarget 2016, 8, 25897–25914. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y.; Xie, C.; Zhu, J.; Wang, X.; Li, Y.; Geng, S.; Wu, J.; Zhong, C.; Li, M. Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J. Cell. Biochem. 2018, 119, 4134–4141. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Rizvi, A.; Cui, T.; Han, C.; Banerjee, A.; Naseem, I.; Zheng, Y.; Wani, A.A.; Wang, Q.-E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget 2018, 9, 14481–14491. [Google Scholar] [CrossRef]
- Fiore, D.; Ramesh, P.; Proto, M.C.; Piscopo, C.; Franceschelli, S.; Anzelmo, S.; Medema, J.P.; Bifulco, M.; Gazzerro, P. Rimonabant Kills Colon Cancer Stem Cells without Inducing Toxicity in Normal Colon Organoids. Front. Pharmacol. 2018, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Nanta, R.; Kumar, D.; Meeker, D.; Rodova, M.; Van Veldhuizen, P.J.; Shankar, S.; Srivastava, R.K. NVP-LDE-225 (Erismodegib) inhibits epithelial–mesenchymal transition and human prostate cancer stem cell growth in NOD/SCID IL2Rγ null mice by regulating Bmi-1 and microRNA-128. Oncogenesis 2013, 2, e42. [Google Scholar] [CrossRef]
- Bar, E.E.; Chaudhry, A.; Lin, A.; Fan, X.; Schreck, K.; Matsui, W.; Piccirillo, S.; Vescovi, A.L.; DiMeco, F.; Olivi, A.; et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 2007, 25, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Fu, J.; Srivastava, R.K.; Shankar, S. Hedgehog Signaling Antagonist GDC-0449 (Vismodegib) Inhibits Pancreatic Cancer Stem Cell Characteristics: Molecular Mechanisms. PLoS ONE 2011, 6, e27306. [Google Scholar] [CrossRef]
- Omene, C.O.; Wu, J.; Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig. New Drugs 2012, 30, 1279–1288. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Yang, L.; Dong, L.; Lin, C.; Zhang, J.; Lin, P.; Wang, X. Morusin inhibits human cervical cancer stem cell growth and migration through attenuation of NF-kappaB activity and apoptosis induction. Mol. Cell. Biochem. 2013, 31, 31. [Google Scholar]
- Yip, N.C.; Fombon, I.S.; Liu, P.; Brown, S.; Kannappan, V.; Armesilla, A.L.; Xu, B.; Cassidy, J.; Darling, J.L.; Wang, W. Disulfiram modulated ROS–MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer 2011, 104, 1564. [Google Scholar] [CrossRef]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol potentiates cisplatin anti-cancer activity through inhibition of ALDH-positive breast cancer stem cells and the NF-κB signaling pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Grothey, A. Napabucasin: An Update on the First-in-Class Cancer Stemness Inhibitor. Drugs 2017, 77, 1091–1103. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Starodub, A.; El-Rayes, B.F.; Shahda, S.; O’Neil, B.H.; Noonan, A.M.; Shaib, W.L.; Hanna, W.T.; Mikhail, S.; Neki, A.S.; et al. Phase 1b/2 trial of cancer stemness inhibitor napabucasin (NAPA) + nab-paclitaxel (nPTX) and gemcitabine (Gem) in metastatic pancreatic adenocarcinoma (mPDAC). J. Clin. Oncol. 2018, 36 (Suppl. S15), 4110. [Google Scholar] [CrossRef]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y.; et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef]
- Hellsten, R.; Johansson, M.; Dahlman, A.; Sterner, O.; Bjartell, A. Galiellalactone Inhibits Stem Cell-Like ALDH-Positive Prostate Cancer Cells. PLoS ONE 2011, 6, e22118. [Google Scholar] [CrossRef]
- Sengupta, S.; Nagalingam, A.; Muniraj, N.; Bonner, M.Y.; Mistriotis, P.; Afthinos, A.; Kuppusamy, P.; Lanoue, D.; Cho, S.; Korangath, P.; et al. Activation of tumor suppressor LKB1 by honokiol abrogates cancer stem-like phenotype in breast cancer via inhibition of oncogenic Stat3. Oncogene 2017, 36, 5709. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2012, 110, 972–977. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Lu, J.; Zhang, P.; Wang, Y.; Xu, Y.; Wang, Z.; Mao, J.H.; Wei, G. Rapamycin inhibits FBXW7 loss-induced epithelial-mesenchymal transition and cancer stem cell-like characteristics in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013, 434, 352–356. [Google Scholar] [CrossRef]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef]
- Kolev, V.N.; Wright, Q.G.; Vidal, C.M.; Ring, J.E.; Shapiro, I.M.; Ricono, J.; Weaver, D.T.; Padval, M.V.; Pachter, J.A.; Xu, Q. PI3K/mTOR Dual Inhibitor VS-5584 Preferentially Targets Cancer Stem Cells. Cancer Res. 2015, 75, 446–455. [Google Scholar] [CrossRef]
- Chen, S.; Fisher, R.C.; Signs, S.; Molina, L.A.; Shenoy, A.K.; Lopez, M.-C.; Baker, H.V.; Koomen, J.M.; Chen, Y.; Gittleman, H.; et al. Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells reduces tumor growth due to apoptosis. Oncotarget 2016, 8, 50476–50488. [Google Scholar] [CrossRef]
- Rotili, D.; Tarantino, D.; Nebbioso, A.; Paolini, C.; Huidobro, C.; Lara, E.; Mellini, P.; Lenoci, A.; Pezzi, R.; Botta, G.; et al. Discovery of Salermide-Related Sirtuin Inhibitors: Binding Mode Studies and Antiproliferative Effects in Cancer Cells Including Cancer Stem Cells. J. Med. Chem. 2012, 55, 10937–10947. [Google Scholar] [CrossRef]
- Rotili, D.; Tarantino, D.; Carafa, V.; Paolini, C.; Schemies, J.; Jung, M.; Botta, G.; Di Maro, S.; Novellino, E.; Steinkühler, C.; et al. Benzodeazaoxaflavins as Sirtuin Inhibitors with Antiproliferative Properties in Cancer Stem Cells. J. Med. Chem. 2012, 55, 8193–8197. [Google Scholar] [CrossRef][Green Version]
- Huddle, B.C.; Grimley, E.; Buchman, C.D.; Chtcherbinine, M.; Debnath, B.; Mehta, P.; Yang, K.; Morgan, C.A.; Li, S.; Felton, J.; et al. Structure-Based Optimization of a Novel Class of Aldehyde Dehydrogenase 1A (ALDH1A) Subfamily-Selective Inhibitors as Potential Adjuncts to Ovarian Cancer Chemotherapy. J. Med. Chem. 2018, 61, 8754–8773. [Google Scholar] [CrossRef]
- Her, N.-G.; Oh, J.-W.; Oh, Y.J.; Han, S.; Cho, H.J.; Lee, Y.; Ryu, G.H.; Nam, D.-H. Potent effect of the MDM2 inhibitor AMG232 on suppression of glioblastoma stem cells. Cell Death Dis. 2018, 9, 792. [Google Scholar] [CrossRef]
- Giustiniano, M.; Daniele, S.; Pelliccia, S.; La Pietra, V.; Pietrobono, D.; Brancaccio, D.; Cosconati, S.; Messere, A.; Giuntini, S.; Cerofolini, L.; et al. Computer-Aided Identification and Lead Optimization of Dual Murine Double Minute 2 and 4 Binders: Structure–Activity Relationship Studies and Pharmacological Activity. J. Med. Chem. 2017, 60, 8115–8130. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Zhang, Z.; Wei, Y.; Xu, Z.; Qin, S.; Liu, N.; Zhao, R.; Peng, J.; Yang, G.; et al. Synthesis and Discovery Novel Anti-Cancer Stem Cells Compounds Derived from the Natural Triterpenoic Acids. J. Med. Chem. 2018, 61, 10814–10833. [Google Scholar] [CrossRef]
- Gunasekara, D.C.; Zheng, M.M.; Mojtahed, T.; Woods, J.R.; Fandy, T.E.; Riofski, M.V.; Glackin, C.A.; Hassan, H.E.; Kirshner, J.; Colby, D.A. 15-Methylene-Eburnamonine Kills Leukemic Stem Cells and Reduces Engraftment in a Humanized Bone Marrow Xenograft Mouse Model of Leukemia. ChemMedChem 2016, 11, 2392–2397. [Google Scholar] [CrossRef]
- Valente, S.; Liu, Y.; Schnekenburger, M.; Zwergel, C.; Cosconati, S.; Gros, C.; Tardugno, M.; Labella, D.; Florean, C.; Minden, S.; et al. Selective Non-nucleoside Inhibitors of Human DNA Methyltransferases Active in Cancer Including in Cancer Stem Cells. J. Med. Chem. 2014, 57, 701–713. [Google Scholar] [CrossRef]
- Idowu, T.; Samadder, P.; Arthur, G.; Schweizer, F. Amphiphilic Modulation of Glycosylated Antitumor Ether Lipids Results in a Potent Triamino Scaffold against Epithelial Cancer Cell Lines and BT474 Cancer Stem Cells. J. Med. Chem. 2017, 60, 9724–9738. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Salerno, M.; Rotili, D.; Valente, S.; Zwergel, C.; Avnet, S.; Lattanzi, G.; Baldini, N.; Mai, A. Novel Histone Deacetylase Inhibitors Induce Growth Arrest, Apoptosis, and Differentiation in Sarcoma Cancer Stem Cells. J. Med. Chem. 2015, 58, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; McNeil, N.M.R.; Albert, M.R.; Ta, V.; Adhikary, G.; Bourgeois, K.; Eckert, R.L.; Keillor, J.W. Structure–Activity Relationships of Potent, Targeted Covalent Inhibitors That Abolish Both the Transamidation and GTP Binding Activities of Human Tissue Transglutaminase. J. Med. Chem. 2017, 60, 7910–7927. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Norman, D.D.; Lee, S.C.; Parrill, A.L.; Pham, T.C.T.; Baker, D.L.; Tigyi, G.J.; Miller, D.D. Highly Potent Non-Carboxylic Acid Autotaxin Inhibitors Reduce Melanoma Metastasis and Chemotherapeutic Resistance of Breast Cancer Stem Cells. J. Med. Chem. 2017, 60, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Akhtar, T.; Rai, B.; Yadav, M.; Akhtar Siddiqui, J.; Dhar Dwivedi, S.K.; Thakur, R.; Singh, A.K.; Singh, A.K.; Kumar, H.; et al. Thioaryl Naphthylmethanone Oxime Ether Analogs as Novel Anticancer Agents. J. Med. Chem. 2014, 57, 8010–8025. [Google Scholar] [CrossRef] [PubMed]
- Mould, D.P.; Alli, C.; Bremberg, U.; Cartic, S.; Jordan, A.M.; Geitmann, M.; Maiques-Diaz, A.; McGonagle, A.E.; Somervaille, T.C.P.; Spencer, G.J.; et al. Development of (4-Cyanophenyl)glycine Derivatives as Reversible Inhibitors of Lysine Specific Demethylase 1. J. Med. Chem. 2017, 60, 7984–7999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Phoa, A.F.; Abbassi, R.H.; Hoque, M.; Reekie, T.A.; Font, J.S.; Ryan, R.M.; Stringer, B.W.; Day, B.W.; Johns, T.G.; et al. Structural Optimization and Pharmacological Evaluation of Inhibitors Targeting Dual-Specificity Tyrosine Phosphorylation-Regulated Kinases (DYRK) and CDC-like kinases (CLK) in Glioblastoma. J. Med. Chem. 2017, 60, 2052–2070. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Rausch, V.; Giese, N.; Giese, T.; Schönsiegel, F.; Labsch, S.; Nwaeburu, C.; Mattern, J.; Gladkich, J.; Herr, I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013, 4, e627. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M.P. δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Zhang, F.; Vlashi, E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 2017, 16, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumor Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Hamaï, A.; Hienzsch, A.; Cañeque, T.; Müller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Nguyen, H.T.; Min, H.-Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Le, T.H.V.; Lee, J.; Park, J.H.; Lee, H.-Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Salamoun, J.; Wipf, P.; Edwards, R.; Van Houten, B.; Qian, W. Combination of a thioxodihydroquinazolinone with cisplatin eliminates ovarian cancer stem cell-like cells (CSC-LCs) and shows preclinical potential. Oncotarget 2017, 9, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, X.; An, Q.; Zhang, Y.; Li, K.; Yao, W.; Shi, F.; Pan, Y.; Jia, Q.; Zhou, W.; et al. Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat. Commun. 2018, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Lin, C.K.; Bai, M.Y.; Hu, T.M.; Wang, Y.C.; Chao, T.K.; Weng, S.J.; Huang, R.L.; Su, P.H.; Lai, H.C. Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer. Oncotarget 2016, 7, 8993–9006. [Google Scholar] [CrossRef]
- Kuhl, S.J.; Kuhl, M. On the role of Wnt/beta-catenin signaling in stem cells. Biochim. Biophys. Acta 2012, 16, 16. [Google Scholar]
- Curtin, J.C.; Lorenzi, M.V. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget 2010, 1, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, N.; Hu, X. Wnt/β-catenin signaling inhibitors. Curr. Top. Med. Chem. 2023, 23, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, S.; Fukuda, K. Recent advances in cardiovascular regenerative medicine: The induced pluripotent stem cell era. Expert Rev. Cardiovasc. Ther. 2008, 6, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Takahashi, M. Neural induction and patterning in Mammalian pluripotent stem cells. CNS Neurol. Disord. Drug Targets 2011, 10, 419–432. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wu, Y.; Chen, J.; Wu, H.; Wei, W.; Yan, S. Human umbilical cord mesenchymal stem cells alleviate rat knee osteoarthritis via activating Wnt/β-catenin signaling pathway. Curr. Stem Cell Res. Ther. 2023, 19, 234–244. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, J.R.; Goss, K.H. A Wnt-ow of Opportunity: Targeting the Wnt/β-Catenin Pathway in Breast Cancer. Curr. Drug Targets 2010, 11, 1074–1088. [Google Scholar] [CrossRef]
- Yao, H.; Ashihara, E.; Maekawa, T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin. Ther. Targets 2011, 15, 873–887. [Google Scholar] [CrossRef]
- Tabnak, P.; Ghasemi, Y.; Natami, M.; Khorram, R.; Ebrahimnezhad, M. Role of m6A modification in dysregulation of Wnt/β-catenin pathway in cancer. Biomed. Pharmacother. 2023, 157, 114023. [Google Scholar] [CrossRef]
- Malanchi, I.; Peinado, H.; Kassen, D.; Hussenet, T.; Metzger, D.; Chambon, P.; Huber, M.; Hohl, D.; Cano, A.; Birchmeier, W.; et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 2008, 452, 650–653. [Google Scholar] [CrossRef]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008, 40, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gibson, P.; Currle, D.S.; Tong, Y.; Richardson, R.J.; Bayazitov, I.T.; Poppleton, H.; Zakharenko, S.; Ellison, D.W.; Gilbertson, R.J. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009, 457, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Welm, B.; Podsypanina, K.; Huang, S.; Chamorro, M.; Zhang, X.; Rowlands, T.; Egeblad, M.; Cowin, P.; Werb, Z.; et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15853–15858. [Google Scholar] [CrossRef]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, F.; Asselin-Labat, M.L.; Shackleton, M.; Forrest, N.C.; Lindeman, G.J.; Visvader, J.E. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008, 68, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Teissedre, B.; Pinderhughes, A.; Incassati, A.; Hatsell, S.J.; Hiremath, M.; Cowin, P. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS ONE 2009, 4, e4537. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, J.; Wu, Y.; Huang, T.L.; Wang, G. Small-molecule inhibitors of Wnt signaling pathway: Towards novel anticancer therapeutics. Future Med. Chem. 2015, 7, 2485–2505. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, G.; Singla, D.; Singh, S.; Kakkar, V.; Gulati, J.S.; Kaur, I.P. Enhanced Oral Absorption of All-trans Retinoic Acid upon Encapsulation in Solid Lipid Nanoparticles. Pharm. Nanotechnol. 2020, 8, 495–510. [Google Scholar] [CrossRef]
- Wei, L.Y.; Zhang, J.K.; Zheng, L.; Chen, Y. The functional role of sulforaphane in intestinal inflammation: A review. Food Funct. 2022, 13, 514–529. [Google Scholar] [CrossRef]
- Hooper, J.E.; Scott, M.P. Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 2005, 6, 306–317. [Google Scholar] [CrossRef]
- Lum, L.; Beachy, P.A. The Hedgehog response network: Sensors, switches, and routers. Science 2004, 304, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Pasca di Magliano, M.; Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 2003, 3, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Kreisingerová, K.; Ondrušová, U.; Horák, P.; Vachtenheim, J. Importance of Aberrantly Activated Hedgehog/Gli Pathway in Tumour Progression. Klin. Onkol. Cas. Ceske A Slov. Onkol. Spol. 2020, 33, 177–183. [Google Scholar]
- Katoh, Y.; Katoh, M. Identification and characterization of rat Desert hedgehog and Indian hedgehog genes in silico. Int. J. Oncol. 2005, 26, 545–549. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. Comparative genomics on Sonic hedgehog orthologs. Oncol. Rep. 2005, 14, 1087–1090. [Google Scholar] [CrossRef]
- Marigo, V.; Roberts, D.J.; Lee, S.M.; Tsukurov, O.; Levi, T.; Gastier, J.M.; Epstein, D.J.; Gilbert, D.J.; Copeland, N.G.; Seidman, C.E.; et al. Cloning, expression, and chromosomal location of SHH and IHH: Two human homologues of the Drosophila segment polarity gene hedgehog. Genomics 1995, 28, 44–51. [Google Scholar] [CrossRef]
- Visbal, A.P.; Lewis, M.T. Hedgehog Signaling in the Normal and Neoplastic Mammary Gland. Curr. Drug Targets 2010, 11, 1103–1111. [Google Scholar] [CrossRef]
- Katoh, M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007, 3, 30–38. [Google Scholar] [CrossRef]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.K.; Kittappa, R.; McKay, R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Zhao, X.; Malhotra, G.K.; Lele, S.M.; Lele, M.S.; West, W.W.; Eudy, J.D.; Band, H.; Band, V. Telomerase-immortalized human mammary stem/progenitor cells with ability to self-renew and differentiate. Proc. Natl. Acad. Sci. USA 2010, 107, 14146–14151. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, J.; Pandey, A.V. Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Witte, K.E.; Greiner, J.F.W.; Weissinger, F.; Kaltschmidt, C. Targeting NF-κB Signaling in Cancer Stem Cells: A Narrative Review. Biomedicines 2022, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, S.J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1alpha activation. Cell. Signal. 2010, 22, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ravindran, J.; Aggarwal, B.B. NF-kappaB and cancer: How intimate is this relationship. Mol. Cell. Biochem. 2010, 336, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-κB and NF-κB-regulated gene expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sandur, S.K.; Lin, X.; Chaturvedi, M.M.; Aggarwal, B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-κB signaling pathway. J. Biol. Chem. 2006, 281, 23425–23435. [Google Scholar] [CrossRef]
- Jiang, R.; Li, Y.; Xu, Y.; Zhou, Y.; Pang, Y.; Shen, L.; Zhao, Y.; Zhang, J.; Zhou, J.; Wang, X.; et al. EMT and CSC-like properties mediated by the IKKβ/IκBα/RelA signal pathway via the transcriptional regulator, Snail, are involved in the arsenite-induced neoplastic transformation of human keratinocytes. Arch. Toxicol. 2012, 16, 16. [Google Scholar] [CrossRef]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ruiz-Ontanon, P.; Vazquez-Barquero, A.; Lafarga, M.; Berciano, M.T.; Aldaz, B.; Grande, L.; Casafont, I.; Segura, V.; Robles, E.F.; et al. Blockade of the NFκB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene 2011, 30, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009, 15, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, W.; Liang, Z.; Han, W.; Li, J.; Ye, L.; Liu, M.; Cai, Z.; Zhao, J.; Chen, Y.; et al. UGT-mediated metabolism plays a dominant role in the pharmacokinetic behavior and the disposition of morusin in vivo and in vitro. J. Pharm. Biomed. Anal. 2018, 154, 339–353. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Tang, M.; Zhang, K.; Gao, X.; Fang, L.; Liu, W. STAT3 and p63 in the Regulation of Cancer Stemness. Front. Genet. 2022, 13, 909251. [Google Scholar] [CrossRef]
- Shih, P.C.; Mei, K.C. Role of STAT3 signaling transduction pathways in cancer stem cell-associated chemoresistance. Drug Discov. Today 2021, 26, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell Mol. Biol. Lett. 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Karol, M.D.; Hitron, M.; Hard, M.L.; Blanchard, J.E.; Eraut, N.; Rich, N.; Gufford, B.T. Mass balance and pharmacokinetics of an oral dose of 14C-napabucasin in healthy adult male subjects. Pharmacol. Res. Perspect. 2021, 9, e00722. [Google Scholar] [CrossRef]
- Steelman, L.S.; Stadelman, K.M.; Chappell, W.H.; Horn, S.; Basecke, J.; Cervello, M.; Nicoletti, F.; Libra, M.; Stivala, F.; Martelli, A.M.; et al. Akt as a therapeutic target in cancer. Expert Opin. Ther. Targets 2008, 12, 1139–1165. [Google Scholar] [CrossRef]
- Wickenden, J.A.; Watson, C.J. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: A play in 3 Akts. Breast Cancer Res. 2010, 12, 202. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Gonzalez-Perez, R.R. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br. J. Cancer 2011, 104, 128–137. [Google Scholar] [CrossRef]
- Carino, C.; Olawaiye, A.B.; Cherfils, S.; Serikawa, T.; Lynch, M.P.; Rueda, B.R.; Gonzalez, R.R. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int. J. Cancer 2008, 123, 2782–2790. [Google Scholar] [CrossRef]
- Sparks, C.A.; Guertin, D.A. Targeting mTOR: Prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene 2010, 29, 3733–3744. [Google Scholar] [CrossRef]
- Ghayad, S.E.; Cohen, P.A. Inhibitors of the PI3K/Akt/mTOR pathway: New hope for breast cancer patients. Recent Pat. Anticancer Drug Discov. 2010, 5, 29–57. [Google Scholar] [CrossRef]
- Hadad, S.M.; Fleming, S.; Thompson, A.M. Targeting AMPK: A new therapeutic opportunity in breast cancer. Crit. Rev. Oncol. Hematol. 2008, 67, 1–7. [Google Scholar] [CrossRef]
- Noh, W.C.; Kim, Y.H.; Kim, M.S.; Koh, J.S.; Kim, H.A.; Moon, N.M.; Paik, N.S. Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Res. Treat. 2008, 110, 477–483. [Google Scholar] [CrossRef]
- Sabbah, M.; Emami, S.; Redeuilh, G.; Julien, S.; Prevost, G.; Zimber, A.; Ouelaa, R.; Bracke, M.; De Wever, O.; Gespach, C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist. Updat. 2008, 11, 123–151. [Google Scholar] [CrossRef]
- Dillon, R.L.; Muller, W.J. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010, 70, 4260–4264. [Google Scholar] [CrossRef]
- Martelli, A.M.; Evangelisti, C.; Follo, M.Y.; Ramazzotti, G.; Fini, M.; Giardino, R.; Manzoli, L.; McCubrey, J.A.; Cocco, L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells. Curr. Med. Chem. 2011, 18, 2715–2726. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, Y.; Zou, F.; Liu, Y.; Wang, S.; Xu, N.; Xu, W.; Cui, C.; Xing, Y.; Cao, B.; et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6829–6834. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The human sirtuin family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [PubMed]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mautone, N.; Menna, M.; D’Acunzo, F.; Mai, A.; Rotili, D. Sirtuin modulators: Past, present, and future perspectives. Future Med. Chem. 2022, 14, 915–939. [Google Scholar] [CrossRef]
- Vasiliou, V.; Pappa, A.; Petersen, D.R. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem. Biol. Interact. 2000, 129, 1–19. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Ginestier, C.; Wicinski, J.; Cervera, N.; Monville, F.; Finetti, P.; Bertucci, F.; Wicha, M.S.; Birnbaum, D.; Charafe-Jauffret, E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle 2009, 8, 3297–3302. [Google Scholar] [CrossRef]
- Burger, P.E.; Gupta, R.; Xiong, X.; Ontiveros, C.S.; Salm, S.N.; Moscatelli, D.; Wilson, E.L. High aldehyde dehydrogenase activity: A novel functional marker of murine prostate stem/progenitor cells. Stem Cells 2009, 27, 2220–2228. [Google Scholar] [CrossRef]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Zeng, D.Z.; Dong, W.G.; Ding, Y.Q.; Rao, J.; Duan, J.J.; Liu, Q.; Yang, J.; Zhan, N.; Liu, Y.; et al. Distinct patterns of ALDH1A1 expression predict metastasis and poor outcome of colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 2976–2986. [Google Scholar] [PubMed]
- Wang, K.; Chen, X.; Zhan, Y.; Jiang, W.; Liu, X.; Wang, X.; Wu, B. Increased expression of ALDH1A1 protein is associated with poor prognosis in clear cell renal cell carcinoma. Med. Oncol. 2013, 30, 574. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Xu, Q.; Fu, X.Y.; Luo, W.S. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer 2014, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Keymoosi, H.; Gheytanchi, E.; Asgari, M.; Shariftabrizi, A.; Madjd, Z. ALDH1 in combination with CD44 as putative cancer stem cell markers are correlated with poor prognosis in urothelial carcinoma of the urinary bladder. Asian Pac. J. Cancer Prev. 2014, 15, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Muller, S.; Nannapaneni, S.; Pan, L.; Wang, Y.; Peng, X.; Wang, D.; Tighiouart, M.; Chen, Z.; Saba, N.F.; et al. Comparison of quantum dot technology with conventional immunohistochemistry in examining aldehyde dehydrogenase 1A1 as a potential biomarker for lymph node metastasis of head and neck cancer. Eur. J. Cancer 2012, 48, 1682–1691. [Google Scholar] [CrossRef]
- Mieog, J.S.; de Kruijf, E.M.; Bastiaannet, E.; Kuppen, P.J.; Sajet, A.; de Craen, A.J.; Smit, V.T.; van de Velde, C.J.; Liefers, G.J. Age determines the prognostic role of the cancer stem cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer 2012, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, V.; Agarwal, S.; Bordeaux, J.; Camp, R.L.; Rimm, D.L. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am. J. Pathol. 2010, 176, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, D.L.; Duan, J.J.; Xu, S.L.; Zhang, J.F.; Yang, X.J.; Zhang, X.; Cui, Y.H.; Bian, X.W.; Yu, S.C. ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: A systematic review and meta-analysis. BMC Cancer 2014, 14, 444. [Google Scholar] [CrossRef]
- Toledo-Guzman, M.E.; Ibanez Hernandez, M.; Gomez-Gallegos, A.A.; Ortiz-Sanchez, E. ALDH as a Stem Cell marker in solid tumors. Curr. Stem Cell Res. Ther. 2018, 13, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, S.; Liu, S.; Zhang, L. Aldehyde dehydrogenase in solid tumors and other diseases: Potential biomarkers and therapeutic targets. MedComm 2023, 4, e195. [Google Scholar] [CrossRef] [PubMed]
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R.; Robertson, G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Moreb, J.S.; Ucar-Bilyeu, D.A.; Khan, A. Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother. Pharmacol. 2017, 79, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Liccardo, D.; Tirino, V. Evaluation and Isolation of Cancer Stem Cells Using ALDH Activity Assay. Methods Mol. Biol. 2018, 1692, 43–48. [Google Scholar] [PubMed]
- Young, M.-J.; Wu, Y.-H.; Chiu, W.-T.; Weng, T.-Y.; Huang, Y.-F.; Chou, C.-Y. All-trans retinoic acid downregulates ALDH1-mediated stemness and inhibits tumour formation in ovarian cancer cells. Carcinogenesis 2015, 36, 498–507. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Giraud, J.; Staedel, C.; Chambonnier, L.; Dubus, P.; Chevret, E.; Bœuf, H.; Gauthereau, X.; Rousseau, B.; Fevre, M.; et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene 2016, 35, 5619. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Juven-Gershon, T.; Moallem, E.; Berger, M.; Vogt Sionov, R.; Lozano, G.; Oren, M.; Haupt, Y. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 1999, 18, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, A.; Jensen, M.H.; Krishna, S. Stress-specific response of the p53-Mdm2 feedback loop. BMC Syst. Biol. 2010, 4, 94. [Google Scholar] [CrossRef]
- Koo, N.; Sharma, A.K.; Narayan, S. Therapeutics Targeting p53-MDM2 Interaction to Induce Cancer Cell Death. Int. J. Mol. Sci. 2022, 23, 5005. [Google Scholar] [CrossRef]
- Bond, G.L.; Hu, W.; Levine, A.J. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr. Cancer Drug Targets 2005, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Olivos, D.J.; Mayo, L.D. Emerging Non-Canonical Functions and Regulation by p53: p53 and Stemness. Int. J. Mol. Sci. 2016, 17, 1982. [Google Scholar] [CrossRef]
- Babaei, G.; Aliarab, A.; Asghari Vostakolaei, M.; Hotelchi, M.; Neisari, R.; Gholizadeh-Ghaleh Aziz, S.; Bazl, M.R. Crosslink between p53 and metastasis: Focus on epithelial-mesenchymal transition, cancer stem cell, angiogenesis, autophagy, and anoikis. Mol. Biol. Rep. 2021, 48, 7545–7557. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Vicente, A.T.S.; Salvador, J.A.R. MDM2-Based Proteolysis-Targeting Chimeras (PROTACs): An Innovative Drug Strategy for Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 11068. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Ye, X.Q.; Li, Q.; Wang, G.H.; Sun, F.F.; Huang, G.J.; Bian, X.W.; Yu, S.C.; Qian, G.S. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int. J. Cancer 2011, 129, 820–831. [Google Scholar] [CrossRef]
- Schulze-Bergkamen, H.; Krammer, P.H. Apoptosis in cancer--implications for therapy. Semin. Oncol. 2004, 31, 90–119. [Google Scholar] [CrossRef] [PubMed]
- Dell’Eva, R.; Pfeffer, U.; Vene, R.; Anfosso, L.; Forlani, A.; Albini, A.; Efferth, T. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem. Pharmacol. 2004, 68, 2359–2366. [Google Scholar] [CrossRef]
- Haraguchi, N.; Ishii, H.; Mimori, K.; Tanaka, F.; Ohkuma, M.; Kim, H.M.; Akita, H.; Takiuchi, D.; Hatano, H.; Nagano, H.; et al. CD13 is a therapeutic target in human liver cancer stem cells. J. Clin. Investig. 2010, 120, 3326–3339. [Google Scholar] [CrossRef]
- Christ, B.; Stock, P.; Dollinger, M.M. CD13: Waving the flag for a novel cancer stem cell target. Hepatology 2011, 53, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Haraguchi, N.; Ishii, H.; Ohkuma, M.; Okano, M.; Mimori, K.; Eguchi, H.; Yamamoto, H.; Nagano, H.; Sekimoto, M.; et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann. Surg. Oncol. 2012, 19 (Suppl. S3), S539–S548. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yuan, T.; Wu, Y.; Wang, Y.; Fan, T.W.; Miriyala, S.; Lin, Y.; Yao, J.; Shi, J.; Kang, T.; et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013, 23, 316–331. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, J.H.; Cho, S.G. Role of Oxidative Stress in Stem, Cancer, and Cancer Stem Cells. Cancers 2010, 2, 859–884. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive oxygen species in cancer stem cells. Antioxid. Redox Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).