Corrosion Behaviors of Ni80A Alloy Valve in Marine Engine Within Ammonia-Rich Environment
Abstract
1. Introduction
2. Experimental Methods
2.1. Service Temperature Identification of Valve
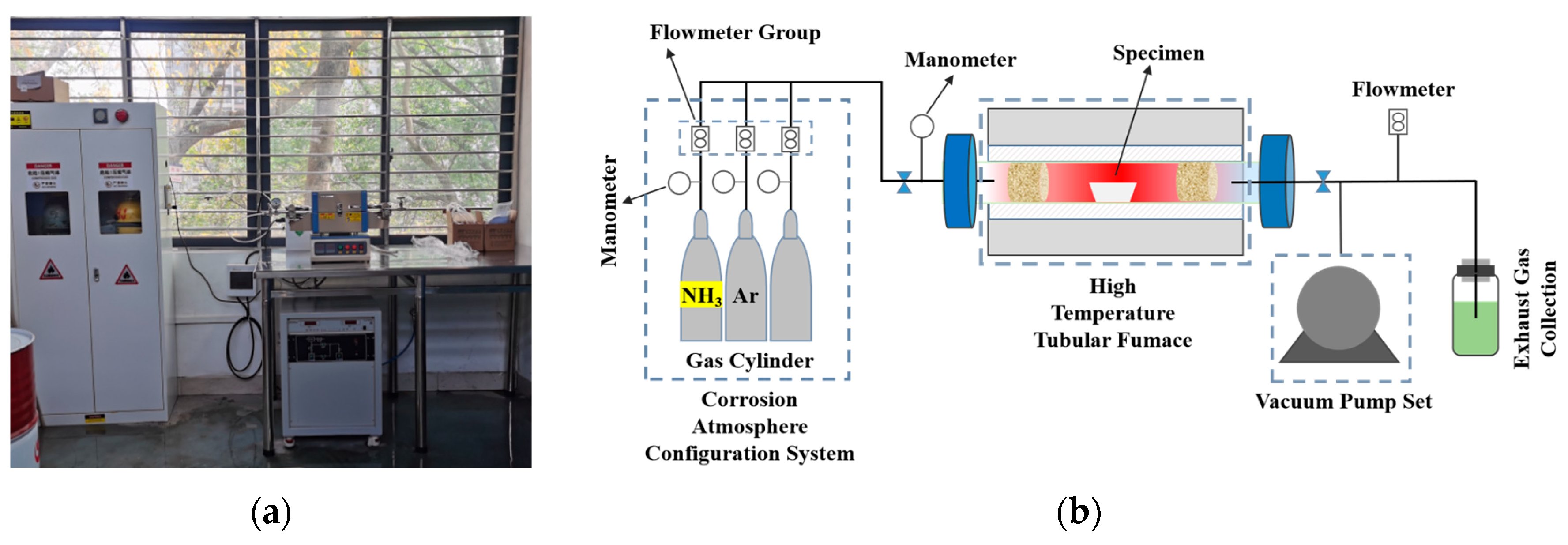
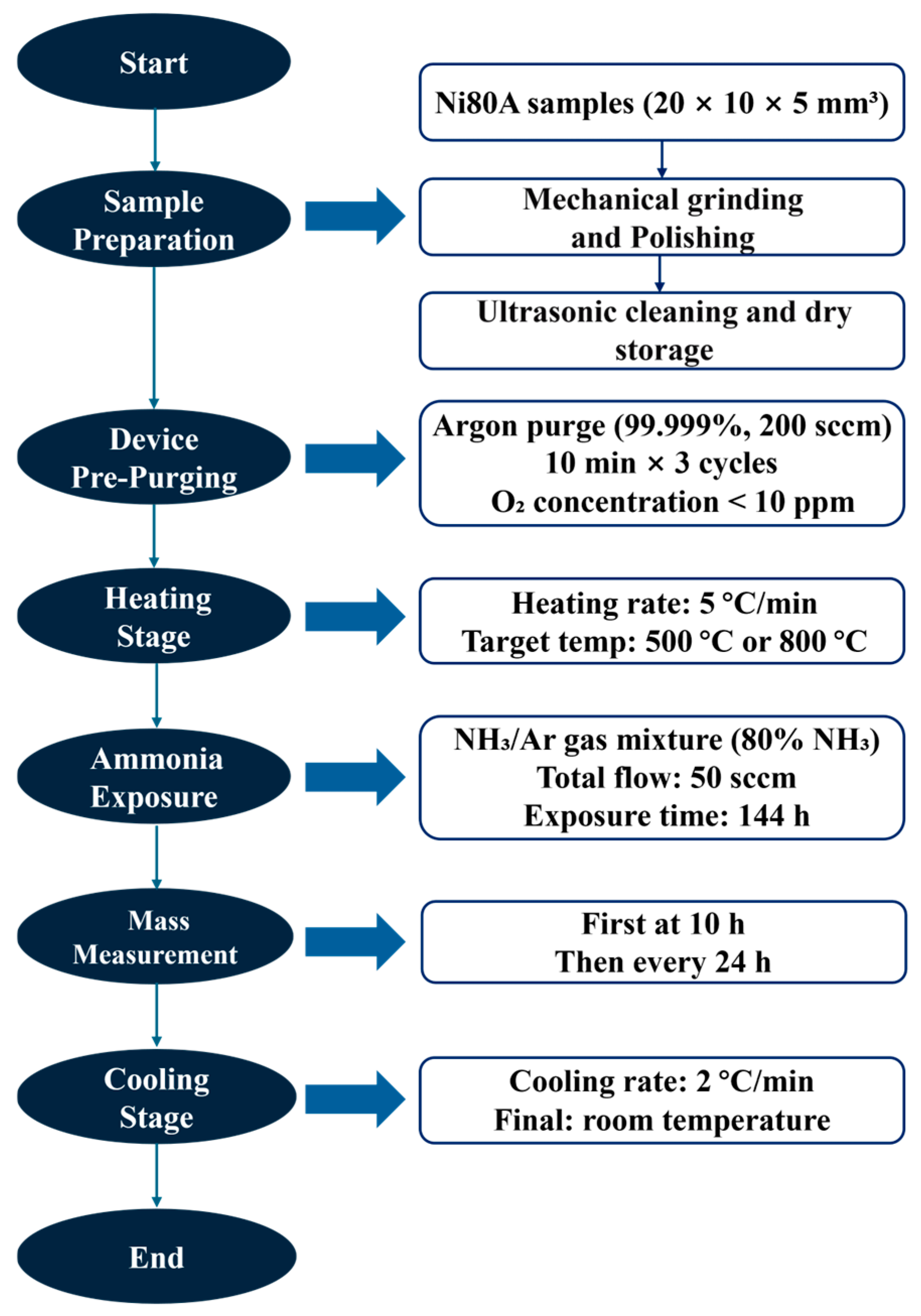
2.2. Ammonia Corrosion Experiments
2.3. Characterization and Testing Methods
3. Results and Discussion
3.1. Analysis of Corrosion Kinetics
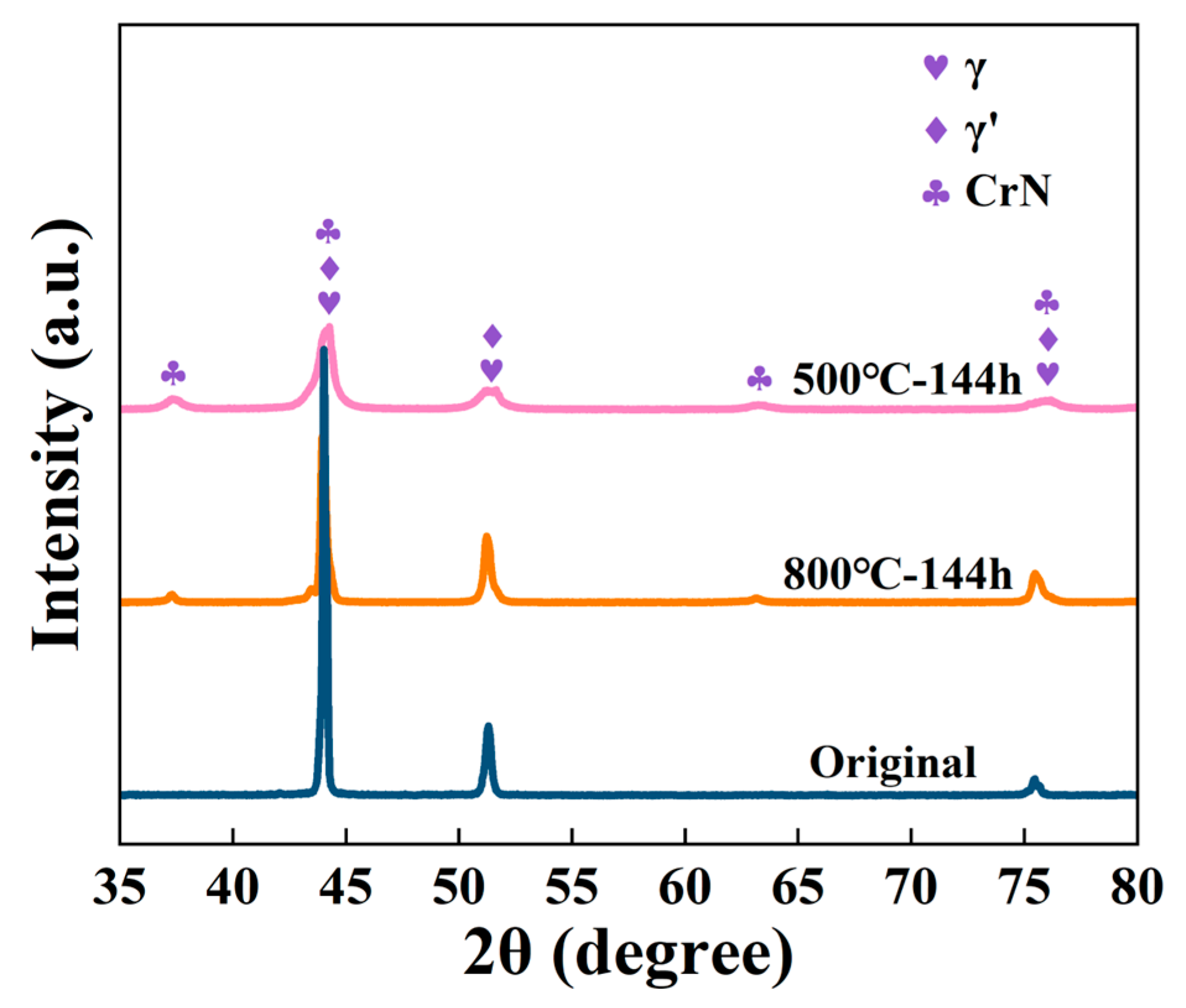
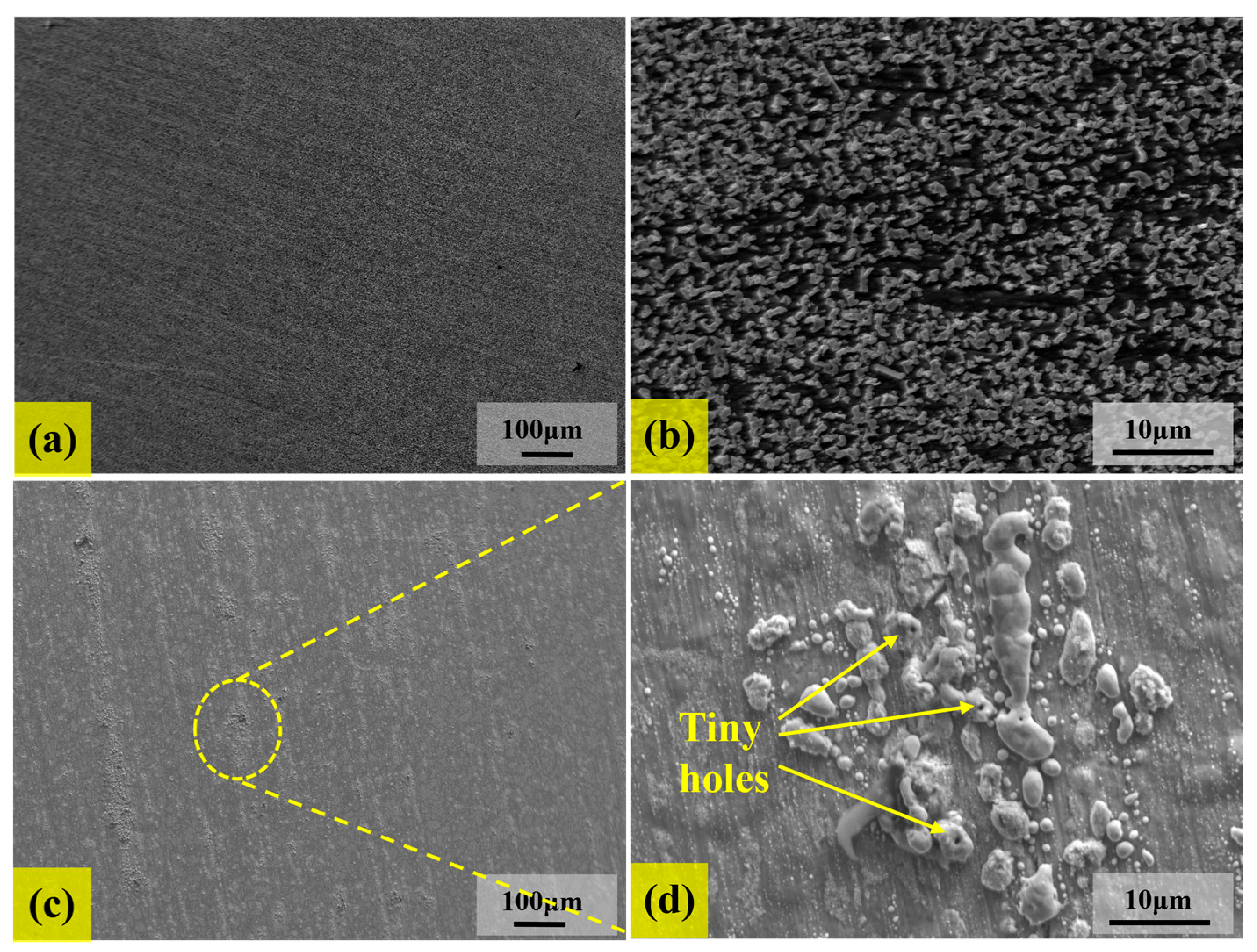
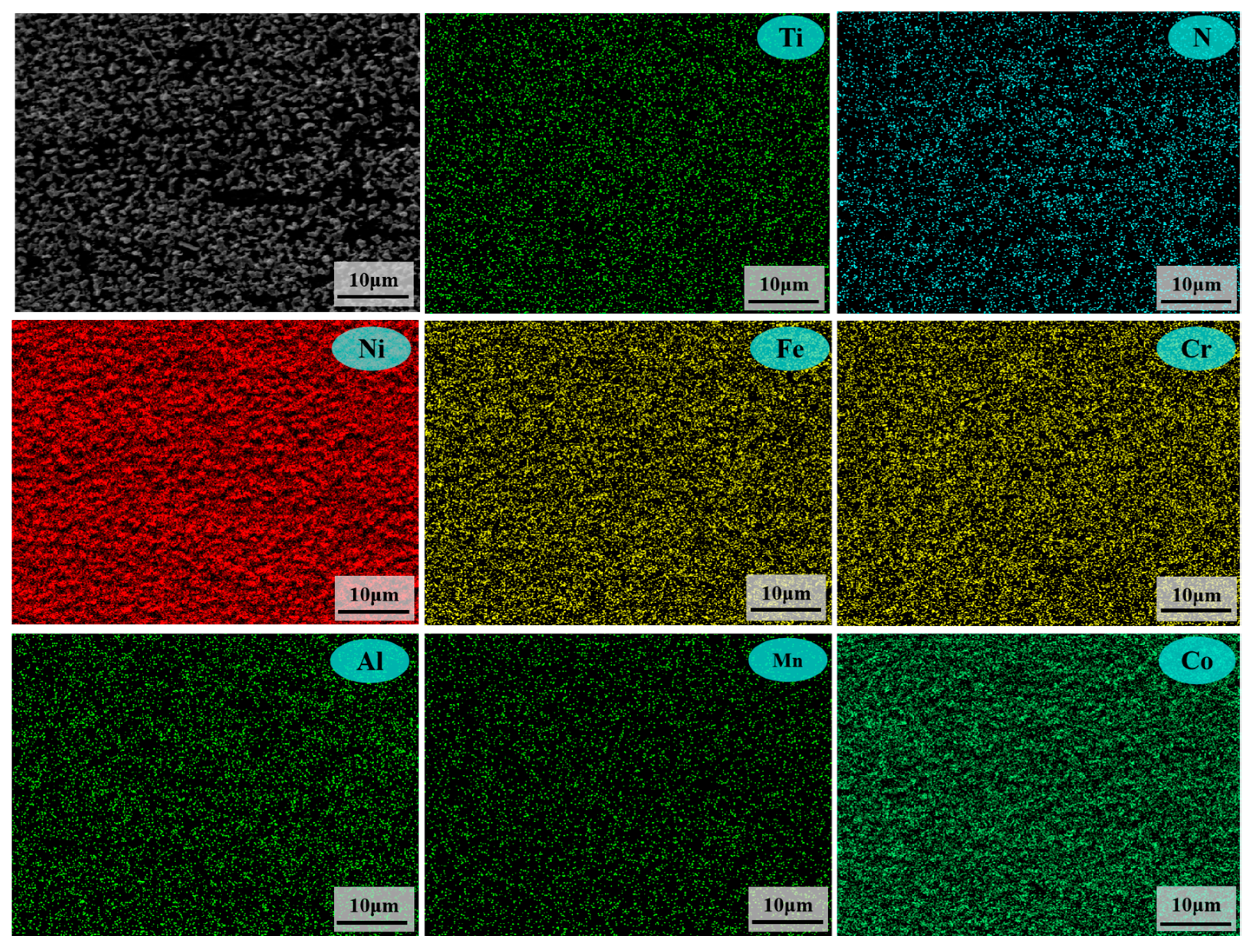
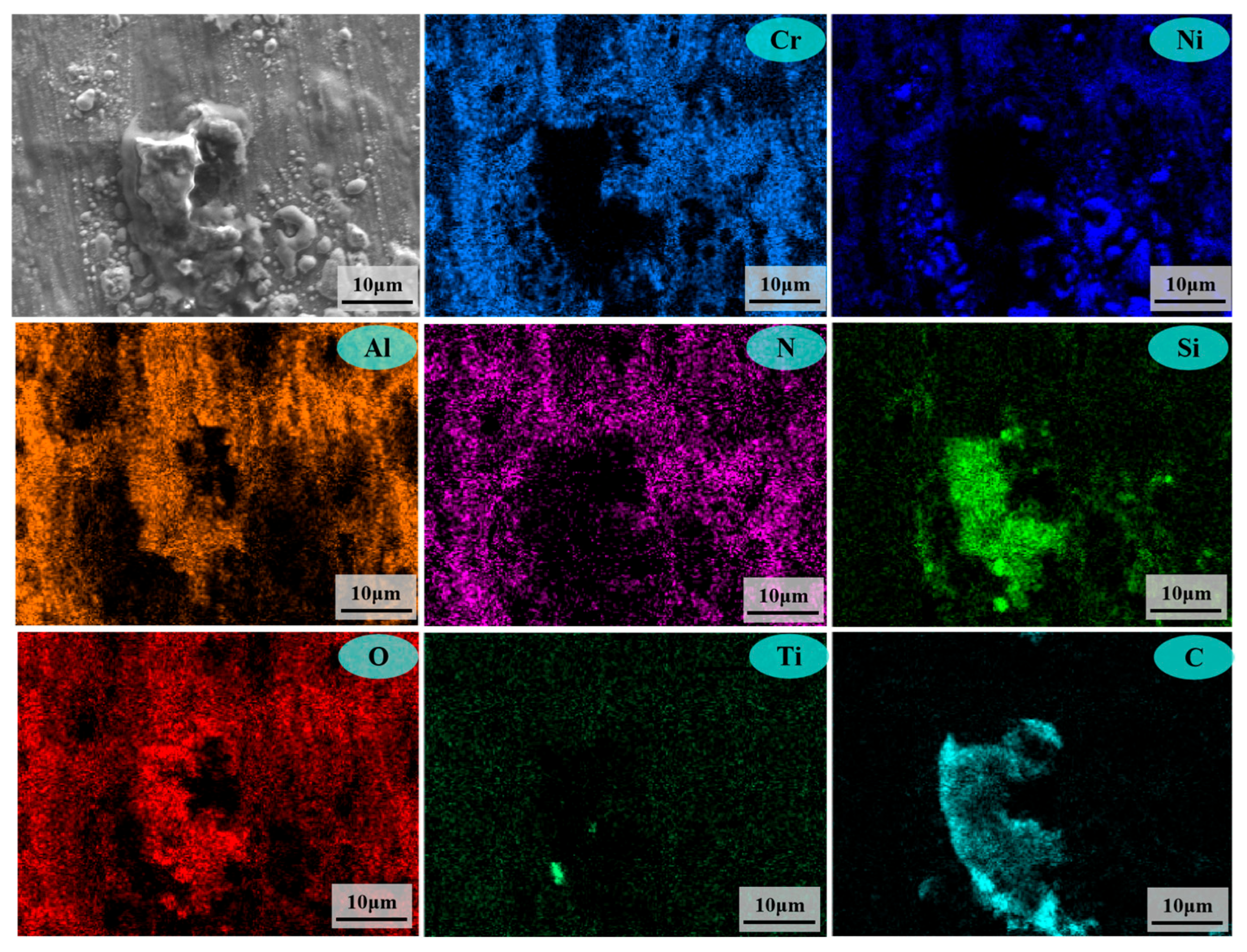
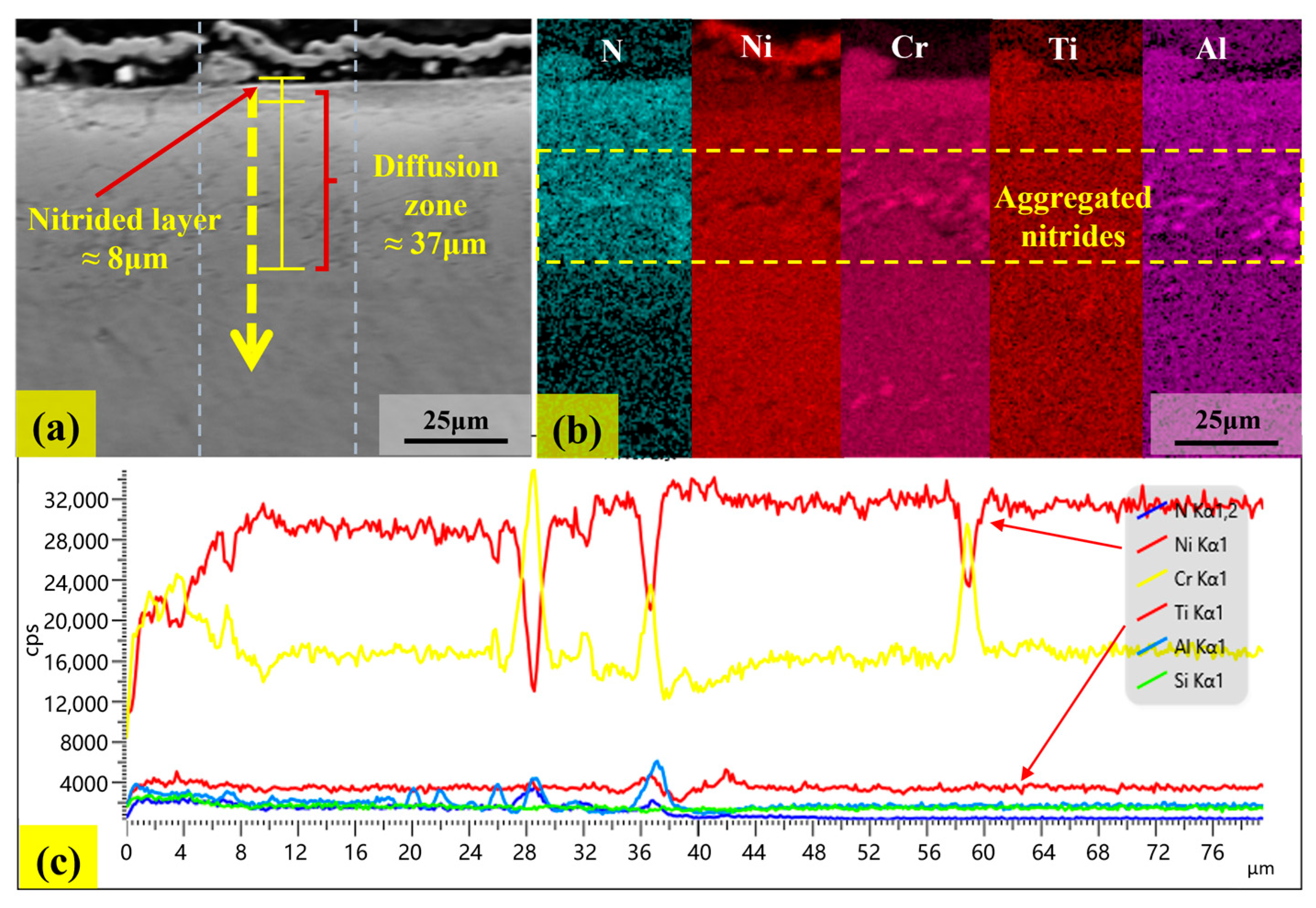
3.2. Analysis of Corrosion Behaviors
3.3. Analysis of Micro-Hardness in Corrosion Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joung, T.H.; Kang, S.G.; Lee, J.K.; Ahn, J. The IMO initial strategy for reducing Greenhouse Gas (GHG) emissions, and its follow-up actions towards 2050. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Jang, H.; Kim, J.; Kim, Y. Regulatory gap analysis for risk assessment of ammonia-fuelled ships. Ocean Eng. 2023, 287, 115751. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Frankl, S.; Gleis, S.; Karmann, S.; Prager, M.; Wachtmeister, G. Investigation of ammonia and hydrogen as CO2-free fuels for heavy duty engines using a high pressure dual fuel combustion process. Int. J. Engine Res. 2021, 22, 3196–3208. [Google Scholar] [CrossRef]
- Frankl, S.G.; Gelner, A.D.; Gleis, S.; Hartl, M.; Wachtmeister, G. Numerical study on renewable and sustainable fuels for HPDF engines. In Proceedings of the ASME 2020 Power Conference, Online, 4–5 August 2020. POWER2020-16438. [Google Scholar]
- Cai, Y.; Wang, Z.; Tang, Y.; Xu, C.; Song, Y.; Dong, K.; Han, E. Corrosion fatigue behavior of cast iron in simulated combustion product solutions of ammonia and methanol fuels. Int. J. Fatigue 2025, 191, 108715. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Vigueras-Zuniga, M.O.; Shi, H.; Mashruk, S.; Alnajideen, M.; Alnasif, A.; Cheng, Y.B. Ammonia combustion in furnaces: A review. Int. J. Hydrogen Energy 2024, 49, 1597–1618. [Google Scholar] [CrossRef]
- Sun, L.; Xiong, Z.; Qiu, J.; Zhu, Y.; Macdonald, D.D. Corrosion behavior of carbon steel in dilute ammonia solution. Electrochim. Acta 2020, 364, 137295. [Google Scholar] [CrossRef]
- Wei, B.; Yang, W.; Wang, J.; Tan, H.; Zhou, S.; Wang, F.; Ma, J. Study on reduction mechanism of Fe2O3 by NH3 under SNCR condition. Fuel 2019, 255, 115814. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Q.; Xu, Y.; Luo, W. Corrosion behavior of bulk nanocrystalline copper in ammonia solution. Mater. Lett. 2011, 65, 857–859. [Google Scholar] [CrossRef]
- Salcedo, A.; Caputo, S.; Loehlé, S.; Steinmann, S.N.; Michel, C. Molecular modeling of the diffusion of ammonia through corrosion inhibitor films on copper. Corros. Sci. 2024, 240, 112491. [Google Scholar] [CrossRef]
- Singh, J.B.; Verma, A.; Paul, B.; Chakravartty, J.K. Failure of Alloy 625 tube stub ends—Effect of primary nitrides. Eng. Fail. Anal. 2013, 32, 236–247. [Google Scholar] [CrossRef]
- Chakravartty, J.K.; Singh, J.B.; Sundararaman, M. Microstructural and mechanical properties of service exposed Alloy 625 ammonia cracker tube removed after 100,000 h. Mater. Sci. Technol. 2012, 28, 702–710. [Google Scholar] [CrossRef]
- Ghara, T.; Kuroda, S.; Yanagisawa, T.; Suzuki, M.; Inoue, T.; Shahien, M.; Shinoda, K. Position-dependent degradation and damage mechanisms of Inconel 600 in an ammonia gas flow environment at elevated temperatures. Corros. Sci. 2024, 240, 112421. [Google Scholar] [CrossRef]
- Perron, M.; Krawczyk, K.; Młotek, M. Plasma-catalytic decomposition of highly concentrated ammonia for a chemical hydrogen storage. Chem. Eng. Sci. 2025, 314, 121815. [Google Scholar] [CrossRef]
- Wang, Z.; He, H.; Deng, J.; Duan, Y.; Zhang, K.; Qiu, X.; Lei, G.; Geng, S.; Zheng, G.; Sun, Q.; et al. Strong metal support interaction in Ru/La2O3 catalyst for plasma enhanced ammonia decomposition to produce hydrogen. J. Rare Earths 2025, in press. [CrossRef]
- Yoon, Y.; Choi, Y.; Bae, Y.; Hong, J.; Kim, K. Ni-based alloy catalysts for cost-effective hydrogen production from ammonia decomposition. Appl. Surf. Sci. Adv. 2025, 27, 100759. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Oxide layer formed on AISI 5140 steel by plasma nitriding and post-oxidation in a mixture of air and ammonia. J. Alloys Compd. 2019, 806, 1517–1521. [Google Scholar] [CrossRef]
- Thierry, D.; Le Bozec, N.; Persson, D. Corrosion of hot-dip-galvanised steel and zinc alloy-coated steel in ammonia and ammonium chloride. Mater. Corros. 2020, 71, 1118–1124. [Google Scholar] [CrossRef]
- Wu, W.; Wei, B.; Li, G.; Chen, L.; Wang, J.; Ma, J. Study on ammonia gas high temperature corrosion coupled erosion wear characteristics of circulating fluidized bed boiler. Eng. Fail. Anal. 2021, 132, 105896. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Qian, F.; Zhang, S.; Wang, J.; Zhu, N.; Bao, X.; Yang, H.; Guo, Z. Ammonia energy fraction effect on the combustion and reduced NOx emission of ammonia/diesel dual fuel. Environ. Res. 2024, 261, 119530. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, S.; Bai, X.; Repo, J.A.; Hautala, S.; Hyvönen, J. Performance and emission characteristics of an ammonia/diesel dual-fuel marine engine. Renew. Sustain. Energy Rev. 2023, 185, 113631. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, R.; Tang, Q.; Lv, Z.; Liu, H.; Yue, Z.; Yao, M. Combustion and emission characteristics of an ammonia–diesel dual-fuel engine under high ammonia substitution ratios. Energy Fuels 2025, 39, 6559–6571. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Zhang, R.; Wei, H.; Pan, J.Y. Flame characteristics and abnormal combustion of ammonia–diesel dual-fuel engine with considering ammonia energy fractions. Appl. Therm. Eng. 2024, 245, 122858. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, W.; Dong, P.; Tian, H.; Tian, J.; Li, B.; Wang, Y. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel 2023, 332 Pt 2, 126086. [Google Scholar] [CrossRef]
- Wang, B.; Yang, C.; Wang, H.; Hu, D.; Duan, B.; Wang, Y. Effects of combustion and emission performance of ammonia/natural gas engines ignited by diesel. Fuel 2024, 358 Pt B, 130323. [Google Scholar] [CrossRef]
- Ghara, T.; Kuroda, S.; Yanagisawa, T.; Shahien, M.; Shinoda, K. Pre-Oxidized HVOF Sprayed CoNiCrAlY Coating as an Environmental Barrier Against Ammonia Corrosion for Pure Ammonia Combustion Gas Turbines. Int. Therm. Spray Conf. 2025, 85045, 450–456. [Google Scholar]
- Xu, X.; Yu, Z.; Cui, L. Microstructure and properties of plasma nitrided layers on Ni-based superalloy Ni-20Cr. Mater. Charact. 2019, 155, 109798. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Li, X. Effect of Hf addition on microstructural evolution and corrosion behavior of nickel-based alloys in hydrochloric acid. Corros. Sci. 2023, 224, 111507. [Google Scholar] [CrossRef]
- Tao, X.; Yang, Y.; Qi, J.; Cai, B.; Rainforth, W.M.; Li, X.; Dong, H. An investigation on nitrogen uptake and microstructure of equimolar quaternary FeCoNiCr high entropy alloy after active-screen plasma nitriding. Mater. Charact. 2024, 208, 113593. [Google Scholar] [CrossRef]
- Meka, S.R.; Bischoff, E.; Schacherl, R.E.; Mittemeijer, E.J. Unusual nucleation and growth of γ′ iron nitride upon nitriding Fe–4.75 at.% Al alloy. Philos. Mag. 2012, 92, 1083–1105. [Google Scholar] [CrossRef]
- Meixner, F.; Ahmadi, M.R.; Sommitsch, C. Cavity nucleation and growth in nickel-based alloys during creep. Materials 2022, 15, 1495. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, P.; Liu, S.; Lei, X.; Wang, R.; Zhang, X.; Tu, S. A novel hole cold-expansion method and its effect on surface integrity of nickel-based superalloy. J. Mater. Sci. Technol. 2020, 59, 129–137. [Google Scholar] [CrossRef]
- Kimmerl, A.C.; Malkowski, T.F.; Griffiths, S.; Hertweck, B.; Steigerwald, T.G.; Freund, L.P.; Schluecker, E. High-temperature corrosion of Inconel® Alloy 718, Haynes®282® Alloy and CoWAlloy1&2 in supercritical ammonia/ammonium chloride solution. J. Cryst. Growth 2018, 498, 289–300. [Google Scholar]
- Zhang, X.; Shang, H.; Gao, Q.; Ma, Q.; Zhang, H.; Li, H.; Sun, L. Coarsening evolution of γ′ phase and failure mechanism of Co–Ni–Al–Ti-based superalloys during isothermal aging. Front. Mater. 2022, 9, 863305. [Google Scholar] [CrossRef]
- Strunz, P.; Petrenec, M.; Polak, J.; Gasser, U.; Farkas, G. Formation and dissolution of γ′ precipitates in IN792 superalloy at elevated temperatures. Metals 2016, 6, 37. [Google Scholar] [CrossRef]
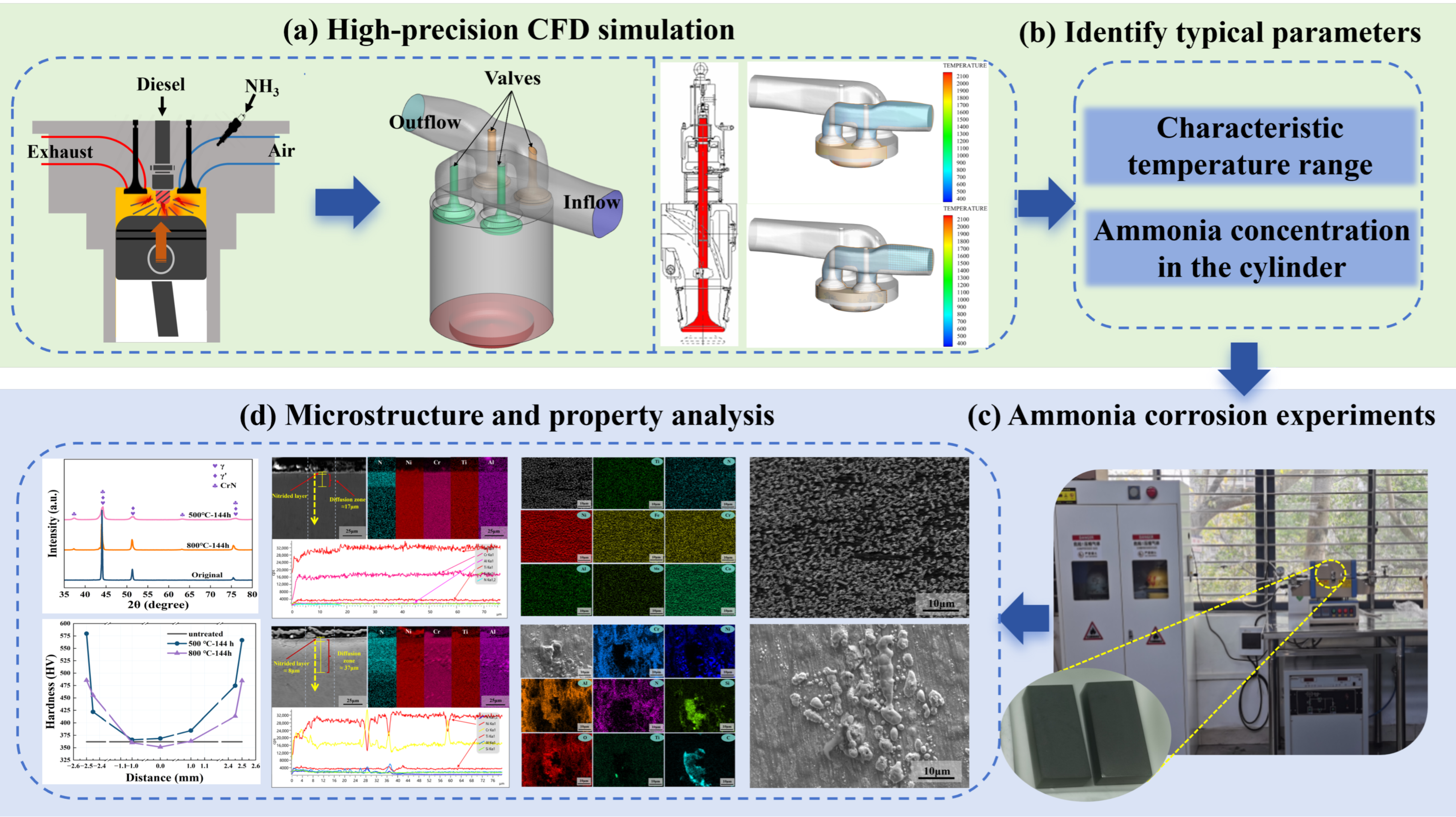
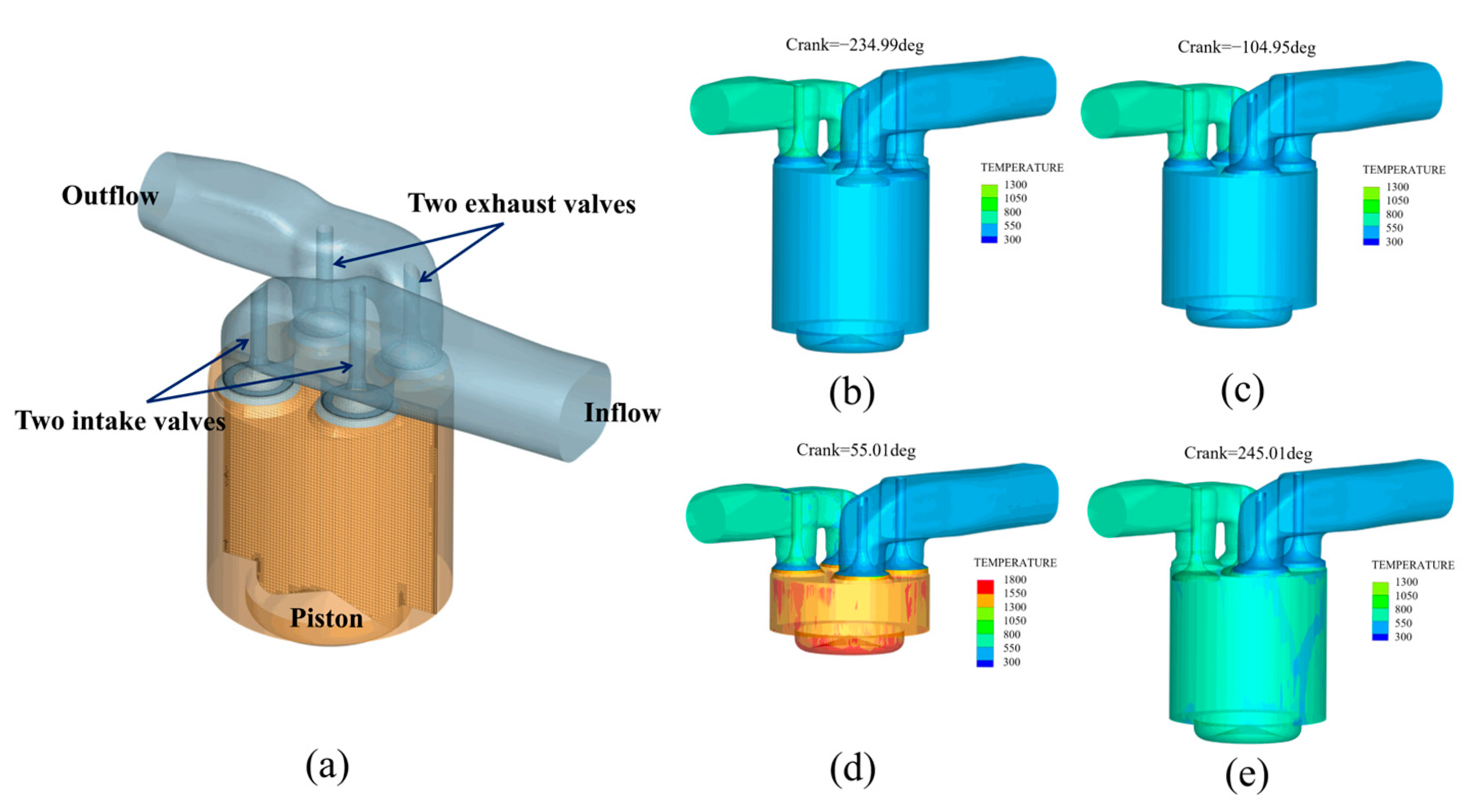
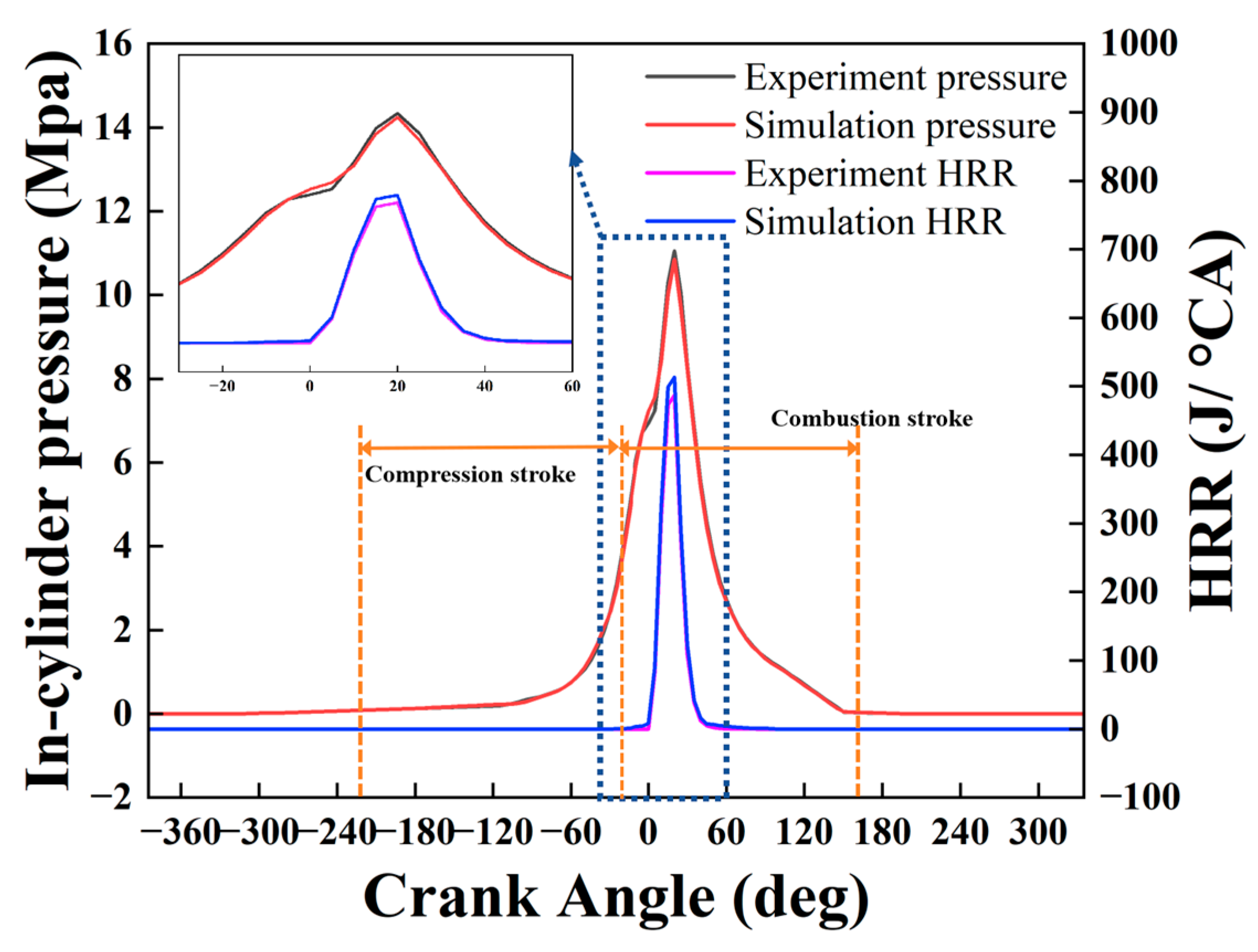
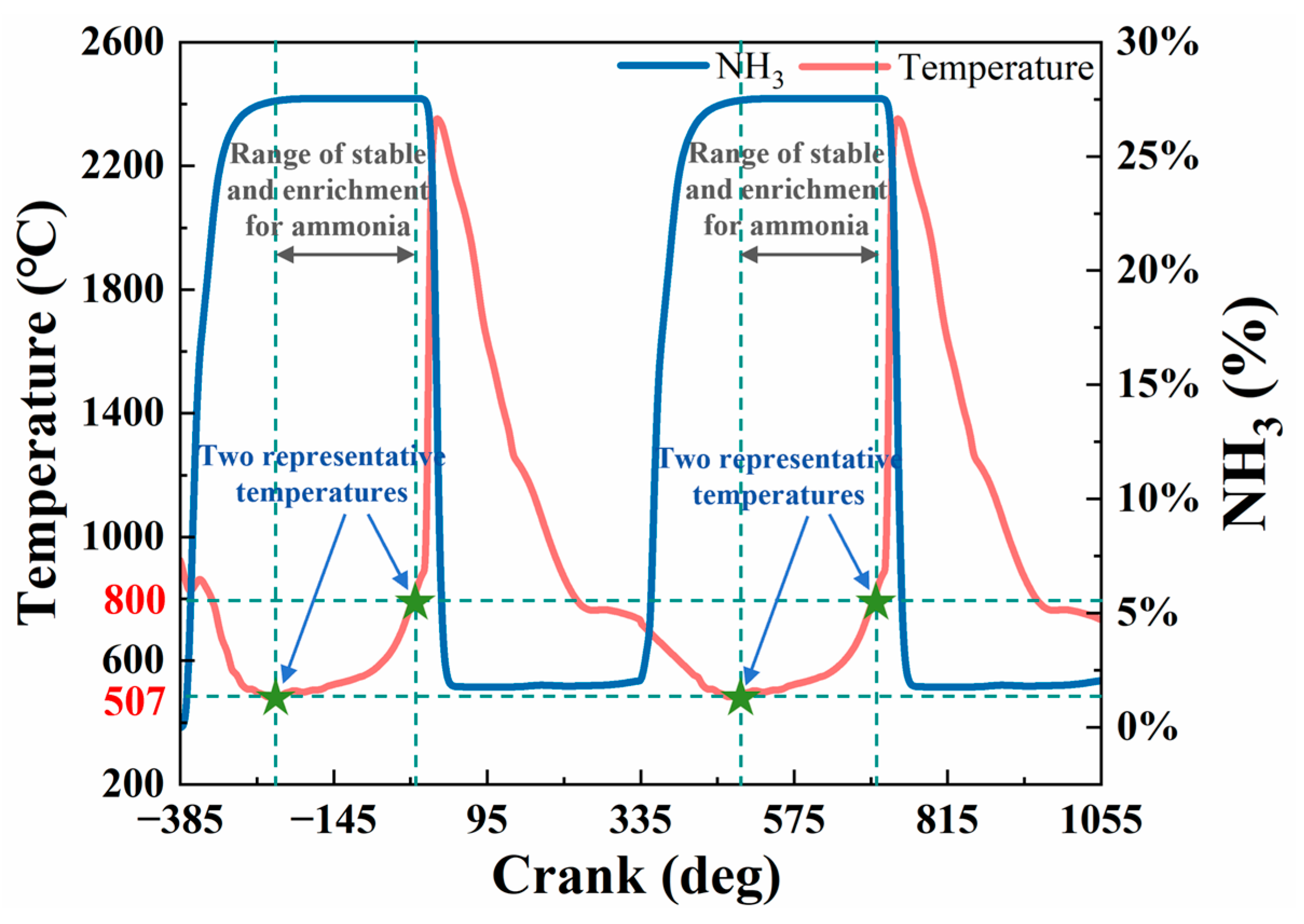



| Model Name | Sub-Models and Justification |
|---|---|
| Turbulence model | RNGκ-ε model—robust for capturing turbulent kinetic energy dissipation in internal combustion engines. |
| Spray breakup model | KH-RT model—simulates both primary and secondary breakup accurately for high-pressure diesel sprays. |
| Spray/wall interaction model | Wall film model/wall film–O’Rourke model—captures droplet–wall interaction and film dynamics under wall impingement conditions. |
| Droplet collision model | NTC collision model—accounts for non-linear droplet collision effects, improving accuracy in dense spray regions. |
| Wall heat transfer model | Han and Reitz model—incorporates film cooling and boiling effects, providing realistic heat transfer prediction at the wall. |
| Combustion model | SAGE model—a detailed chemical kinetics solver suitable for multi-component fuel combustion in diesel engines. |
| NOx Emission Model | Extended Zeldovich NOx model—widely validated for thermal NOx formation in high-temperature combustion environments. |
| Evaporation | Frossling model—considers convective heat and mass transfer effects, appropriate for fuel droplet evaporation under transient engine conditions. |
| Element | Cr | Ti | Al | Fe | Mn | Si | C | Ni |
|---|---|---|---|---|---|---|---|---|
| Content | 20.88 | 2.02 | 1.48 | 1.15 | 0.52 | 0.61 | 0.063 | Balance |
| Temperature | S (m2) | M0 (g) | M10h (g) | M144h (g) | V10h g/(m2·h) | V144h g/(m2·h) |
|---|---|---|---|---|---|---|
| 500 °C | 0.0007 | 7.6092 | 7.6112 | 7.6152 | 0.2975 | 0.0620 |
| 800 °C | 0.0007 | 7.4735 | 7.4818 | 7.4883 | 1.1857 | 0.1468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-y.; Quan, G.-z.; Yu, Y.-z.; Ran, W.-j.; Xiong, W. Corrosion Behaviors of Ni80A Alloy Valve in Marine Engine Within Ammonia-Rich Environment. Materials 2025, 18, 3006. https://doi.org/10.3390/ma18133006
Liu Y-y, Quan G-z, Yu Y-z, Ran W-j, Xiong W. Corrosion Behaviors of Ni80A Alloy Valve in Marine Engine Within Ammonia-Rich Environment. Materials. 2025; 18(13):3006. https://doi.org/10.3390/ma18133006
Chicago/Turabian StyleLiu, Ying-ying, Guo-zheng Quan, Yan-ze Yu, Wen-jing Ran, and Wei Xiong. 2025. "Corrosion Behaviors of Ni80A Alloy Valve in Marine Engine Within Ammonia-Rich Environment" Materials 18, no. 13: 3006. https://doi.org/10.3390/ma18133006
APA StyleLiu, Y.-y., Quan, G.-z., Yu, Y.-z., Ran, W.-j., & Xiong, W. (2025). Corrosion Behaviors of Ni80A Alloy Valve in Marine Engine Within Ammonia-Rich Environment. Materials, 18(13), 3006. https://doi.org/10.3390/ma18133006







