Abstract
This study synthesized two eco-friendly inhibitors—a chitosan–copper metal–organic framework (CS@Cu MOF) and chitosan–Schiff base–Cu complex (Schiff–CS@Cu)—for Q345 steel protection in 3.5% NaCl/1M HCl. Electrochemical and weight loss analyses demonstrated exceptional corrosion inhibition: untreated specimens showed a 25.889 g/(m2·h) corrosion rate, while 100 mg/L of CS@Cu MOF and Schiff–CS@Cu reduced rates to 2.50 g/(m2·h) (90.34% efficiency) and 1.67 g/(m2·h) (93.56%), respectively. Schiff–CS@Cu’s superiority stemmed from its pyridine–Cu2+ chelation forming a dense coordination barrier that impeded Cl−/H+ penetration, whereas CS@Cu MOF relied on physical adsorption and micro-galvanic interactions. Surface characterization revealed that Schiff–CS@Cu suppressed pitting nucleation through chemical coordination, contrasting with CS@Cu MOF’s porous film delaying uniform corrosion. Both inhibitors achieved optimal performance at 100 mg/L concentration. This work establishes a molecular design strategy for green inhibitors, combining metal–organic coordination chemistry with biopolymer modification, offering practical solutions for marine infrastructure and acid-processing equipment protection.
1. Introduction
With the rapid development of marine engineering and infrastructure construction, Q345 low-alloy high-strength steel has been widely used in bridges, offshore platforms, and other key structures due to its excellent mechanical properties; however, its corrosion in chloride-containing acidic environments poses a serious threat to structural durability [1,2,3]. Although traditional organic corrosion inhibitors can effectively inhibit corrosion, they have issues such as high toxicity and poor biodegradability, which are not consistent with the development needs of green chemistry [4,5,6].
As a natural cationic polysaccharide, chitosan has demonstrated great potential in the field of environmentally friendly corrosion inhibitors by virtue of its abundant hydroxyl/amino functional groups and metal coordination ability [7,8]. In detail, chitosan can inhibit corrosion by adsorbing on the metal surface to form a protective film [9,10,11]. Studies have revealed that chitosan and its derivatives have a good corrosion inhibition effect on carbon steel in acidic media [12,13,14,15]. For instance, water-soluble chitosan derivatives exhibited excellent corrosion inhibition properties on mild steel in 1 M HCl solution [12]. Another study also confirmed the potential of modified chitosan as an efficient corrosion inhibitor for carbon steel in acidic media [10,13]. However, its inherent defects such as poor solubility and insufficient adsorption stability have restricted practical engineering applications [16,17]. In recent years, metal–organic frameworks (MOFs) and Schiff base compounds have attracted much attention in the field of functional materials due to their unique porous structure, high specific surface area, and designability [18,19,20,21,22].
It has been revealed that MOF materials can synergistically enhance corrosion inhibition performance through physical adsorption and chemical bonding [19,20]. The application of MOFs in the field of corrosion inhibition can hinder the penetration of corrosive media by forming a dense protective layer on the metal surface through physical or chemical adsorption [23,24]. It was found that MOFs synthesized from different metal precursors have enhanced corrosion resistance for mild steel in acidic media [24]. The structural properties of MOFs enable them to effectively trap and segregate corrosive ions, which further enhances the corrosion inhibition effect [23]. For example, excellent impedance matching can be achieved through compositional modulation engineering, and rich heterogeneous interfaces can be constructed to realize long-term corrosion protection [25].
Schiff base compounds have been widely used as metal corrosion inhibitors because of their ease of synthesis, variety of molecular structures, and the presence of heteroatoms (i.e., N, O, S) [26,27,28,29,30]. They usually form coordination bonds between the lone pair of electrons in the molecule and the empty orbitals on the metal surface, adsorb on the metal surface, and form a monomolecular or multimolecular protective film, thus inhibiting the electrochemical reaction [26,27,28,31]. Phenolic Schiff bases showed effective corrosion inhibition properties on mild steel in acidic environments [26]. Thiophene-based chitosan Schiff bases also exhibited corrosion inhibition in 1 M HCl solution [15]. Meanwhile, the corrosion inhibition efficiency of Schiff base compounds is closely related to its molecular structure, substituent type and position, concentration and ambient temperature, etc. [29,32].
The introduction of a Schiff base structure into chitosan chain through a Schiff base reaction could endow chitosan with better metal coordination ability and adsorption properties [33,34,35]. Through combining chitosan or Schiff base-modified chitosan with metal ions or MOFs, it is expected to construct composite corrosion inhibitors with synergistic effects, integrating the environmental friendliness of biopolymers, the porous structure of MOFs, and the strong adsorption ability of Schiff base to realize more efficient and long-lasting corrosion inhibition effects [33,36,37,38].
In this paper, two types of corrosion inhibitors, a chitosan–copper metal–organic framework (CS@Cu MOF) and a chitosan–Schiff Base–copper functional material (chitosan–3-pyridinecarbaldehyde Schiff Base–CuSO4 complex, Schiff–CS@Cu), were innovatively designed and synthesized, and their corrosion inhibition behaviors were systematically investigated on Q345 steel in a mixture of 3.5% NaCl and 1 mol/L HCl. Through the weight loss method, corrosion morphology characterization, and corrosion inhibition efficiency calculation, the concentration–performance correlation law was revealed, and the differences in the mechanism of action of the two types of corrosion inhibitors were compared, which provided a theoretical basis and technical path for the development of highly efficient and environmentally friendly protective materials for steel structures.
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Steel Specimens
Q345 low-alloy high-strength steel (composition: C 0.18%, Mn 1.50%, Si 0.55%, P ≤ 0.025%, S ≤ 0.020%, Fe balance) was machined into rectangular plates (30 mm × 20 mm × 3 mm). Prior to testing, all specimens were sequentially polished with 400–2000 grit SiC abrasive paper to achieve a mirror finish (Ra ≤ 0.1 μm), degreased ultrasonically in acetone for 15 min, and dried under nitrogen flow. The specimen image is depicted in Figure 1.
Figure 1.
The images of steel specimen.
2.1.2. Corrosion Inhibitors
- (a)
- Chitosan–copper MOF (CS@CU MOF): A chitosan–copper precursor solution was initially prepared by completely dissolving 2.0 g of chitosan powder in 50 mL of a 1 wt% acetic acid aqueous solution. This dissolution occurred under constant magnetic agitation at 400 rpm and 25 °C for 12 h. Subsequently, 5.7 g of Cu(NO3)2·3H2O was introduced into this homogeneous mixture, with stirring maintained at 400 rpm for an additional 2 h to achieve full complexation. The resulting solution underwent controlled gelation by careful dripping into a 3 M NaOH solution held at 25 °C. Following a 6 h reaction period, this process yielded spherical porous beads, which were then collected via vacuum filtration. A rigorous purification protocol ensued, involving rinsing with distilled water until neutral pH was attained, followed by sequential 20 min ultrasonication treatments in ethanol/water solutions with progressively increasing ethanol concentrations (10/90, 30/70, 50/50, 70/30, and 90/10 v/v), culminating in a final 20 min immersion in anhydrous ethanol to stabilize the pore structure. To induce MOF crystallization, the purified beads were immersed in a 0.16 g/L dimethylimidazole (DMIM) ethanol solution and reacted at 40 °C for 24 h under a nitrogen atmosphere. The final CS@Cu MOF material was obtained after vacuum drying at 60 °C for 12 h [39,40].
- (b)
- Chitosan Schiff base (chitosan–3-pyridinecarbaldehyde Schiff base–CuSO4) complex (Schiff–CS@Cu): The preparation of chitosan–Schiff base–copper functional material (Schiff–CS@Cu) involves a two-step procedure. Initially, chitosan powder was dissolved in acetic acid solution to form a homogeneous system, which was then mixed with 3-pyridinecarboxaldehyde in anhydrous ethanol. The mixture underwent 12 h reflux condensation at 75 °C to form Schiff base linkages. The resultant Schiff–CS product was purified through sequential ethanol washing until colorless filtrate and deionized water rinsing to neutrality, followed by vacuum drying at 50 °C. Subsequently, the Schiff–CS material was immersed in saturated copper sulfate solution under 50 °C stirring for 6 h to achieve copper ion adsorption. After filtration and thorough washing to remove unbound ions, the final Schiff–CS@Cu composite was obtained through 12 h drying at 50 °C. This methodology integrates covalent modification through Schiff base formation with subsequent metal ion coordination, demonstrating the effective integration of organic–inorganic hybrid functionalities [41,42].
2.1.3. Corrosive Medium
A simulated industrial marine environment was prepared by mixing 3.5 wt% NaCl (analytical grade) and 1 mol/L HCl (37% purity), yielding a solution with pH = 1.2 ± 0.1 (measured by pH meter, Mettler Toledo, Changzhou, China) [43].
2.2. Testing Methods
2.2.1. Experimental Design
Six inhibitor concentrations (30, 50, 80, 100, 200, 300 mg/L) were tested for each inhibitor type, with triplicate specimens per group. Control groups (no inhibitor) were included for baseline comparison. Specimens were coded as M-30 to M-300 (CS@Cu MOF) and X-30 to X-300 (Schiff–CS@Cu), corresponding to their concentrations.
2.2.2. Corrosion Testing
In this study, different concentrations of corrosion inhibitors were first added to the acidic corrosion medium (3.5 wt% NaCl (analytical grade) and 1 mol/L HCl (37% purity)) to form a mixed solution, and subsequently, the Q345 steel samples were immersed to a corrosion chamber configured with the above mixed solution and were subjected to a continuous corrosion process under the given conditions (25 ± 0.5 °C, 95 ± 2% RH) for 4 h. Post-corrosion, specimens were subjected to derusting per “Corrosion of metals and alloys: Removal of corrosion products from corroded specimens” (Chinese industry standard, GB/T 16545-2015) [44].
In detail, the specimen was firstly immersed in 10% HCl + 3.5 g hexamethylenetetramine solution (1 L) for 5 min to remove corrosion products. Then, it was ultrasonically cleaned in deionized water (10 min) and absolute ethanol (5 min). Finally, it was dried at 50 °C for 2 h and weighed (analytical balance, ±0.1 mg, Sartorius CPA225D, Changzhou, China).
2.2.3. Data Analysis
Assuming that the specimen was uniformly corroded, the corrosion rate, v (g/(m2·h)) can be expressed as the weight loss of the metal after corrosion per unit of time and per unit of area, which was calculated as shown in Equation (1):
where m1 stands for the mass of the specimen before corrosion, g;
m2 is the mass of the specimen after corrosion, g;
S refers to the exposed area of the specimen, m2;
t denotes the time of exposure, h.
The corrosion inhibition rate, η (%), was calculated in Equation (2):
where v0 and v1 denote corrosion rates of control and inhibited groups, respectively.
Statistical significance was assessed via one-way ANOVA (p < 0.05) using OriginPro 2018.
3. Results and Discussion
3.1. Corrosion Inhibition Effect
According to the data presented in Table 1, the corrosion rate of the blank control group (B-0) reached 25.889 g/(m2·h), indicating a severe degradation of the metal substrate in the corrosive medium. In contrast, experimental groups incorporating corrosion inhibitors at varying concentrations demonstrated remarkable improvements. The CS@Cu MOF group exhibited a corrosion rate which reduced to 2.000~3.167 g/(m2·h), representing a substantial reduction of 87.74~92.27%, while the Schiff–CS@Cu group showed a further decrease to 1.333~2.833 g/(m2·h), corresponding to an impressive inhibition efficiency of 89.06~94.85%. This concentration-dependent inhibitory effect confirms that both organic–inorganic composite corrosion inhibitors effectively interrupted the corrosion process through synergistic mechanisms. Specifically, the CS@Cu MOF likely utilizes the porous structure of the metal–organic framework to adsorb corrosive ions and establish a physical barrier layer, whereas the Schiff base groups in Schiff–CS@Cu form a chemisorbed protective layer on the metal surface via coordination interactions. These dual mechanisms collaboratively suppress both anodic metal dissolution and cathodic oxygen reduction reactions.

Table 1.
Corrosion rates of CS@Cu MOF and Schiff–CS@Cu groups based on mass loss method.
As illustrated in Figure 2a, the corrosion behavior of the CS@Cu MOF group exhibited a distinct non-monotonic concentration dependence. At 80 mg/L, the corrosion rate unexpectedly increased to 3.167 g/(m2·h), suggesting a critical threshold where insufficient inhibitor coverage or competitive adsorption between inhibitor molecules and corrosive species might compromise protection efficiency. However, at elevated concentrations (100–300 mg/L), the corrosion rates progressively decreased, reaching a minimum of 2.000 g/(m2·h) at 300 mg/L. This recovery implies that higher concentrations enable complete surface coverage through multilayer adsorption, effectively blocking active corrosion sites. In contrast, the Schiff–CS@Cu group demonstrated a linearly decreasing corrosion rate (slope k = −0.00167, R2 = 1) across the tested concentration range (100~300 mg/L), achieving optimal protection (1.333 g/(m2·h)) at 300 mg/L. This linear trend highlights its superior dose-responsive behavior, likely attributed to the Schiff base’s strong chemisorption capability that ensures uniform monolayer formation even at lower concentrations.
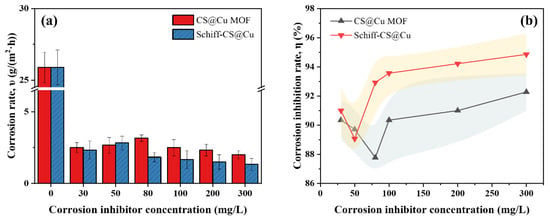
Figure 2.
Corrosion rate and corrosion inhibition rate of samples with different corrosion inhibitors: (a) corrosion rate, (b) corrosion inhibition rate.
Furthermore, the corrosion inhibition efficiency (η, %) was quantitatively evaluated using Equation (2). As depicted in Figure 2b, both inhibitors showed concentration-dependent η enhancement. CS@Cu MOF exhibited fluctuating efficiency (peak η = 92.27% at 300 mg/L), aligning with its non-monotonic corrosion rate profile, while Schiff–CS@Cu displayed near-linear efficiency growth (η = 94.85% at 300 mg/L). The divergence in η trends underscores mechanistic differences: CS@Cu MOF’s porous framework may require critical concentration for optimal pore filling and ion trapping, whereas Schiff–CS@Cu’s planar molecular geometry facilitates rapid surface passivation through covalent bonding.
From Figure 2b, it can be found that CS@Cu MOF has the highest corrosion inhibition efficiency (η = 92.27%) at 300 mg/L, while Schiff–CS@Cu has a η of 94.85% at 300 mg/L, which is better than the former. Specifically, for the CS@Cu MOF group, the corrosion inhibition rate showed a “U-shape” change with the increase in concentration; the trough of the corrosion inhibition efficiency (η = 87.77%) occurred at 80 mg/L, which was mainly due to the fact that CS@Cu MOF physically adsorbed Cl− and H+, while Cu2+ formed microcells with Fe atoms on the steel surface, promoting the oxidation of Fe2+ to Fe3+ and generating dense oxide films [45] (e.g., FeOOH) to inhibit anodic dissolution [18,19]. However, the MOF particles may agglomerate due to van der Waals forces at 80 mg/L [19], resulting in a decrease in the effective adsorption area and a decrease in corrosion inhibition efficiency.
As for the Schiff–CS@Cu group, the corrosion inhibition rate increased approximately monotonically with the increase in concentration, and η peaked at 300 mg/L, indicating a more stable corrosion inhibition performance. It was attributed to the fact that the pyridine ring in the chitosan Schiff bases chelated with Cu2+ to form a stable ligand layer, which was chemically bonded to Fe atoms on the steel surface via the N and O heteroatoms [21]. It constructed a bilayer barrier to block the penetration of corrosive media [22]. Its corrosion inhibition efficiency increased linearly with concentration, indicating that the coordination film coverage was positively correlated with the corrosion inhibition performance.
It should be noted that once the concentration of the two inhibitors exceeded 100 mg/L, the growth rate of their corrosion inhibition efficiencies slowed down, which may imply the approaching of their respective critical effects of corrosion inhibition.
3.2. Corrosion Morphology Analysis
3.2.1. The Morphology Under the Same Corrosion Inhibitors Concentration
Figure 3 present the corrosion morphologies of Q345 steel specimens with an inhibitor concentration of 80 mg/L. The distinct differences in surface degradation between CS@Cu MOF and Schiff–CS@Cu groups highlighted their contrasting inhibition mechanisms.
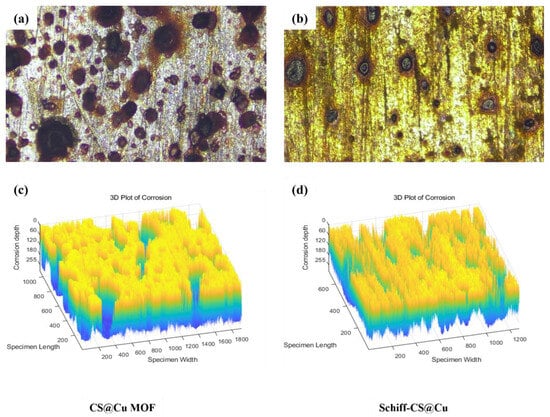
Figure 3.
Micro-corrosion morphology of Q345 steel specimens with corrosion inhibitor concentration of 80 mg/L at 20× magnification: (a,c) CS@Cu MOF; (b,d) Schiff–CS@Cu MOF.
As shown in Figure 3a,c, the CS@Cu MOF-treated specimen exhibited widespread pitting corrosion with irregularly distributed pits at 20× magnification, which indicated insufficient barrier protection. This aligned with the elevated corrosion rate (v = 3.167 g/(m2·h)) observed at this concentration. Meanwhile, Figure 3b,d presents the Schiff–CS@Cu group shallow pits and sparse corrosion products at 80 mg/L, which explained the lower corrosion rate (v = 1.833 g/(m2·h)) despite there being a suboptimal concentration.
3.2.2. The Morphology at Lower Corrosion Inhibition Effect
The corrosion morphology of Q345 steel samples under the worst corrosion inhibition effect of the two corrosion inhibitors was presented in Figure 4. In this case, the concentrations of CS@Cu MOF and Shichill-CS@Cu were 80 mg/L and 50 mg/L, respectively, which originated from the test results in Figure 2.
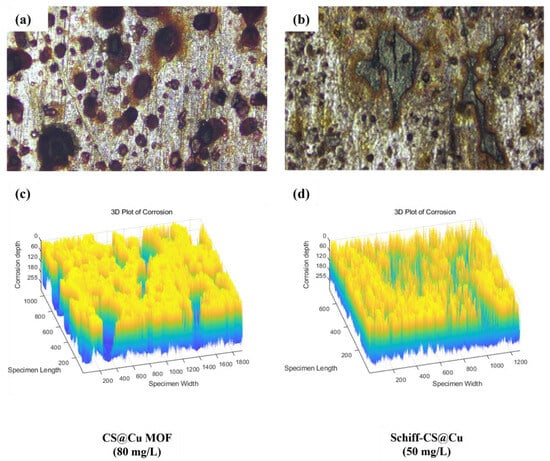
Figure 4.
Micro-corrosion morphology of Q345 steel specimens with lower corrosion inhibition effect at 20× magnification: (a,c) CS@Cu MOF; (b,d) Schiff–CS@Cu MOF.
From Figure 4a,c, it can be seen that the black corrosion spots on the surface of the steel samples at a CS@Cu MOF concentration of 80 mg/L were denser and larger in area, and accompanied by deeper corrosion pits. However, Figure 4b,d confirms that the number and area of black spots on the sample surface at Schiff–CS@Cu concentration of 50 mg/L were smaller, and the depth of corrosion was shallower (except for a few lumpy and shallow corrosion pits) compared to CS@Cu MOF. This phenomenon indicated that the corrosion inhibition effect of Schiff–CS@Cu on the steel was superior to that of CS@Cu MOF in the respective most unfavorable corrosion inhibition effect, which was consistent with the conclusion of Section 3.1.
The corrosion inhibition performance of CS@Cu MOF and Schiff–CS@Cu composites was evaluated by quantifying corrosion areas at different magnifications (20× and 50×), as presented in Table 2.

Table 2.
Corrosion area of the samples under the effect of different corrosion inhibitors.
The corrosion area of CS@Cu MOF at 80 mg/L was 833,361 and 1,420,465 pixels at 20× and 50×, respectively, which was significantly higher than that of Schiff–CS@Cu at the same concentration of 118,731 and 28,033 pixels. In addition, the corrosion area of Schiff–CS@Cu at 50 mg/L increased to 239,232 and 472,139 pixels. This indicated that the corrosion inhibition effect of Schiff–CS@Cu was superior to that of CS@Cu MOF, especially at high concentrations, and the effect was weakened when the concentration decreased.
The high corrosion area of CS@Cu MOF at 80 mg/L might be due to the aggregation of particles, which resulted in the reduction in the effective surface area and the inability to effectively adsorb corrosive ions. Meanwhile, the porous structure of MOF may adsorb the electrolytes and promote localized corrosion instead [18]. The microcell effect (coupling of Cu2+ to Fe) mentioned in the literature may have exacerbated the localized corrosion [19].
The excellent performance of Schiff–CS@Cu at 80 mg/L may have been due to the formation of a dense chemisorption film between Schiff base and Cu2+, which effectively blocked the corrosive medium. Conversely, the membrane coverage was incomplete when the concentration was reduced to 50 mg/L, resulting in an increase in the corrosion area [20,21,22].
The above analysis revealed that CS@Cu MOF mainly relied on physical adsorption, while Schiff–CS@Cu formed a protective film through chemical coordination, and the latter was more stable and effective in protecting steel.
4. Conclusions
This study focused on the corrosion inhibition performance of chitosan MOF (CS@Cu MOF) and chitosan Schiff base (Schiff–CS@Cu) on Q345 steel in corrosive solution. The corrosion inhibition effect of the two corrosion inhibitors was comparatively analyzed through the design of different concentrations, and the following main conclusions were obtained:
- (1)
- Chitosan MOF (CS@Cu MOF) and chitosan Schiff base (Schiff–CS@Cu) have a favorable corrosion inhibition effect on Q345 steel; the concentration of 100 mg/L is recommended, considering its efficiency and economy.
- (2)
- Schiff–CS@Cu has better corrosion inhibition performance than physical adsorption-dominated CS@Cu MOF due to the chemical ligand-dominated dense film layer.
The dispersibility of MOF needs to be improved by surface functionalization or the development of a MOF-Schiff base composite system to synergistically enhance the corrosion inhibition performance.
Author Contributions
Conceptualization, L.H.; Methodology, J.L.; Software, X.W.; Validation, L.W.; Formal analysis, B.L.; Investigation, L.H.; Data curation, L.W., B.L. and X.W.; Writing – original draft, J.L.; Writing – review & editing, L.H.; Supervision, S.K. and L.Z.; Funding acquisition, L.H. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by Natural Science Foundation of Hubei Province (2022CFB547, 2023AFA108), Key Project of Science and Technology Research of Hubei Provincial Department of Education (D20232702, D20232701), and Natural Science Foundation of Ningxia Province (2024AAC03316).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simoncelli, M.; Aloisio, A.; Zucca, M.; Venturi, G.; Alaggio, R. Intensity and location of corrosion on the reliability of a steel bridge. J. Constr. Steel Res. 2023, 206, 107937. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, Q.; Zhang, D.; Lao, W.; Wu, L.; Jiang, Z. Monitoring and detection of steel bridge diseases: A review. J. Traffic Transp. Eng. (Engl. Ed.) 2024, 11, 188–208. [Google Scholar] [CrossRef]
- Bao, A.; Guillaume, C.; Satter, C.; Moraes, A.; Williams, P.; Kelly, T.; Guo, Y. Testing and evaluation of web bearing capacity of corroded steel bridge girders. Eng. Struct. 2021, 238, 112276. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Corrosion inhibitors: Natural and synthetic organic inhibitors. Lubricants 2023, 11, 174. [Google Scholar] [CrossRef]
- Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Redkina, G.V. Thin protective coatings on metals formed by organic corrosion inhibitors in neutral media. Coatings 2022, 12, 149. [Google Scholar] [CrossRef]
- Lai, X.; Hu, J.; Ruan, T.; Zhou, J.; Qu, J. Chitosan derivative corrosion inhibitor for aluminum alloy in sodium chloride solution: A green organic/inorganic hybrid. Carbohydr. Polym. 2021, 265, 118074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Rajesh, T.; Obot, I.B.; Bin Sharfan, I.I.; Abdulhamid, M.A. Water-soluble chitosan salt as ecofriendly corrosion inhibitor for N80 pipeline steel in artificial sea water: Experimental and theoretical approach. Int. J. Biol. Macromol. 2024, 254, 127697. [Google Scholar] [CrossRef]
- Dou, X.; Fan, N.; Yang, J.; Zhang, Z.; Wu, B.; Wei, X.; Shi, S.; Zhang, W.; Feng, Y. Research progress on chitosan and its derivatives in the fields of corrosion inhibition and antimicrobial activity. Environ. Sci. Pollut. Res. 2024, 31, 30353–30369. [Google Scholar] [CrossRef]
- Farag, A.A.; Al-Shomar, S.M.; Abdelshafi, N.S. Eco-friendly modified chitosan as corrosion inhibitor for carbon steel in acidic medium: Experimental and in-depth theoretical approaches. Int. J. Biol. Macromol. 2024, 279, 135408. [Google Scholar] [CrossRef]
- Pawariya, V.; De, S.; Dutta, J. Chitosan-based Schiff bases: Promising materials for biomedical and industrial applications. Carbohydr. Polym. 2024, 323, 121395. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Mathew, Z.P.; Augustine, C.; George, J.B.; Joseph, B.; Josh, M.S. Corrosion inhibition of mild steel in 1 M HCl using water soluble chitosan derivative of vanillin. Int. J. Biol. Macromol. 2024, 262, 130024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Xu, N.; Jiang, Z.N.; Liu, H.; Zhang, G. Chitosan derivatives as promising green corrosion inhibitors for carbon steel in acidic environment: Inhibition performance and interfacial adsorption mechanism. J. Colloid Interface Sci. 2023, 640, 1052–1067. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, B.; Li, J.; Zhou, C.; Guo, M.; Peng, K.; Dai, H.; Lan, B.; Xiong, W.; Liu, Y. Zwitterion modified chitosan as a high-performance corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Biol. Macromol. 2024, 267, 131429. [Google Scholar] [CrossRef]
- Menaka, R.; Nijarubini, V.; Sakthivel, K.; Kavitha, P.; Firdhouse, M.J. Electrochemical investigation and surface interaction of thiophene based Chitosan Schiff base on mild steel surface in 1M HCl. Mater. Today Proc. 2024, 88, 684–692. [Google Scholar] [CrossRef]
- Sun, A.; Cui, G.; Liu, Q. Capsule corrosion inhibitor loaded with hyperbranched chitosan: Carbon dioxide corrosion protection for downhole pipelines in oil fields. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131106. [Google Scholar] [CrossRef]
- Dalhatu, S.N.; Modu, K.A.; Mahmoud, A.A.; Zango, Z.U.; Umar, A.B.; Usman, F.; Dennis, J.O.; Alsadig, A.; Ibnaouf, K.H.; Aldaghri, O.A. L-arginine grafted chitosan as corrosion inhibitor for mild steel protection. Polymers 2023, 15, 398. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and applications of metal-organic frameworks (MOFs) in emerging technologies: A comprehensive review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Poonia, K.; Patial, S.; Raizada, P.; Ahamad, T.; Khan, A.A.P.; Van Le, Q.; Nguyen, V.-H.; Hussain, C.M.; Singh, P. Recent advances in Metal Organic Framework (MOF)-based hierarchical composites for water treatment by adsorptional photocatalysis: A review. Environ. Res. 2023, 222, 115349. [Google Scholar] [CrossRef]
- Shahraki, S. Schiff base compounds as artificial metalloenzymes. Colloids Surf. B Biointerfaces 2022, 218, 112727. [Google Scholar] [CrossRef] [PubMed]
- Afshari, F.; Ghomi, E.R.; Dinari, M.; Ramakrishna, S. Recent advances on the corrosion inhibition behavior of schiff base compounds on mild steel in acidic media. ChemistrySelect 2023, 8, e202203231. [Google Scholar] [CrossRef]
- Ramu, A.G.; Yang, D.; Song, M.; Choi, D. Advancements in integrating MOFs into micro-arc oxidation coatings on Mg alloys: A perspective on PEO-MOF coatings as innovative corrosion inhibitors. J. Magnes. Alloys 2024, 12, 4363–4394. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; El-Shahat, M.; Abdelhameed, R.M. Role of different metal precursors based MOFs for boosting anti-corrosion performance of mild steel in acid media. Mater. Today Sustain. 2023, 23, 100460. [Google Scholar] [CrossRef]
- Guo, Z.; Lan, D.; Jia, Z.; Gao, Z.; Shi, X.; He, M.; Guo, H.; Wu, G.; Yin, P. Multiple Tin Compounds Modified Carbon Fibers to Construct Heterogeneous Interfaces for Corrosion Prevention and Electromagnetic Wave Absorption. Nano-Micro Lett. 2024, 17, 23. [Google Scholar] [CrossRef]
- Lahhit, A.; Azghay, I.; Elyoussfi, A.; Ghalit, M.; Ouzidan, Y.; El Massaoudi, M.; Mourabit, F.; Akichouh, E.H.; Ahari, M.; Amhamdi, H.; et al. Exploring the potent corrosion inhibition properties of phenolic Schiff bases on mild steel in acidic environments, part A: Coupling experimental and theoretical investigations. Environ. Sci. Pollut. Res. 2024, 31, 63652–63670. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, C.P.; Boekom, E.J.; Akpan, G.J.; Asuquo, I.G.; Edet, E.K.; Jacob, A.E. Computational Modelling and Comparative Analysis of a Schiff Base Ligand and Its Analog as Inhibitors Against Mild Steel Corrosion in 1M HCl. J. Sustain. Mater. Process. Manag. 2024, 4, 71–88. [Google Scholar] [CrossRef]
- Iranpour, M.; Babaei, A.; Bagherzadeh, M. Corrosion Inhibition of Carbon Steel by Some Schiff Base Compounds in HCl and H2SO4 Solutions. ChemistrySelect 2024, 9, e202305180. [Google Scholar] [CrossRef]
- Jafari, H.; Ameri, E.; Vakili, M.H.; Berisha, A. Effect of OH position on adsorption behavior of Schiff-base derivatives in corrosion inhibition of carbon steel in 1 M HCl. Electrochem. Commun. 2024, 159, 107653. [Google Scholar] [CrossRef]
- Shashirekha, K.; Aithal, S.; Praveen, B.M.; Pavithra, M.K.; Guruprasad, A.M.; Devendra, B.K.; Rathod, M.R. Experimental, electrochemical and DFT simulation studies of a novel Schiff base derivative as an efficient mild steel corrosion inhibitor in acidic environments. Results Surf. Interfaces 2024, 16, 100246. [Google Scholar] [CrossRef]
- Azzouzi, M.; Daoudi, W.; Dagdag, O.; Berisha, A.; Kim, H.; Oussaid, A.; El Aatiaoui, A.; Oussaid, A. Corrosion inhibition and in silico toxicity assessment of imidazo[1,2-a]pyrimidine-Schiff base derivatives as effective and environmentally friendly corrosion inhibitors for mild steel. RSC Adv. 2025, 15, 12342–12363. [Google Scholar] [CrossRef] [PubMed]
- Soliz, A.; Zamora, P.P.; Muena, J.P.; Bieger, K.; Haribabu, J.; Landaeta, E.; Arulraj, A.; Mangalaraja, R.V. Experimental and DFT analysis of the concentration dependency of corrosion inhibition by a pyridine Schiff base for mild steel in HCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135283. [Google Scholar] [CrossRef]
- El Mahamdi, M.; Daoudi, W.; Naguib, I.A.; Benhadi, L.; Dagdag, O.; Berisha, A.; Kim, H.; Noureddine, B.; El Aatiaoui, A. Enhanced corrosion protection of copper in saline environments using bio-nanocomposite coatings based on chitosan and chitosan Schiff base. Int. J. Biol. Macromol. 2024, 282, 136702. [Google Scholar] [CrossRef]
- Çelikçi, N.; Zıba, C.A.; Tümer, M. Chitosan-Based Schiff Base Compounds: Synthesis, Chemical Characterization and Antibacterial Properties. J. Fluoresc. 2025, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Li, F.; Hu, J.; Cui, N.; Su, H.; Li, L.; Wang, Z.; Sun, S.; Hu, S. Preparation and corrosion inhibition mechanism of a chitosan ionic liquid Schiff base for the protection of N80 in HCl solution. New J. Chem. 2024, 48, 3064–3079. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, C.; Shen, P.; Liu, Z.; Hu, J.; Zhang, Y. Facile synthesis of recyclable Mn-crosslinked chitosan schiff base composites as heterogeneous catalysts for selective oxidation of methyl phenyl sulfide with H2O2. Int. J. Biol. Macromol. 2025, 311, 144119. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Quraishi, M.A.; Alfantazi, A.; Rhee, K.Y. Corrosion inhibition potential of chitosan based Schiff bases: Design, performance and applications. Int. J. Biol. Macromol. 2021, 184, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Miladi, R.; Salahshoori, I.; Golriz, M.; Raji, M.; Ranjbarzadeh-Dibazar, A.; Naderi, G.; Khonakdar, H.A. Computational insights into pectin and chitosan-enhanced MOFs: A green pathway for pollutant remediation. Process Saf. Environ. Prot. 2024, 192, 862–877. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akitsu, T.; Furuya, M.; Sekiguchi, T.; Shoji, S.; Tanii, T.; Tanaka, D. Efficient Synthesis of a Schiff Base Copper(II) Complex Using a Microfluidic Device. Micromachines 2023, 14, 890. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Li, B.; Zhao, X.; Cai, Z.; Wang, X.; Zuo, Z.; Liu, H.; Zhu, L. Chitosan-copper MOF for corrosion inhibition of Q345 steel: Performance, mechanism, and temperature effects. Mater. Des. 2025, 255, 114227. [Google Scholar] [CrossRef]
- Telmenbayar, L.; Gopal Ramu, A.; Yang, D.; Choi, D. Development of mechanically robust and anticorrosion slippery PEO coating with metal–organic framework (MOF) of magnesium alloy. Chem. Eng. J. 2023, 458, 141397. [Google Scholar] [CrossRef]
- Umoren, S.A.; Banera, M.J.; Alonso-Garcia, T.; Gervasi, C.A.; Mirífico, M.V. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013, 20, 2529–2545. [Google Scholar] [CrossRef]
- Costa, T.G.; Cunha Ostroski, V.W.; de Souza, F.S. “Self arranged Cactis” as new goethite morphology from the natural corrosion process of SAE 1020 carbon steel. Heliyon 2019, 5, e02771. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16545-2015; Corrosion of Metals and Alloys. Removal of Corrosion Products from Corrosion Test Specimens. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2015.
- de Souza, F.S.; Costa, T.G.; Feldhaus, M.J.; Szpoganicz, B.; Spinelli, A. Nonenzymatic Amperometric Sensors for Hydrogen Peroxide Based on Melanin-Capped Fe3+, Cu2+, or Ni2+ Modified Prussian Blue Nanoparticles. IEEE Sens. J. 2015, 15, 4749–4757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).