In Situ Phase Separation Strategy to Construct Zinc Oxide Dots-Modified Vanadium Nitride Flower-like Heterojunctions as an Efficient Sulfur Nanoreactor for Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Preparation of Zn3(OH)2(V2O7)(H2O)2
2.1.2. Preparation of Porous ZnO-QDs-VN and Other Specimens for Comparison
2.1.3. Preparation of ZnO-QDs-VN@S, VN@S and ZnO@S
2.1.4. Analysis of Synthetic Mechanism
2.1.5. Preparation of Li2S6 Solution for the Adsorption Experiments
2.1.6. Lithium Sulfide Deposition and Dissolution Experiments
2.1.7. Voltammograms of Symmetric Cells
2.1.8. Constant Current Titration Interval (GITT) Test
2.1.9. Electrochemical Performance Test of the Coin Battery
2.2. Material Characterization
3. Results and Discussion
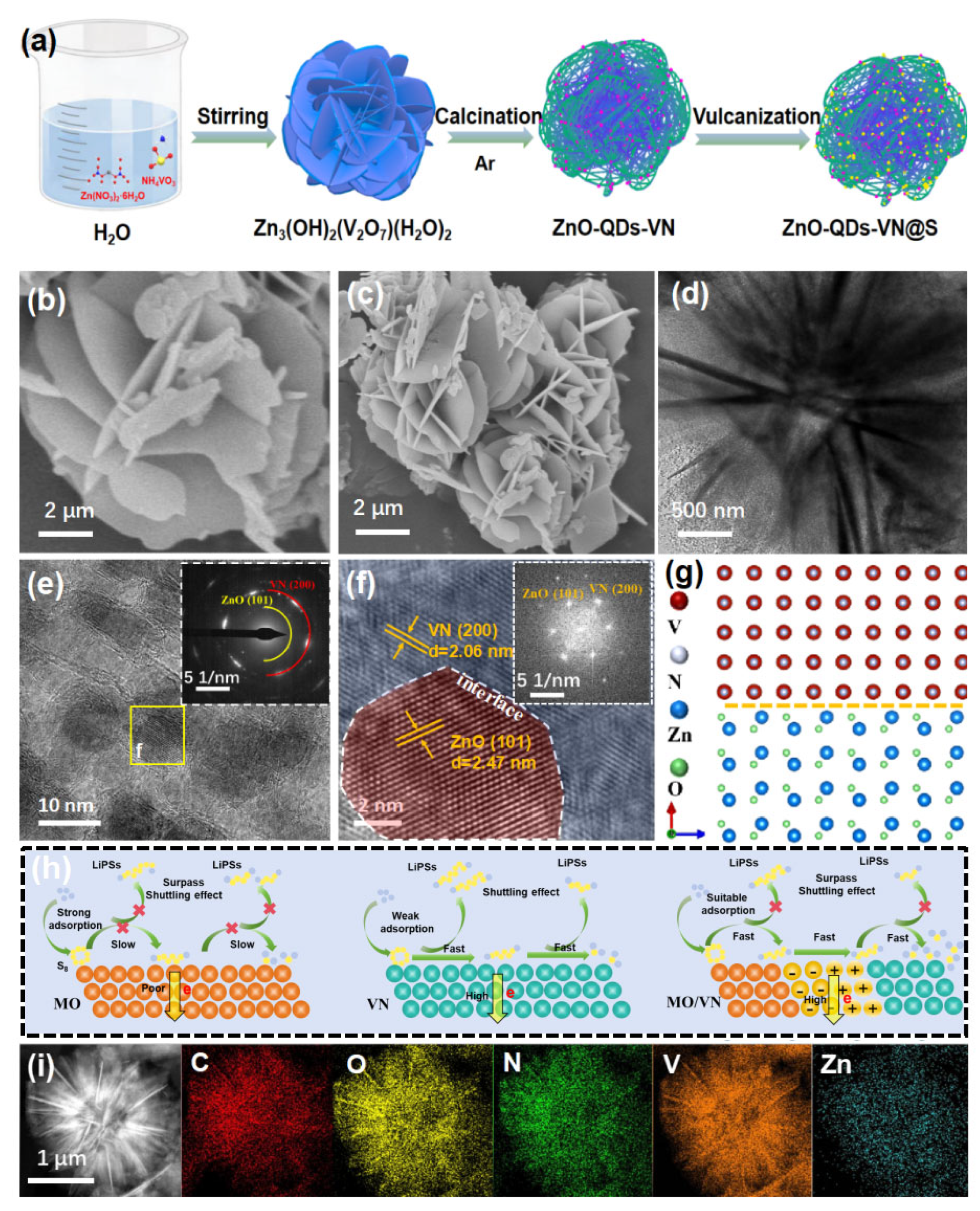
3.1. Physical Characterization and Component Analysis of ZnO-QDs-VN Electrocatalyst
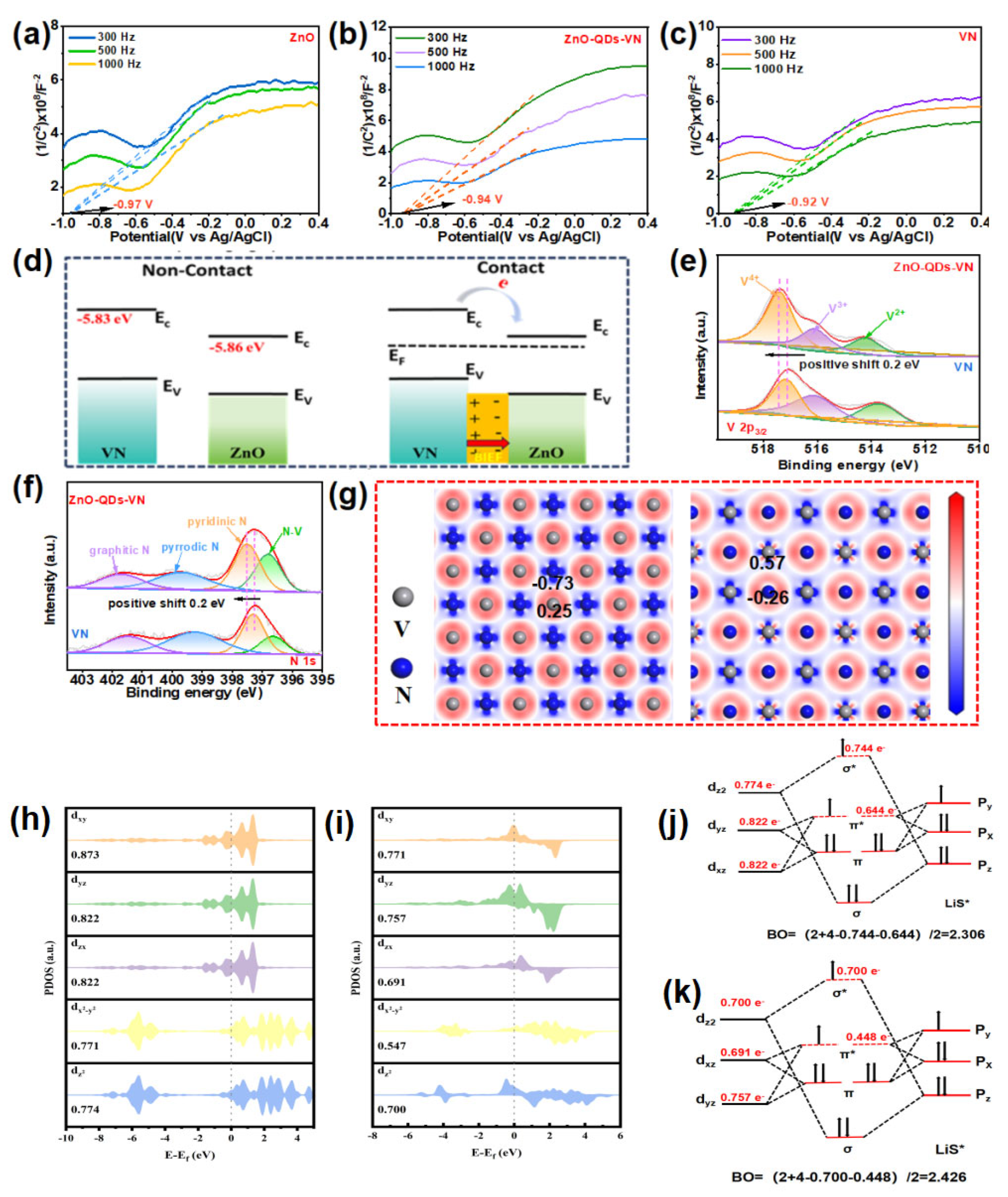
3.2. Overview of Calculation Details and Methods
3.3. Spin Configuration−Orbital Orientation
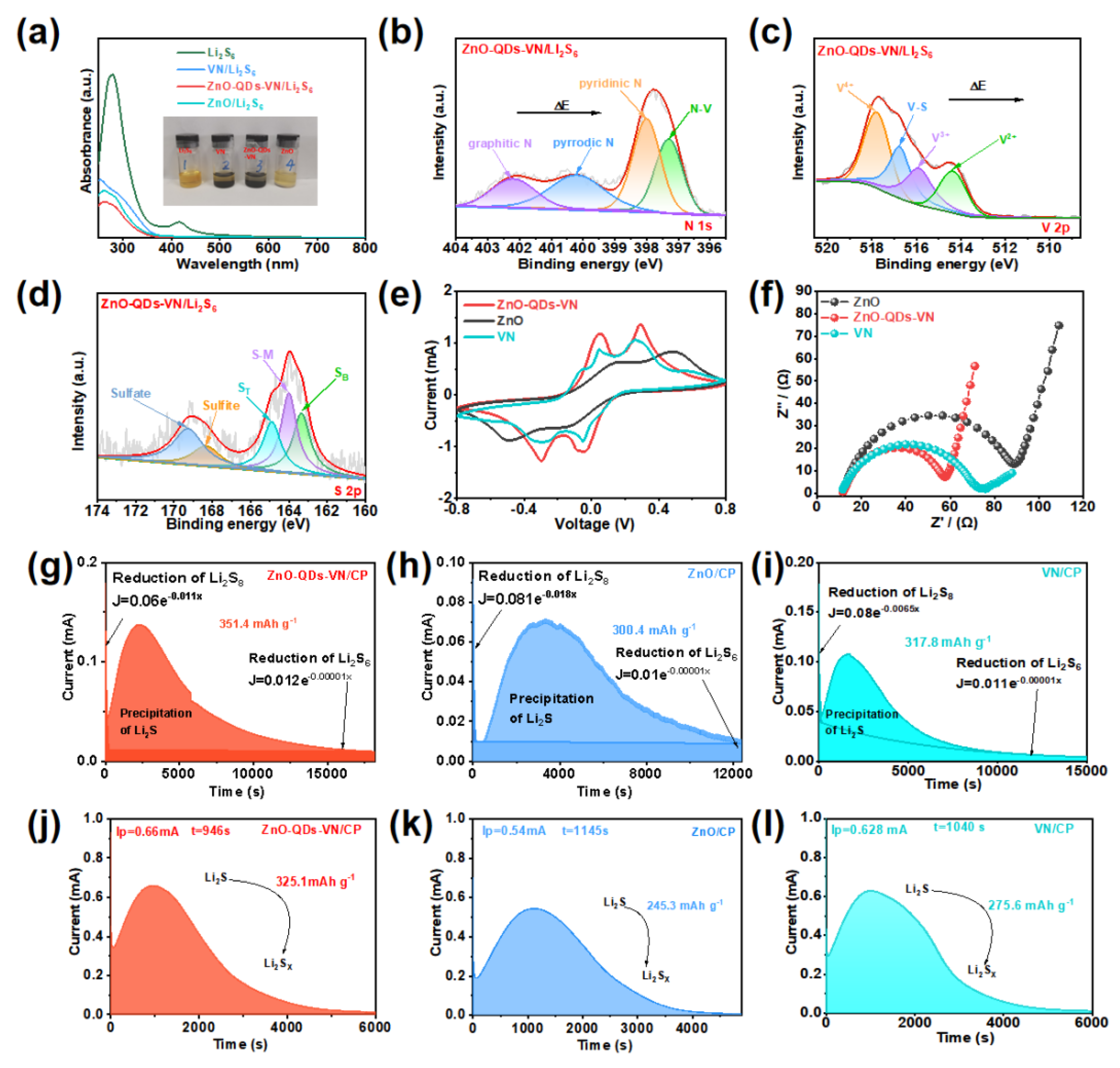
3.4. Absorption and Catalytic Conversion Mechanism
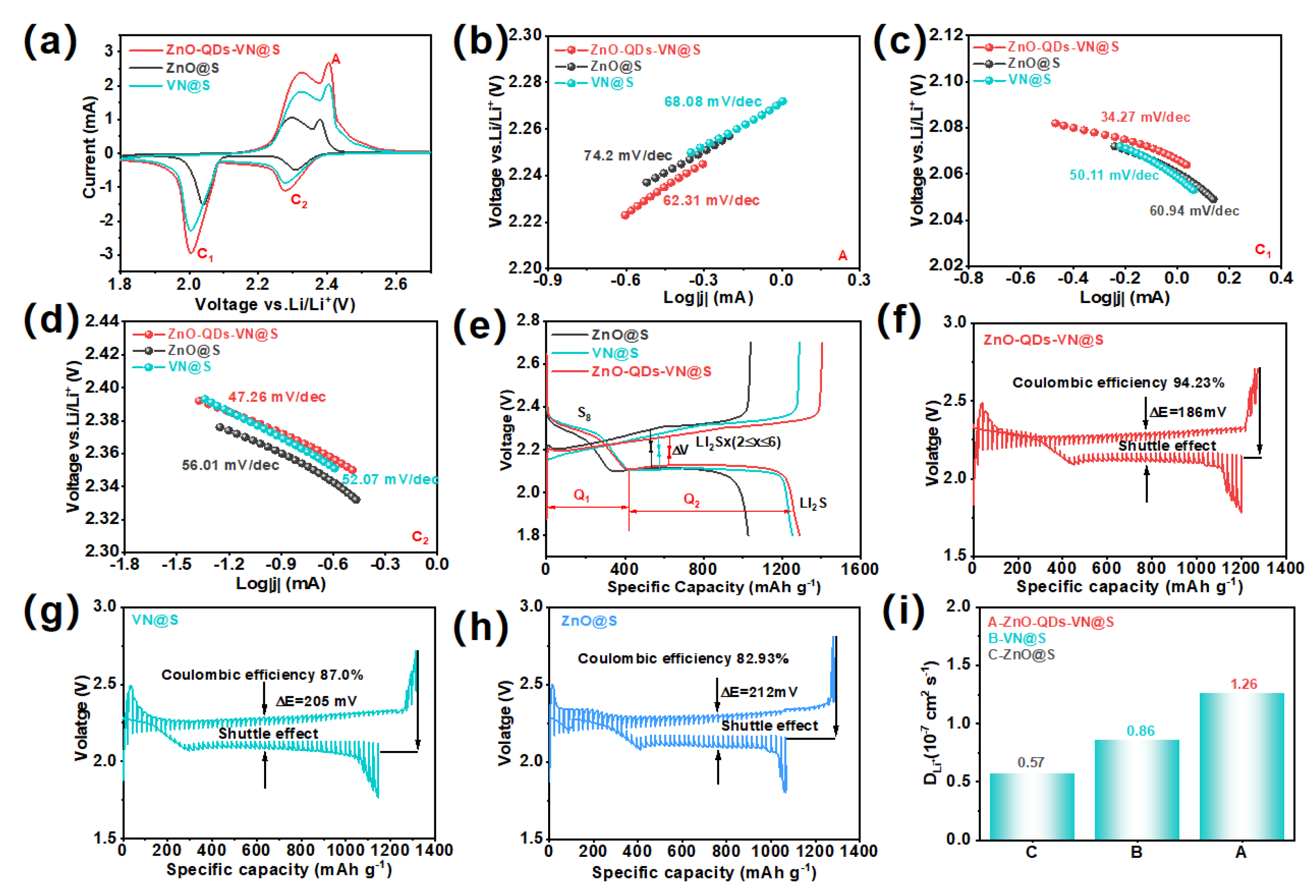
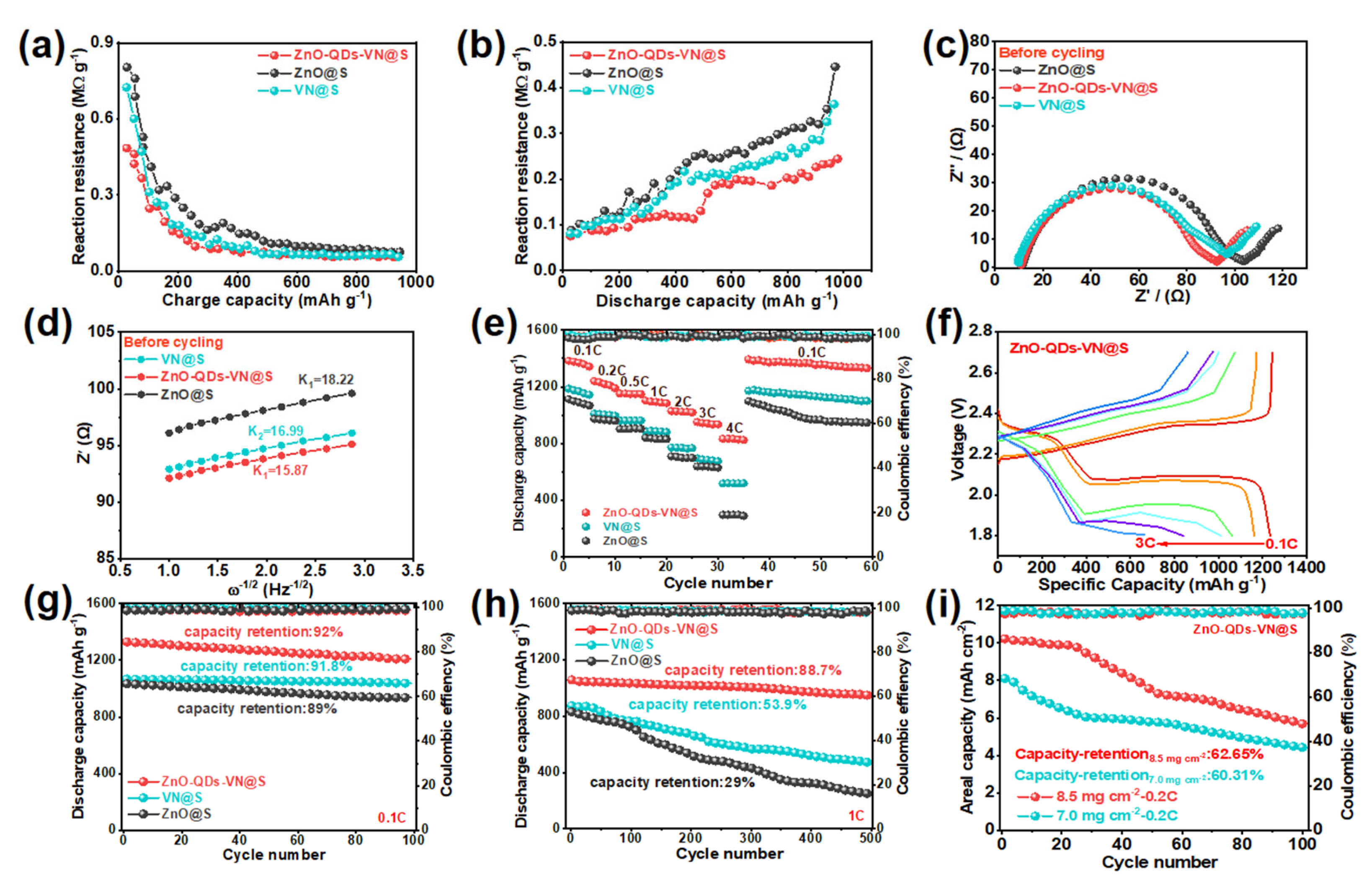
3.5. Electrochemical Performance of ZnO-QDs-VN Heterostructure Composite Materials
4. Conclusions
5. Prospects
- Deepen the analysis of application potential. Through systematic research on industry data (such as production costs and process parameters of similar materials), combined with existing experimental data, conduct a preliminary feasibility assessment of the synthetic method from the perspectives of raw material economy and process complexity, and clarify the gap between current research and practical application.
- Strengthen cross-disciplinary cooperation. We have reached initial cooperation agreements with certain enterprises and plan to jointly conduct pilot-scale amplification experiments in subsequent research, optimize the synthesis process in line with the demands of the enterprises, and verify the performance of the materials under actual working conditions.
- Adjust the research focus. In line with recommendations, we will reduce some repetitive electrochemical characterization content and instead focus on the synthesis mechanism during the synthesis process, providing theoretical support for subsequent process optimization through mechanism analysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Nong, S.; Huang, D.; Li, Y.; Yang, R.; Xie, J.; Li, J.; Huang, H.; Liang, X.; Li, G.; Lan, Z.; et al. Multifunction-balanced porous carbon and its application in sulfur-loading host and separator modification for lithium–sulfur batteries. J. Energy Storage 2024, 81, 110296. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Z.-H.; Chen, X.; Zhang, Q. A review on theoretical models for lithium–sulfur battery cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Wei, Y.; Tao, Y.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Layered carbide-derived carbon with hierarchically porous structure for high rate lithium-sulfur batteries. Electrochim. Acta 2016, 188, 385–392. [Google Scholar] [CrossRef]
- Gu, Q.; Qi, Y.; Chen, J.; Lu, M.; Zhang, B. Cobalt Nanoparticles Loaded on MXene for Li-S Batteries: Anchoring Polysulfides and Accelerating Redox Reactions. Small 2022, 18, 2204005. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, Y.; Du, X.; Liu, G.; Li, J.; Li, G. Ordered macroporous V-doped ZnO framework impregnated with microporous carbon nanocages as multifunctional sulfur reservoir in lithium-sulfur batteries. Chem. Eng. J. 2022, 431, 134242. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lou, X.W. Hollow Carbon Nanofibers Filled with MnO2 Nanosheets as Efficient Sulfur Hosts for Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2015, 54, 12886–12890. [Google Scholar] [CrossRef]
- Wang, N.; Chen, B.; Qin, K.; Liu, E.; Shi, C.; He, C.; Zhao, N. Rational design of Co9S8/CoO heterostructures with well-defined interfaces for lithium sulfur batteries: A study of synergistic adsorption-electrocatalysis function. Nano Energy 2019, 60, 332–339. [Google Scholar] [CrossRef]
- Tian, K.; Wei, C.; Wang, Z.; Li, Y.; Xi, B.; Xiong, S.; Feng, J. Heterogenization-Activated Zinc Telluride via Rectifying Interfacial Contact to Afford Synergistic Confinement-Adsorption-Catalysis for High-Performance Lithium−Sulfur Batteries. Small 2024, 20, 2309422. [Google Scholar] [CrossRef]
- Zhong, Y.; Chao, D.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, Y.; Wang, X.; Xia, X.; Tu, J. Confining Sulfur in Integrated Composite Scaffold with Highly Porous Carbon Fibers/Vanadium Nitride Arrays for High-Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1706391. [Google Scholar] [CrossRef]
- Ye, C.; Jiao, Y.; Jin, H.; Slattery, A.D.; Davey, K.; Wang, H.; Qiao, S.-Z. 2D MoN-VN Heterostructure To Regulate Polysulfides for Highly Efficient Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 16703–16707. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, S.; Gong, W.; Meng, X.; Ma, J.; Zhang, J.; Zheng, L.; Abruña, H.D.; Geng, J. Kinetic Enhancement of Sulfur Cathodes by N-Doped Porous Graphitic Carbon with Bound VN Nanocrystals. Small 2020, 16, 2004950. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Zhuang, Y.; Fei, B.; Zhang, C.; Wang, Y.; Chen, Q.; Zhan, H. Self-supported VN arrays coupled with N-doped carbon nanotubes embedded with Co nanoparticles as a multifunctional sulfur host for lithium-sulfur batteries. Chem. Eng. J. 2022, 430, 132931. [Google Scholar] [CrossRef]
- Jing, E.; Chen, L.; Xu, S.; Tian, W.; Zhang, D.; Wang, N.; Bai, Z.; Zhou, X.; Liu, S.; Duan, D.; et al. Dual redox catalysis of VN/nitrogen-doped graphene nanocomposites for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 64, 574–582. [Google Scholar] [CrossRef]
- Ma, W.; Shao, Z.; Yao, J.; Zhao, K.; Ma, X.; Wu, L.; Zhang, X. Mott-Schottky electrocatalyst selectively mediates the sulfur species conversion in lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 631, 114–124. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, Y.; Fan, M.; Liu, H.; Li, X.; Zhang, R.; Li, M.; Qiao, Z.-A. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Storage Mater. 2019, 23, 678–683. [Google Scholar] [CrossRef]
- Xiong, W.; Lin, J.; Wang, H.; Li, S.; Wang, J.; Mao, Y.; Zhan, X.; Wu, D.-Y.; Zhang, L. Construction of strong built-in electric field in binary metal sulfide heterojunction to propel high-loading lithium-sulfur batteries. J. Energy Chem. 2023, 81, 492–501. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Zhang, Q.; Wu, X.; Cheng, J.; Zhang, N.; Feng, Y.; Sun, K. Graphene Aerogels with Anchored Sub-Micrometer Mulberry-Like ZnO Particles for High-Rate and Long-Cycle Anode Materials in Lithium Ion Batteries. Small 2016, 12, 5208–5216. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Chen, D.; Li, Z.; Yan, L.; Li, S.; Liang, C.; Ling, M.; Peng, X. Nitrogen-doped porous carbon sponge-confined ZnO quantum dots for metal collector-free lithium ion battery. J. Electroanal. Chem. 2019, 848, 113275. [Google Scholar] [CrossRef]
- Wei, S.; Wang, C.; Chen, S.; Zhang, P.; Zhu, K.; Wu, C.; Song, P.; Wen, W.; Song, L. Dial the Mechanism Switch of VN from Conversion to Intercalation toward Long Cycling Sodium-Ion Battery. Adv. Energy Mater. 2020, 10, 1903712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Wang, J.; Zhang, Z.; Yu, J.; Yang, Z. Enhanced chemisorption and catalytic conversion of polysulfides via CoFe@NC nanocubes modified separator for superior Li–S batteries. Chem. Eng. J. 2022, 433, 133792. [Google Scholar] [CrossRef]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 Nanowire/Graphene Electrodes for Aqueous Rechargeable Zinc Ion Batteries with High Rate Capability and Large Capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, D.; Wu, Q.; Dan, J.; Zhu, X.; Lei, W.; Ma, L.-J.; Li, L. Rational Design of the Lotus-Like N-Co2VO4-Co Heterostructures with Well-Defined Interfaces in Suppressing the Shuttle Effect and Dendrite Growth in Lithium–Sulfur Batteries. Small 2021, 17, 2104109. [Google Scholar] [CrossRef]
- Wang, W.-P.; Zhang, J.; Chou, J.; Yin, Y.-X.; You, Y.; Xin, S.; Guo, Y.-G. Solidifying Cathode–Electrolyte Interface for Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2000791. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Wu, H.; Wu, Y.; Shi, K.; Li, J.; Zhang, W.; Liu, Q. 3d-Orbital High-Spin Configuration Driven From Electronic Modulation of Fe3O4/FeP Heterostructures Empowering Efficient Electrocatalyst for Lithium−Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2409303. [Google Scholar] [CrossRef]
- Cui, M.; Zheng, Z.; Wang, J.; Wang, Y.; Zhao, X.; Ma, R.; Liu, J. Rational design of Lithium-Sulfur battery cathodes based on differential Atom Electronegativity. Energy Storage Mater. 2021, 35, 577–585. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, X.; Zhou, Z.; Hu, Z. A first-principles study on the electrochemical reaction activity of 3d transition metal single-atom catalysts in nitrogen-doped graphene: Trends and hints. eScience 2022, 2, 219. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Yang, H.; Zeng, S.; Xu, R.; Liu, F.; Shi, P.; Feng, Y.; Wang, K.; Yang, W.; et al. A Dual-Functional Conductive Framework Embedded with TiN-VN Heterostructures for Highly Efficient Polysulfide and Lithium Regulation toward Stable Li–S Full Batteries. Adv. Mater. 2020, 32, 1905658. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Zhao, D.; Zhu, C.; Wang, N.; Lei, W.; Guo, Y.; Li, L. Engineering Spin State in Spinel Co3O4 nanosheets by V-Doping for Bidirectional Catalysis of Polysulfides in Lithium–Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2402114. [Google Scholar] [CrossRef]
- Dan, J.; Zhou, W.; Chen, M.; Zhu, C.; Li, S.; Zhao, D.; Li, L.; An, M. Cobalt-Carbon nanotubes supported on V2O3 nanorods as sulfur hosts for High-performance Lithium-Sulfur batteries. J. Colloid Interface Sci. 2023, 640, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Z.; Von Lim, Y.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent Advances in Heterostructure Engineering for Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Xia, J.; Hua, W.; Wang, L.; Sun, Y.; Geng, C.; Zhang, C.; Wang, W.; Wan, Y.; Yang, Q.-H. Boosting Catalytic Activity by Seeding Nanocatalysts onto Interlayers to Inhibit Polysulfide Shuttling in Li–S Batteries. Adv. Funct. Mater. 2021, 31, 2101980. [Google Scholar] [CrossRef]
- Chu, F.; Wang, M.; Liu, J.; Guan, Z.; Yu, H.; Liu, B.; Wu, F. Low Concentration Electrolyte Enabling Cryogenic Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2205393. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Zhang, Z.; Tsuji, M.; Miyazaki, M.; Kitano, M.; Hosono, H. Barium Oxynitride Electride as Highly Enhanced Promotor for Ruthenium Catalyst in Ammonia Synthesis: Comparative Study with Barium Oxide. Adv. Energy Mater. 2023, 13, 2302424. [Google Scholar] [CrossRef]
- Capkova, D.; Knap, V.; Fedorkova, A.S.; Stroe, D.-I. Analysis of 3.4 Ah lithium-sulfur pouch cells by electrochemical impedance spectroscopy. J. Energy Chem. 2022, 72, 318–325. [Google Scholar] [CrossRef]
- Yi, T.-F.; Wei, T.-T.; Li, Y.; He, Y.-B.; Wang, Z.-B. Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Mater. 2020, 26, 165–197. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Sun, F.; Jiang, G.; Zheng, N.; Xu, C.; Li, Y. Core-shell polyhedrons of carbon nanotubes-grafted graphitic carbon@nitrogen doped carbon as efficient sulfur immobilizers for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 450, 364–371. [Google Scholar] [CrossRef]
- Wu, K.; Lu, G.; Huang, B.; Hu, Z.; Lv, Y.; Younus, H.A.; Wang, X.; Liu, Z.; Zhang, S. Entropy-Driven Highly Chaotic MXene-Based Heterostructures as an Efficient Sulfur Redox Electrocatalysts for Li-S Battery. Adv. Funct. Mater. 2024, 34, 2404976. [Google Scholar] [CrossRef]
- Zeng, P.; Yu, H.; Chen, M.; Xiao, W.; Li, Y.; Liu, H.; Luo, J.; Peng, J.; Shao, D.; Zhou, Z.; et al. Flower-like ZnO modified with BiOI nanoparticles as adsorption/catalytic bifunctional hosts for lithium–sulfur batteries. J. Energy Chem. 2020, 51, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Estevez, L.; Kumar, J. Kinetics of all-solid-state sulfur cathodes. Energy Storage Mater. 2020, 27, 232–243. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Zhang, Y.; Luo, D.; Yu, A.; Wang, X.; Chen, Z. Amorphizing metal-organic framework towards multifunctional polysulfide barrier for high-performance lithium-sulfur batteries. Nano Energy 2021, 86, 106094. [Google Scholar] [CrossRef]
- Jian, Z.; Zhang, S.; Guan, X.; Li, J.; Li, H.; Wang, W.; Xing, Y.; Xu, H. ZnO quantum dot-modified rGO with enhanced electrochemical performance for lithium–sulfur batteries. RSC Adv. 2020, 10, 32966–32975. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shen, Z.; Cai, D.; Fei, B.; Zhang, C.; Wang, Y.; Chen, Q.; Zhan, H. A hierarchical VN/Co3ZnC@NCNT composite as a multifunctional integrated host for lithium–sulfur batteries with enriched adsorption sites and accelerated conversion kinetics. J. Mater. Chem. A 2022, 10, 20525–20534. [Google Scholar] [CrossRef]
- Li, N.; Xu, Z.; Wang, P.; Zhang, Z.; Hong, B.; Li, J.; Lai, Y. High-rate lithium-sulfur batteries enabled via vanadium nitride nanoparticle/3D porous graphene through regulating the polysulfides transformation. Chem. Eng. J. 2020, 398, 125432. [Google Scholar] [CrossRef]
- Xu, P.; Liu, H.; Zeng, Q.; Li, X.; Li, Q.; Pei, K.; Zhang, Y.; Yu, X.; Zhang, J.; Qian, X.; et al. Yolk−Shell Nano ZnO@Co-Doped NiO with Efficient Polarization Adsorption and Catalysis Performance for Superior Lithium−Sulfur Batteries. Small 2021, 17, 2005227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Zhou, W.; Chen, M.; Yuan, K.; Zuo, H.; Wang, A.; Zhao, D.; Wang, N.; Li, L. In Situ Phase Separation Strategy to Construct Zinc Oxide Dots-Modified Vanadium Nitride Flower-like Heterojunctions as an Efficient Sulfur Nanoreactor for Lithium-Sulfur Batteries. Materials 2025, 18, 2639. https://doi.org/10.3390/ma18112639
Chen N, Zhou W, Chen M, Yuan K, Zuo H, Wang A, Zhao D, Wang N, Li L. In Situ Phase Separation Strategy to Construct Zinc Oxide Dots-Modified Vanadium Nitride Flower-like Heterojunctions as an Efficient Sulfur Nanoreactor for Lithium-Sulfur Batteries. Materials. 2025; 18(11):2639. https://doi.org/10.3390/ma18112639
Chicago/Turabian StyleChen, Ningning, Wei Zhou, Minzhe Chen, Ke Yuan, Haofeng Zuo, Aocheng Wang, Dengke Zhao, Nan Wang, and Ligui Li. 2025. "In Situ Phase Separation Strategy to Construct Zinc Oxide Dots-Modified Vanadium Nitride Flower-like Heterojunctions as an Efficient Sulfur Nanoreactor for Lithium-Sulfur Batteries" Materials 18, no. 11: 2639. https://doi.org/10.3390/ma18112639
APA StyleChen, N., Zhou, W., Chen, M., Yuan, K., Zuo, H., Wang, A., Zhao, D., Wang, N., & Li, L. (2025). In Situ Phase Separation Strategy to Construct Zinc Oxide Dots-Modified Vanadium Nitride Flower-like Heterojunctions as an Efficient Sulfur Nanoreactor for Lithium-Sulfur Batteries. Materials, 18(11), 2639. https://doi.org/10.3390/ma18112639








