Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes
Abstract
1. Introduction
2. Results and Discussion
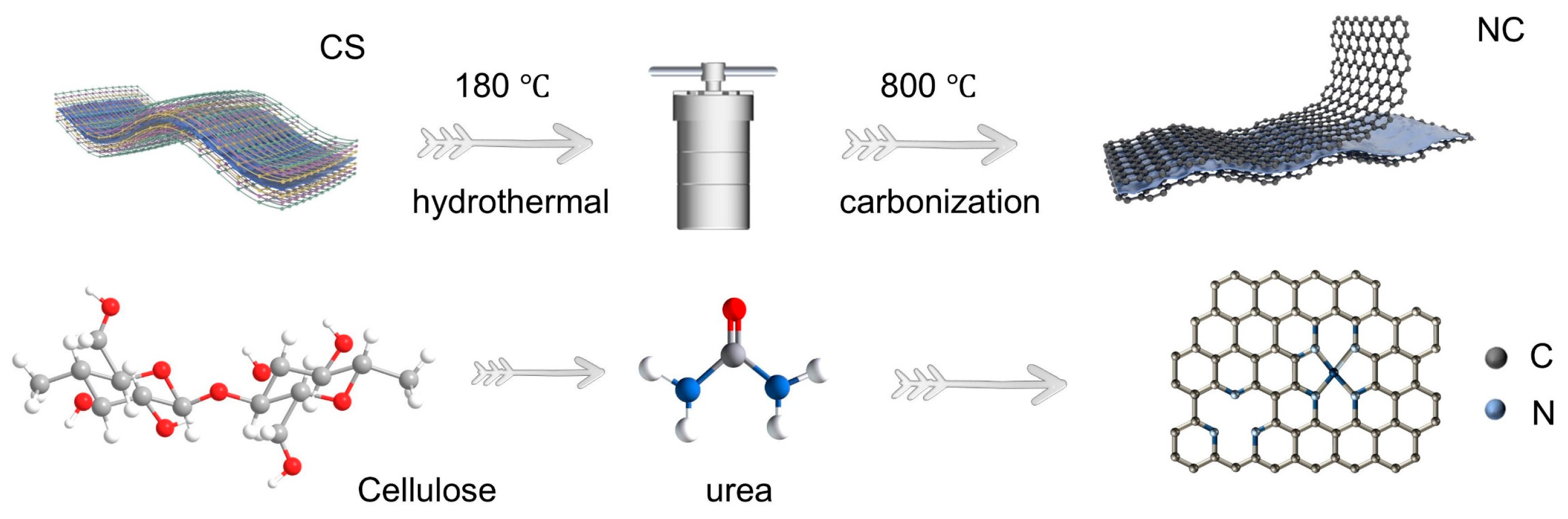
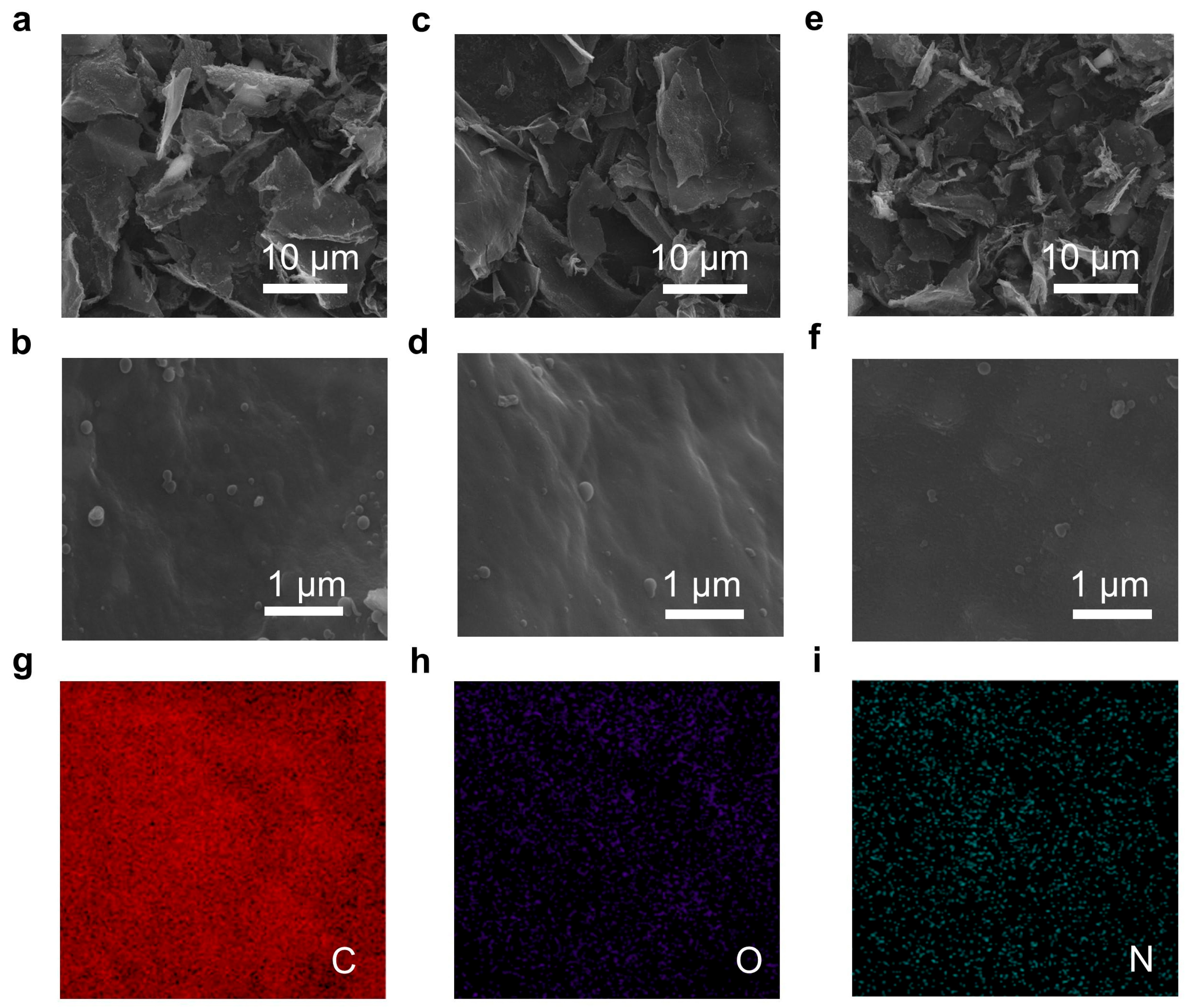
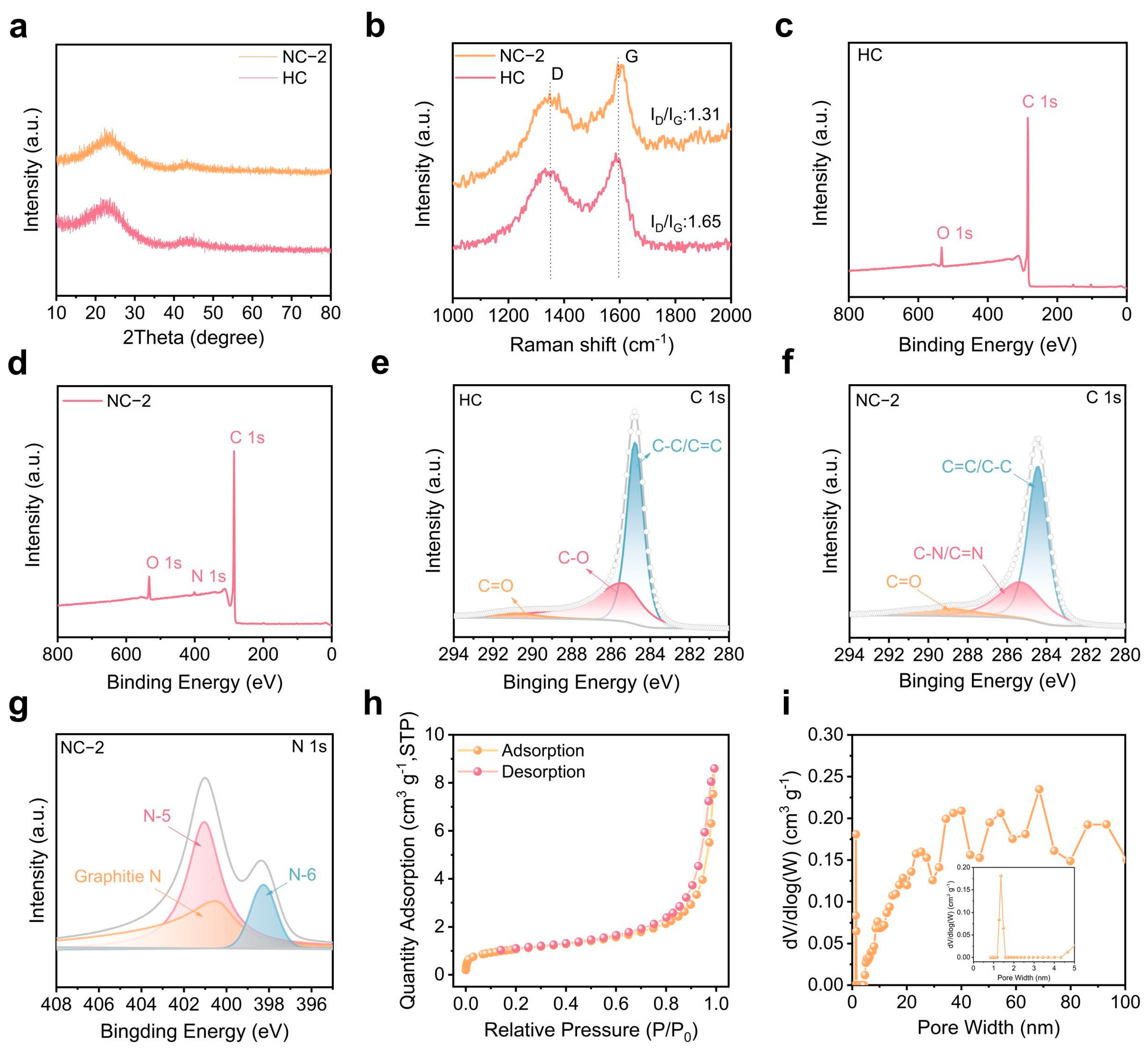
2.1. Structural Evolution and Chemical Modulation of N-Doped Carbon (NC)
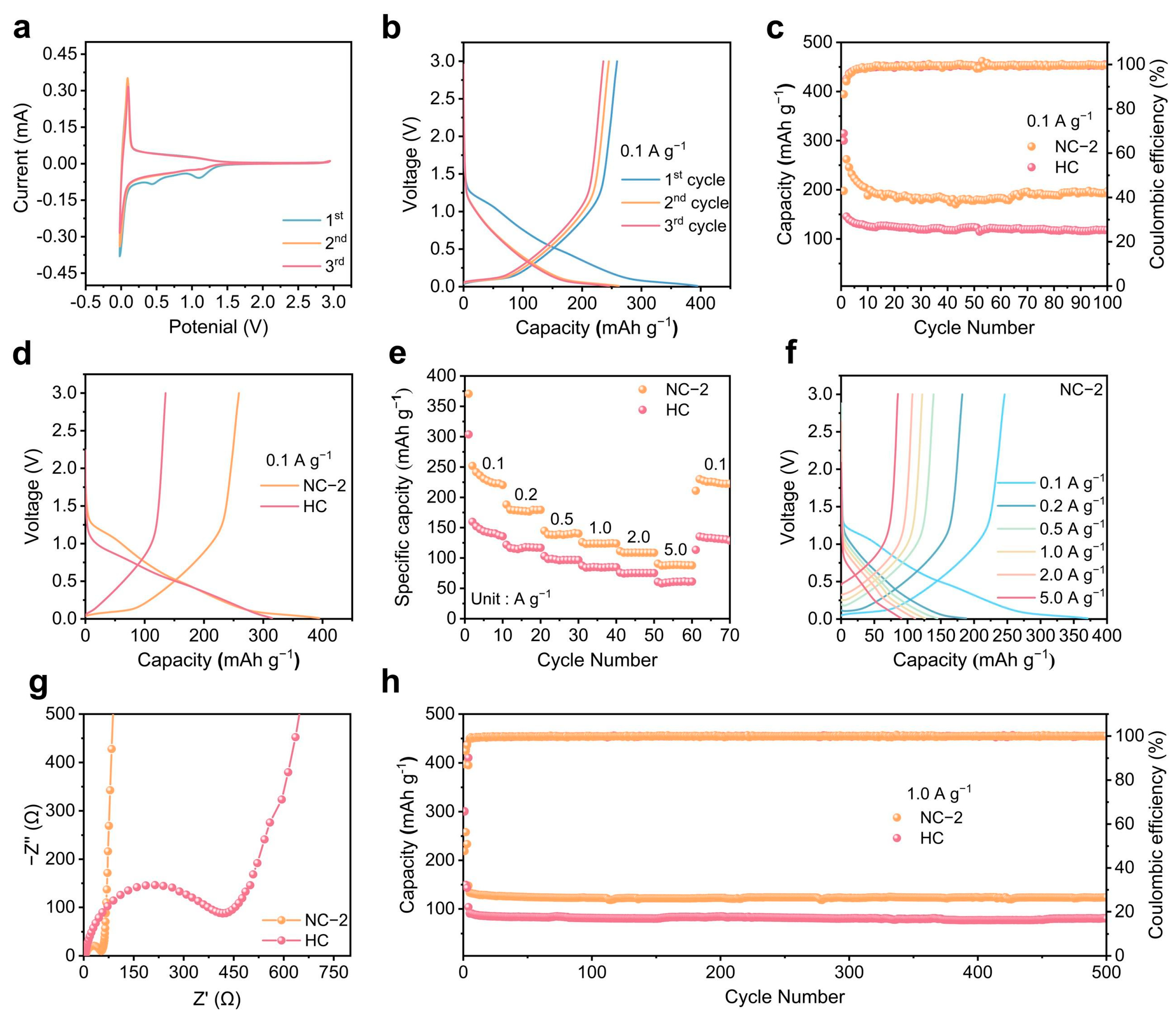
2.2. Electrochemical Performance of the NC Anode of SIBs
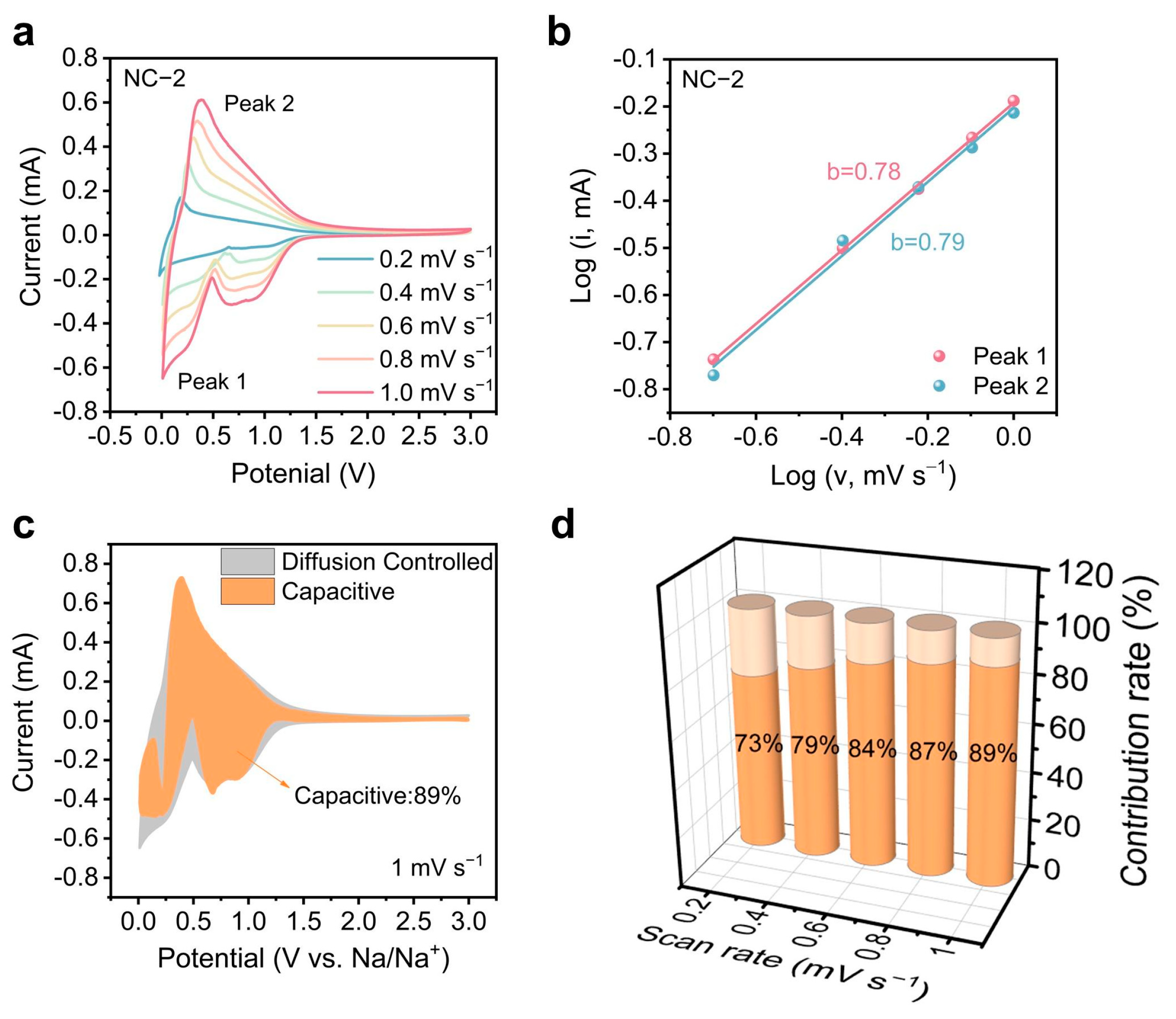
2.3. Elucidating Sodium Storage Mechanisms Through Kinetic Analysis
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 94–143. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard carbon anodes for next-generation Li-ion batteries: Review and perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Zhang, H.; Liu, Y.; Yang, L.; Li, F.; Liu, W.; Dong, X.; Li, X.; Li, Z.; et al. Revealing the multiple cathodic and anodic involved charge storage mechanism in an FeSe2 cathode for aluminium-ion batteries by in situ magnetometry. Energy Environ. Sci. 2022, 15, 311–319. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, L.; Lu, J. The Tuning of Strain in Layered Structure Oxide Cathodes for Lithium-Ion Batteries. Research 2024, 7, 0489. [Google Scholar] [CrossRef]
- Xiang, L.; Li, X.; Xiao, J.; Zhu, L.; Zhan, X. Interface issues and challenges for NASICON-based solid-state sodium-metal batteries. Adv. Powder Mater. 2024, 3, 100181. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; de Larramendi, I.R.; Guo, X.; Wang, G.; Rojo, T. Na-ion batteries-approaching old and new challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Yuan, M.; Meng, C.; Li, A.; Cao, B.; Dong, Y.; Wang, D.; Liu, X.; Chen, X.; Song, H. A general multi-interface strategy toward densified carbon materials with enhanced comprehensive electrochemical performance for Li/Na-ion batteries. Small 2022, 18, 2105738. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rogach, A.L. Anodes and sodium-free cathodes in sodium ion batteries. Adv. Energy Mater. 2020, 10, 2000288. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Liu, L.; Du, Z.; Wang, J.; Du, H.; Wu, S.; Li, M.; Zhang, Y.; Sun, J.; Sun, Z.; Ai, W. Fast-Charging Sodium-Ion Batteries Enabled by Molecular-Level Designed Nitrogen and Phosphorus Codoped Mesoporous Soft Carbon. Research 2023, 6, 0209. [Google Scholar] [CrossRef]
- Salaheldeen, M.; M. Abu-Dief, A.; El-Dabea, T. Functionalization of Nanomaterials for Energy Storage and Hydrogen Production Applications. Materials 2025, 18, 768. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today. 2019, 23, 87–104. [Google Scholar] [CrossRef]
- He, X.-X.; Zhao, J.-H.; Lai, W.-H.; Li, R.; Yang, Z.; Xu, C.-M.; Dai, Y.; Gao, Y.; Liu, X.-H.; Li, L.; et al. Soft-Carbon-Coated, Free-Standing, Low-Defect, Hard-Carbon Anode to Achieve a 94% Initial Coulombic Efficiency for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 44358–44368. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Ma, S.; Yan, W.; Dong, Y.; Su, Y.; Ma, L.; Li, Y.; Fang, Y.; Wang, B.; Wu, S.; Liu, C.; et al. Recent Advances in Carbon-Based Anodes for High-Performance Sodium-Ion Batteries: Mechanism, Modification and Characterizations. Mater. Today 2024, 75, 334–358. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Wang, Y.; Xu, Z.; Chen, L.; Yang, H. N/P/O co-doped porous carbon derived from agroindustry waste of peanut shell for sodium-ion storage. J. Energy Storage 2024, 100, 113682. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Zhao, Y.; Xu, G.; Zhang, F.; Zhang, X. A sustainable route from corn stalks to N, P-dual doping carbon sheets toward high performance sodium-ion batteries anode. Carbon 2018, 130, 664–671. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Tao, L.; Xu, Z.; Yu, D.; Jin, Q.; Lin, F.; Huang, H. Hydrothermally Assisted Conversion of Switchgrass into Hard Carbon as Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 28461–28472. [Google Scholar] [CrossRef]
- Liu, L.; Tian, Y.; Abdussalam, A.; Gilani, M.R.H.S.; Zhang, W.; Xu, G. Hard carbons as anodes in sodium-ion batteries: Sodium storage mechanism and optimization strategies. Molecules 2022, 27, 6516. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Song, J.; Maulana, A.Y.; Kim, H.; Yun, B.; Gim, H.; Jeong, Y.; An, N.; Futalan, C.M.; Kim, J. N-doped graphitic carbon coated Fe2O3 using dopamine as an anode material for sodium-ion batteries. J. Alloys Compd. 2022, 921, 166082–166103. [Google Scholar] [CrossRef]
- Cui, L.; Tan, C.; Pan, Q.; Huang, Y.; Li, Y.; Wang, H.; Zheng, F.; Li, Q. SnS/Fe7S8 heterojunction embedded in three-dimensional N, S co-doped carbon nanosheets as anode material for sodium-ion batteries with long-term cycle life. Appl. Surf. Sci. 2022, 613, 155992. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Fan, L.; Yan, L.; Wang, Z.; Lei, W.; Ruan, D.; Shi, Z. Boosting the High Capacitance-Controlled Capacity of Hard Carbon by Using Surface Oxygen Functional Groups for Fast and Stable Sodium Storage. ACS Appl. Energy Mater. 2021, 4, 11436–11446. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, J.; Zhang, Y.; Chen, M.; Ji, K.; Wang, C. Facile synthesis of N, P-codoped hard carbon nanoporous microspheres from Lignin for high-performance anodes of sodium-ion batteries. ChemElectroChem 2021, 8, 3544–3552. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Lei, C.; Qiu, H.; Jiang, W.; Sun, X.; Zhang, Y.; He, W. P-Doped Hard Carbon Material for Anode of Sodium Ion Battery was Prepared by Using Polyphosphoric Acid Modified Petroleum Asphalt as Precursor. Electrochim. Acta 2024, 477, 143812. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous—Containing Activated Carbon Derived From Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem.—Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.M.; Shim, J.-J.; Chong, K.F. Cobalt-doped tungsten suboxides for supercapacitor applications. Chem. Eng. J. 2023, 473, 145341. [Google Scholar] [CrossRef]
- Zhong, W.; Cheng, D.; Zhang, M.; Zuo, H.; Miao, L.; Li, Z.; Qiu, G.; Cheng, A.; Zhang, H. Boosting ultrafast and durable sodium storage of hard carbon electrode with graphite nanoribbons. Carbon 2022, 198, 278–288. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Li, S.; Chen, Y.; Wang, G.; Yang, H.; Tian, H. Biomass-derived B/N/P co-doped porous carbons as bifunctional materials for supercapacitors and sodium-ion batteries. J. Mater. Chem. A 2024, 12, 18324–18337. [Google Scholar] [CrossRef]
- Pei, Z.; Meng, Q.; Wei, L.; Fan, J.; Chen, Y.; Zhi, C. Toward efficient and high rate sodium-ion storage: A new insight from dopant-defect interplay in textured carbon anode materials. Energy Storage Mater. 2020, 28, 55–63. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, G.; Zhang, H.; Zhang, Z.; Liu, Y.; Gao, F.; Shang, Z.; Gao, C.; Zhou, Y.; Fu, S.; et al. Soft template-induced self-assembly strategy for sustainable production of porous carbon spheres as anode towards advanced sodium-ion batteries. Chem. Eng. J. 2024, 495, 153646–153656. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zhang, Y.; Zhang, N. Hard Carbon for Sodium Storage: Mechanism and Performance Optimization. Nano Res. 2024, 17, 6038–6057. [Google Scholar] [CrossRef]
- Ma, W.; Yu, L.; Miao, X.; An, X.; Zhang, J.; Kong, Q.; Wang, Q.; Yao, W. Simple Synthesis and Sodium Storage Analysis of Ultra-High Nitrogen-Doped Core-Shell Mesoporous Carbon Nanospheres. Adv. Funct. Mater. 2025, 21, 2421790. [Google Scholar] [CrossRef]
- Xiang, J.; Ma, L.; Sun, Y.; Dong, S.; Xu, Q.; He, X.; Zhou, Y.; Hai, C. Ball-Milling-Assisted N/O Codoping for Enhanced Sodium Storage Performance of Coconut-Shell-Derived Hard Carbon Anodes in Sodium-Ion Batteries. Langmuir 2024, 40, 23853–23863. [Google Scholar] [CrossRef]
- Xu, C.; Yang, W.; Ma, G.; Che, S.; Li, Y.; Jia, Y.; Chen, N.; Huang, G.; Li, Y. Edge-Nitrogen Enriched Porous Carbon Nanosheets Anodes with Enlarged Interlayer Distance for Fast Charging Sodium-Ion Batteries. Small 2022, 18, 2204375. [Google Scholar] [CrossRef]
- Jin, Q.; Li, W.; Wang, K.; Li, H.; Feng, P.; Zhang, Z.; Wang, W.; Jiang, K. Tailoring 2D Heteroatom-Doped Carbon Nanosheets with Dominated Pseudocapacitive Behaviors Enabling Fast and High-Performance Sodium Storage. Adv. Funct. Mater. 2020, 30, 1909907. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Lee, G.W.; Shim, G.H.; Lim, S.T.; Kim, K.M.; Nguyen Vo, T.T.; Kweon, B.; Wongwises, S.; Jerng, D.W.; et al. Advanced Boiling—A Scalable Strategy for Self-Assembled Three-Dimensional Graphene. ACS Nano 2021, 15, 2839–2848. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Tan, L. Hydrogen-bonding crosslinking MXene to highly mechanically stable and super-zincophilic host for stable Zn metal anode. Chem. Eng. J. 2023, 472, 145056. [Google Scholar] [CrossRef]
- Chavhan, M.P.; Kryeziu, A.; Ganguly, S.; Parmentier, J. Monolithic metal-based/porous carbon nanocomposites made from dissolved cellulose for use in electrochemical capacitor. Green Carbon 2024, 2, 109–117. [Google Scholar] [CrossRef]
- Mao, H.; Yang, S.; Yang, Y.; Yang, J.; Yuan, G.; Zheng, M.; Hu, H.; Liang, Y.; Yu, X. Hybrid catalyst-assisted synthesis of multifunctional carbon derived from Camellia shell for high-performance sodium-ion batteries and sodium-ion hybrid capacitors. Carbon Neutralization 2024, 3, 673–688. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, L.; Liu, Y.; Li, D.; Xia, Q.; Wang, J.; Zhang, Z.; Han, X.; Long, Y.; Zhang, Y.; et al. 2H-MoS2 modified nitrogen-doped hollow mesoporous carbon spheres as the efficient catalytic cathode catalyst for the aprotic lithium-oxygen batteries. Renewables 2023, 1, 100–111. [Google Scholar] [CrossRef]
- Li, H.; Guo, C.; Zhang, T.; Xue, P.; Zhao, R.; Zhou, W.; Li, W.; Elzatahry, A.; Zhao, D.; Chao, D. Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery. Nano Lett. 2022, 22, 4223–4231. [Google Scholar] [CrossRef]
- Ding, J.; Qiao, J.; Zheng, Z.; Song, Z.; Ding, S.; Luo, J.; Wang, F.; Li, F.; Li, H. Assembling MOF on CNTs into 0D-1D heterostructures for enhanced volatile organic compounds detection. Talanta 2025, 285, 127444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Li, H.; Huang, H. Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes. Materials 2025, 18, 2397. https://doi.org/10.3390/ma18102397
Li N, Li H, Huang H. Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes. Materials. 2025; 18(10):2397. https://doi.org/10.3390/ma18102397
Chicago/Turabian StyleLi, Na, Hongpeng Li, and Haibo Huang. 2025. "Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes" Materials 18, no. 10: 2397. https://doi.org/10.3390/ma18102397
APA StyleLi, N., Li, H., & Huang, H. (2025). Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes. Materials, 18(10), 2397. https://doi.org/10.3390/ma18102397





