Fishing for the Virome of Tropical Tuna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Procedure
2.2. Sampling of the Skin Mucus, Gut and Liver
2.3. Viral Nucleic Acid Extraction and Sequencing
2.4. Viral Sequences Treatment and Analysis
2.5. Statistical Analysis
3. Results and Discussion
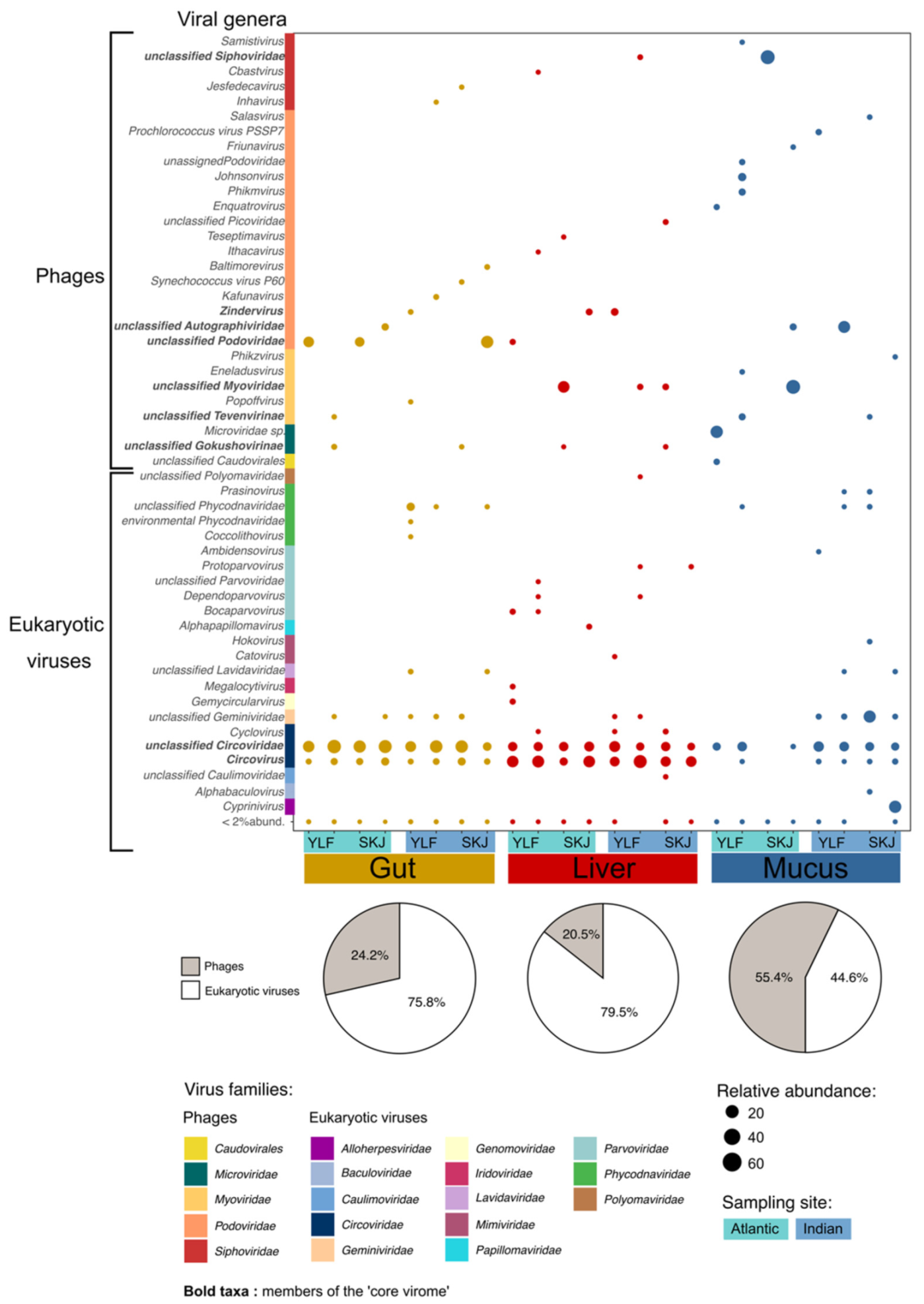
3.1. Tuna Host Specific Viral Niches
3.2. The Tuna ‘Core Virome’
3.3. Tuna Virome Composition Is Not Governed by Species or Sex
3.4. The Skin Virome Differs between Oceans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef]
- Knowles, B.; Silveira, C.; Bailey, B.A.; Barott, K.; Cantu, V.A.; Cobián-Güemes, A.G.; Coutinho, F.; Dinsdale, E.; Felts, B.; Furby, K.A.; et al. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470. [Google Scholar] [CrossRef]
- Laffy, P.W.; Botté, E.S.; Wood-Charlson, E.M.; Weynberg, K.D.; Rattei, T.; Webster, N.S. Thermal stress modifies the marine sponge virome. Environ. Microbiol. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.; Ye, J.; Ziegler, M.; Payet, J.P.; McMinds, R.; Thurber, R.V.; Voolstra, C.R. Coral-Associated Viral Assemblages from the Central Red Sea Align with Host Species and Contribute to Holobiont Genetic Diversity. Front. Microbiol. 2020, 11, 572534. [Google Scholar] [CrossRef] [PubMed]
- Thurber, R.V.; Payet, J.P.; Thurber, A.R.; Correa, A.M.S. Virus–host interactions and their roles in coral reef health and disease. Nat. Rev. Microbiol. 2017, 15, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Leong, J.-A.; Gates, R.D. Coral-virus interactions: A double-edged sword? Symbiosis 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Pascelli, C.; Laffy, P.W.; Botté, E.; Kupresanin, M.; Rattei, T.; Lurgi, M.; Ravasi, T.; Webster, N.S. Viral ecogenomics across the Porifera. Microbiome 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Filipa-Silva, A.; Parreira, R.; Martínez-Puchol, S.; Bofill-Mas, S.; Barreto Crespo, M.T.; Nunes, M. The Unexplored Virome of Two Atlantic Coast Fish: Contribution of Next-Generation Sequencing to Fish Virology. Foods 2020, 9, 1634. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Wille, M.; Ortiz-Baez, A.S.; Costa, V.A.; Ghaly, T.; Mifsud, J.C.; Turnbull, O.M.; Bellwood, D.R.; Williamson, J.E.; et al. Virome composition in marine fish revealed by meta-transcriptomics. Virus Evol. 2021, 7, veab005. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Monteil-Bouchard, S.; Temmam, S.; Desnues, C. Protocol for Generating Infectious RNA Viromes from Complex Biological Samples. In The Human Virome; Moya, A., Pérez Brocal, V., Eds.; Springer: New York, NY, USA, 2018; Volume 1838, pp. 25–36. [Google Scholar]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.J.; Tappu, R. MEGAN Community Edition-Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Colston, T.J.; Jackson, C.R. Microbiome evolution along divergent branches of the vertebrate tree of life: What is known and unknown. Mol. Ecol. 2016, 25, 3776–3800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geoghegan, J.L.; Di Giallonardo, F.; Cousins, K.; Shi, M.; Williamson, J.E.; Holmes, E.C. Hidden diversity and evolution of viruses in market fish. Virus Evol. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, W.; Liu, C.; Wang, W.; Li, N.; Chen, Y.; Lu, C.; Sun, X.; Han, Y.; Kuang, D.; et al. Characteristics of the tree shrew gut virome. PLoS ONE 2019, 14, e0212774. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.A.; Silva, J.M.F.; Brito, C.R.; Teixeira, D.S.; Melo, F.L.; Ribeiro, B.M.; Nagata, T.; Campos, F.S. Faecal Virome Analysis of Wild Animals from Brazil. Viruses 2019, 11, 803. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ling, Y.; Shan, T.; Yang, S.; Xu, H.; Deng, X.; Delwart, E.; Zhang, W. Gut virome of mammals and birds reveals high genetic diversity of the family Microviridae. Virus Evol. 2019, 5, vez013. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Martínez, L.A.; Loza-Rubio, E.; Mosqueda, J.; González-Garay, M.L.; García-Espinosa, G. Fecal virome composition of migratory wild duck species. PLoS ONE 2018, 13, e0206970. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.; Dishon, A.; Kotler, M. Herpesviruses that Infect Fish. Viruses 2011, 3, 2160–2191. [Google Scholar] [CrossRef] [Green Version]
- Hanson, L.; Doszpoly, A.; van Beurden, S.J.; de Oliveira Viadanna, P.H.; Waltzek, T. Alloherpesviruses of Fish. Aquaculture Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–172. [Google Scholar]
- Gregory, A.C.; Zayed, A.; Conceição-Neto, N.; Temperton, B.; Bolduc, B.; Alberti, A.; Ardyna, M.; Arkhipova, K.; Carmichael, M.; Cruaud, C.; et al. Marine DNA Viral Macro- and Microdiversity from Pole to Pole. Cell 2019, 177, 1109–1123.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carding, S.R.; Davis, N.; Hoyles, L. Review article: The human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Graves, M.V.; Müller, D.G.; Boland, W.; Delaroque, N. Phycodnaviridae–large DNA algal viruses. Arch. Virol. 2002, 147, 1479–1516. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, J.; Jiang, N.; Fan, Y.; Zhou, Y.; Cain, K.; Yi, M.; Jia, K.; Wen, H.; et al. Determination of a novel parvovirus pathogen associated with massive mortality in adult tilapia. PLoS Pathog. 2020, 16, e1008765. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Shariff, M.; Omar, A.R.; Hair-Bejo, M. Megalocytivirus infection in fish. Rev. Aquac. 2012, 4, 221–233. [Google Scholar] [CrossRef]
- Labella, A.M.; Leiva-Rebollo, R.; Alejo, A.; Castro, D.; Borrego, J.J. Lymphocystis disease virus (LCDV-Sa), polyomavirus 1 (SaPyV1) and papillomavirus 1 (SaPV1) in samples of Mediterranean gilthead seabream. Dis. Aquat. Org. 2019, 132, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Dickson, K.A. Tuna comparative physiology. J. Exp. Biol. 2004, 207, 4015–4024. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, K.M.; Fuller, D.W.; Block, B.A. Vertical Movements and Habitat Utilization of Skipjack (Katsuwonus pelamis), Yellowfin (Thunnus albacares), and Bigeye (Thunnus obesus) Tunas in the Equatorial Eastern Pacific Ocean, Ascertained Through Archival Tag Data. In Tagging and Tracking of Marine Animals with Electronic Devices; Nielsen, J.L., Arrizabalaga, H., Fragoso, N., Hobday, A., Lutcavage, M., Sibert, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 9, pp. 121–144. [Google Scholar]
- Jaquemet, S.; Potier, M.; Ménard, F. Do drifting and anchored Fish Aggregating Devices (FADs) similarly influence tuna feeding habits? A case study from the western Indian Ocean. Fish. Res. 2011, 107, 283–290. [Google Scholar] [CrossRef]
- Schaefer, K.M. Reproductive Biology of Tunas. Fish Physiology; Academic Press: New York, NY, USA, 2001; Volume 19, pp. 225–270. [Google Scholar]
- Ross, A.A.; Rodrigues Hoffmann, A.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Chiarello, M.; Paz-Vinas, I.; Veyssière, C.; Santoul, F.; Loot, G.; Ferriol, J.; Boulêtreau, S. Environmental conditions and neutral processes shape the skin microbiome of European catfish (Silurus glanis) populations of Southwestern France. Environ. Microbiol. Rep. 2019, 11, 605–614. [Google Scholar] [CrossRef]
| Organs | Ocean | Species | Sex | |
|---|---|---|---|---|
| All samples | p = 0.001 | p = 0.138 | p = 0.718 | p = 0.999 |
| Skin | p = 0.023 | p = 0.884 | p = 0.863 | |
| Gut | p = 0.122 | p = 0.159 | p = 0.919 | |
| Liver | p = 0.136 | p = 0.308 | p = 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadoin, E.; Desnues, C.; Monteil-Bouchard, S.; Bouvier, T.; Auguet, J.-C.; Roque d’Orbcastel, E.; Bettarel, Y. Fishing for the Virome of Tropical Tuna. Viruses 2021, 13, 1291. https://doi.org/10.3390/v13071291
Gadoin E, Desnues C, Monteil-Bouchard S, Bouvier T, Auguet J-C, Roque d’Orbcastel E, Bettarel Y. Fishing for the Virome of Tropical Tuna. Viruses. 2021; 13(7):1291. https://doi.org/10.3390/v13071291
Chicago/Turabian StyleGadoin, Elsa, Christelle Desnues, Sonia Monteil-Bouchard, Thierry Bouvier, Jean-Christophe Auguet, Emmanuelle Roque d’Orbcastel, and Yvan Bettarel. 2021. "Fishing for the Virome of Tropical Tuna" Viruses 13, no. 7: 1291. https://doi.org/10.3390/v13071291
APA StyleGadoin, E., Desnues, C., Monteil-Bouchard, S., Bouvier, T., Auguet, J.-C., Roque d’Orbcastel, E., & Bettarel, Y. (2021). Fishing for the Virome of Tropical Tuna. Viruses, 13(7), 1291. https://doi.org/10.3390/v13071291






