Abstract
An ultra-highly efficient Graphene Oxide/TiO2/Bentonite (GO/TiO2/Bent) sponge was synthesized using an in situ hydrothermal method. GO/TiO2/Bent sponge with a GO mass concentration of 10% exhibited the highest treatment efficiency of methylene blue (MB), combining adsorption and photocatalytic degradation, and achieved a maximum removal efficiency of 100% within about 70 min. To further prove the ultra-high removal capacity of the sponge, the concentration of MB in water increased to ten times the original concentration. At so high a MB concentration, the removal rate was still as high as 80% in 90 min. The photocatalytic mechanism of GO/TiO2/Bent sponge was discussed through XPS, PL and radicals quenching experiments. Here Bent can immobilize TiO2 and react with a photo-generated hole to increase the amount of hydroxyl radical; effectively enhancing the degradation of MB.GO sponge enlarges the sensitivity range of TiO2 to visible light by increasing the charge separation of TiO2 and reducing the recombination of photo-generated electron–hole pairs. Additionally, GO sponge with an interconnected porous structure provides an effective platform to immobilize TiO2/bent and makes them be easily recovered. The as-prepared sponge develops a simple and cost-effective strategy to realize the ultra-highly efficient treatment of dyes in wastewater.
1. Introduction
Dyes are commonly used in many industrial applications, including textiles, printing, plastics, leather and papermaking. When these pollutants are discharged into the environment, they present a serious challenge to aquatic life, the food chain and human health [1,2,3,4]. Owing to the difficulty of dye degradation, it is difficult for normal treatment by biochemical methods to meet the requirements for their treatment. Photocatalytic decomposition of organic compounds has been widely studied to elevate the degradation efficiency of dyes in aqueous solution, wherein TiO2 has received much attention and has been reported in various studies [5,6,7,8]. TiO2 is one of the most popular types of photocatalyst, and it is utilized in many fields, such as wastewater degradation and energy fuel, because of its high efficiency, nontoxicity, good chemical stability, and low cost [9,10].
However, because of its 3.2 eV electronic band gap, TiO2 is only sensitive to ultraviolet light, and cannot take full advantage of solar energy. In addition, TiO2 usually also has a small surface area and low absorbability, and its photocatalytic effect is low in solutions [11,12,13,14,15]. Additionally, commercial nano-TiO2 is of super-hydrophilicity, and easily agglomerates in water. The conglomeration of TiO2 particles not only weakens its photocatalytic performance, but is also unfavorable to its reutilization. In recent years, many efforts have been directed towards facilitating the use of this cost-effective photocatalyst [16,17,18]. For example, graphene oxide (GO) or reduced graphene oxide (RGO) has been used to modify TiO2 for photocatalytic degradation of organics, such as MB and Rhodamine B (RhB), by improving charge separation of TiO2 and reducing the combination of photo-generated electron–hole pairs [19,20,21,22,23]. However, the photocatalytic effect is far from satisfactory. Wang et al. prepared several graphene-titanium composites by a sol–gel method using titanium isopropoxide as Ti-precursors [24]. In the first 30 min (dark reaction), the maximum removal rate of MB reached only about 31%. Meanwhile, MB was completely degraded in about 90 min (Content of MB: 10 mg/L, 200mL; Mass of photocatalyst: 100 mg). Li et al. obtained TiO2-graphene nano-composite photocatalyst using a facile one-step hydrothermal method [25]. Under simulated sunlight, the maximum degradation rate is only 63% in 65 min (content of MB: 10 mg/L, 40 mL; mass of photocatalyst: 30 mg). Fan’s group reported that TiO2 doped by carbon could extend TiO2 light absorption cut off wavelength. However, the agglomeration of TiO2 nanoparticles on graphene prohibited the direct chemical contact between the two components [26]. In addition, Su-Il researcher groups used a TiO2 nanotube array photoanode coupled with a conventional bioanode, achieving simultaneous degradation of methylene blue (MB) dye and improved power generation [9].
In recent years, clays, such as bentonite, rectorite and kaolinite, have been widely used in adsorption fields [27,28,29]. These natural and low-cost materials provide layered structures, large surface areas and a high cation exchange capacity [30,31,32,33]. It was reported in our previous paper that the clay bentonite can exhibit an improved adsorption capacity and reusability after modification [34]. Additionally, in the early work of our group [35], three-dimensional GO sponge was found to have a much larger surface area than two-dimensional GO sheets. Meanwhile, other groups have also performed the similar works on three-dimensional GO sponge [36,37].
In this work, to improve the adsorption and photocatalytic properties of TiO2, as well as the reusability of the material for removing MB in water, the sponge-like composite made of GO, TiO2 and bentonite is designed (see Figure 1). Firstly, in order to further enhance the degradation ability of photocatalyst to organic pollutants by a simple and cost-effective method, bentonite is introduced to load TiO2. The important role of bentonite and the photocatalytic mechanism are expounded in detail. On the one hand, bentonite, as an excellent carrier with a large surface area, can effectively immobilize TiO2 nanoparticles and prevent TiO2 from agglomeration. On the other hand, the surface of bentonite has abundant hydroxyl functional groups. TiO2, as a kind of semiconductor, will produce electron–hole pairs under the excitation of photons. The photo-generated holes have strong oxidation properties and can oxidize the hydroxyl groups on the bentonite surface to hydroxyl radicals and thus improve the photocatalytic efficiency. In addition, bentonite can also strongly adsorb pollutants on its surface, particularly organic cationic dyes, playing a role as a medium, which is also advantageous to the photocatalytic process.
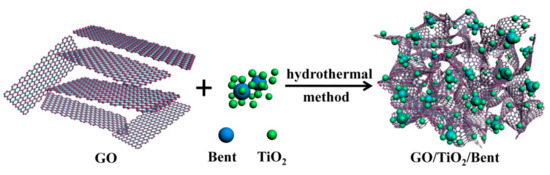
Figure 1.
Scheme for the preparation of GO/TiO2/Bent sponge.
Secondly, as mentioned above, the introduction of GO sponge, which has an interconnected structure to promote the transfer of electrons, favors the increase of TiO2 photocatalytic properties by improving the charge separation of TiO2, reducing their combination of photo-generated electron–hole pairs and strengthening the response of TiO2 to the sun light.
The third, three-dimensional GO sponge provides plenty of space to accommodate as many TiO2/bent particles as possible. In addition, it acts as an efficient platform for immobilizing the particles and prevents them from aggregation and losing in water. In addition, more notably, as a whole sponge which can be easily recovered, the GO/TiO2/bent composite exhibits an excellent reusability which is a difficult problem that troubles us for a long time in this field.
2. Experimental
2.1. Materials
Graphite flakes (>99.7%, weight percent) were purchased from Qingdao Chemical Reagent Co. Ltd. (Qingdao, China). Sodium nitrate, sulphuric acid (98%), potassium permanganate, hydrochloric acid (36%), barium sulfate, and TiO2 (P25) were purchased from Shanghai Maclin Biochemistry Technology Co., Ltd. (Shanghai, China). All chemicals were used as received without further purification.
2.2. Preparation of GO Sponge
GO was prepared based on Hummers’ method [35,38]. Graphite flakes (0.6 g) and sodium nitrate (1 g) were added slowly into concentrated sulfuric acid (35 mL), maintaining the temperature of the ice water bath between 0 and 4 °C. Then, the potassium permanganate was added to the solution very slowly, while simultaneously maintaining the temperature at no more than 20 °C. Next, the solution was put into a water bath of 35 °C, with stirring, for 2 h. After that, 150 mL deionized water was slowly added to the solution and the temperature was maintained at 98 °C for 15 min. The liquid was further diluted with 200 mL water, and stirred for 2 h. At the same time, 10 mL of hydrogen peroxide was added, until the color of the sample turned golden. Subsequently, the solution was stirred for 3 h and the supernatant was poured out. Hydrochloric acid solution (VHCl: Vwater = 1:10) and water were used to wash the golden solutions several times until the pH was greater than 5. Then, the golden sample was diluted to 4 mg/mL with water and the 4 mg/mL golden sample was diluted with 50 mL water. The suspension was placed into a 100 mL Teflon-sealed autoclave and kept at 180 °C for 10 h. Finally, the composite was freeze-dried. Then, the three-dimensional GO sponge was successfully prepared.
2.3. Preparation of GO/TiO2 and TiO2/Bent
The GO/TiO2 sample was prepared as followed. The bright-yellow GO solution was diluted to 4 mg/mL by water and then 50 mL of such sample was used to disperse TiO2. In GO/TiO2 samples, GO loading was kept at 5%, 10%, 15% by adjusting TiO2 amount to 1.33, 2, and 4 g, respectively. The suspension was placed in a 100 mL Teflon-sealed autoclave and kept at 175 °C for 10 h. Finally, the composite was obtained by freeze-drying.
TiO2/Bent sample was prepared as follows. Bent (0.5 g) and TiO2 (0.5 g) were mixed into water (20 mL), kept in a water bath at 60 °C, and stirred for 2 h. Then, the dispersion was aged for 12 h at 70 °C. The TiO2/Bent sample was obtained by centrifuging, washing and drying at 80 °C for 12 h.
2.4. Preparation of GO/TiO2/Bent Sponge
The bright-yellow GO solution was diluted to 4 mg/mL with water and then 50 mL of this sample was used to disperse TiO2/Bent. In GO/TiO2/Bent samples, GO loading was kept at 5%, 10%, and 15% by adjusting TiO2/Bent amount to 1.33, 2, and 4 g, respectively. The suspension was placed in a 100 mL Teflon-sealed autoclave and kept at 175 °C for 10 h. Finally, the composite was obtained by freeze-drying, and then fully ground for further characterization.
2.5. Photocatalytic Tests
All the adsorption experiments were conducted three times and the averaged values were used as the experimental data to make the results reliable. Photocatalytic performances of various catalysts were evaluated by the photo-degradation of methylene blue (MB) under artificial solar light (λ = 220–1050 nm). In a typical process, aqueous solution of MB (10 mg/L or 100 mg/L, 200 mL) and the photocatalysts (10 mg) were put into a beaker under constant stirring. The intensity of artificial solar light was 86 mW/cm2 in each test. Firstly, the reaction solution was stirred for 30 min in dark to achieve adsorption equilibrium. The photocatalytic reaction was started by turning on a Xeon lamp. The suspension solution was centrifuged, and the MB solution was analyzed at 10-minute intervals to obtain the diffuse reflectance spectra by a UV-vis (Beijing Persee General Instrument Co., Ltd., Beijing, China) spectrophotometer at a wavelength of 664 nm.
2.6. Characterization of Materials
The crystalline structure of samples was analyzed by Bruker AXS D8 Focus X-ray diffraction (XRD, Bruker AXS, Karlsruhe, Germany) operating at 40 kV and 40 mA. The scan angle 2θ varied from 5° to 80°, and the scan speed was 0.04°·s−1. FTIR analysis was performed on a Bruker VECTOR-22 and the spectra were collected over the spectral range of 500–4000 cm−1. Scanning electron microscopy (SEM, JSM-6700F, JEOL Ltd., Sartorius, Göttingen, Germany) was used to evaluate the morphology and size information of the samples. X-ray photoelectron spectroscopy (XPS, ESCALAB 250, Thermo Fisher Scientific, Waltham, MA, USA) can be used in qualitative and quantitative analysis to characterize the surface composition of samples. Photoluminescence (PL) spectra were performed by F-4600 FL (Hitachi, Tokyo, Japan) using excitation wavelength of 320 nm. Nitrogen adsorption–desorption isotherms were measured at −196·°C with a Tristar II 3020 (Micromeritics Inc., Norcross, GA, USA).
3. Results and Discussion
3.1. Characterization of Materials
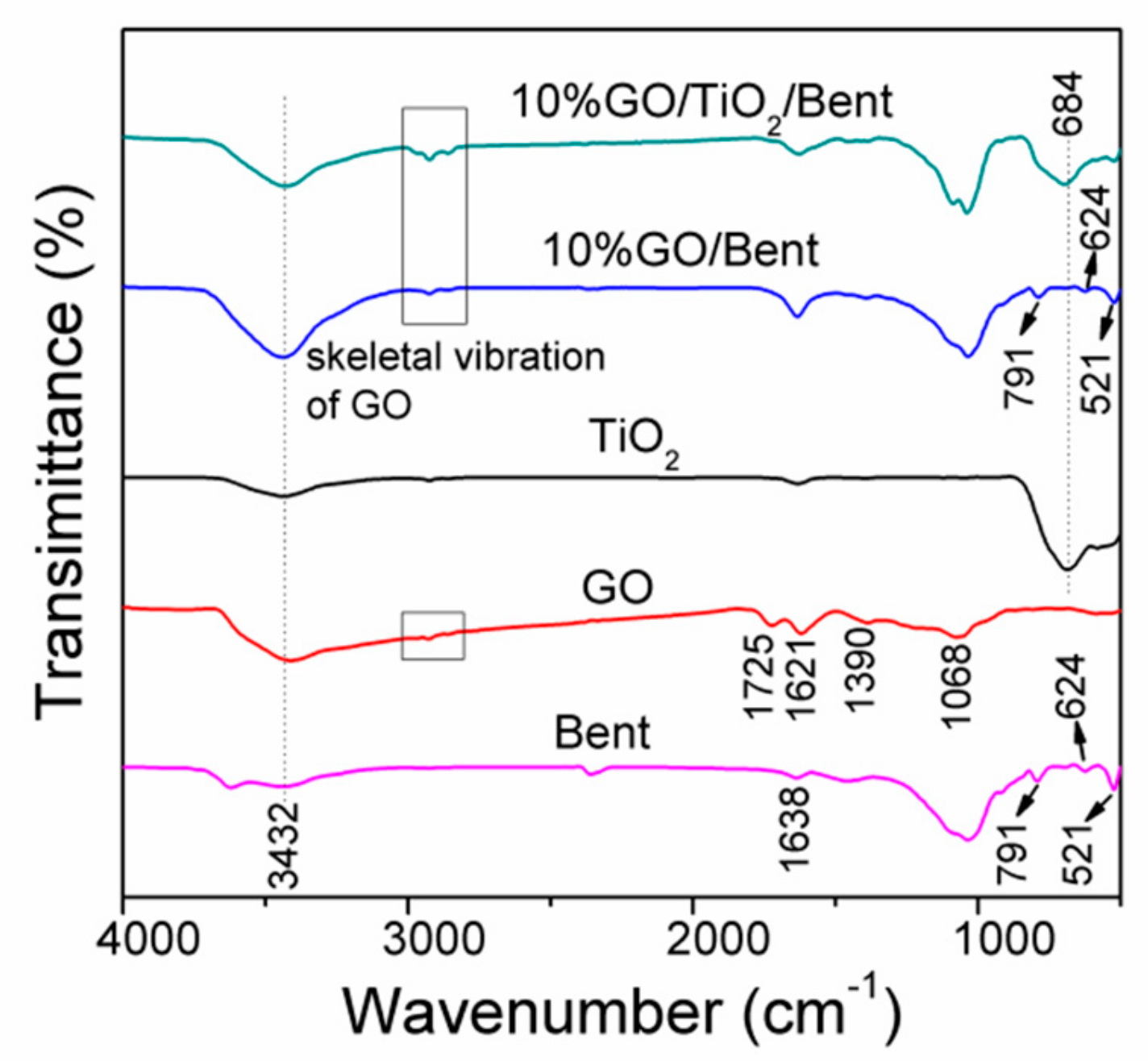
The FTIR spectra of Bent, GO, TiO2, 10% GO/Bent and 10% GO/TiO2/Bent are shown in Figure 2. The band at 3432 cm−1 is ascribed to a stretching vibration of the hydroxyl group (O–H) of the adsorbed water molecules in all the samples. For Bent, the band at 1638 cm−1 is due to the bending vibrations of the H–O–H bonds of the water molecules intercalated in the clay mineral. The strong absorption peak at 1033 cm−1 is the stretching vibration absorption peak of the Si–O–Si group, while the absorption peaks at 791, 624 and 521 cm−1 are caused by the deformation and bending vibration of the Si–O bond [39].
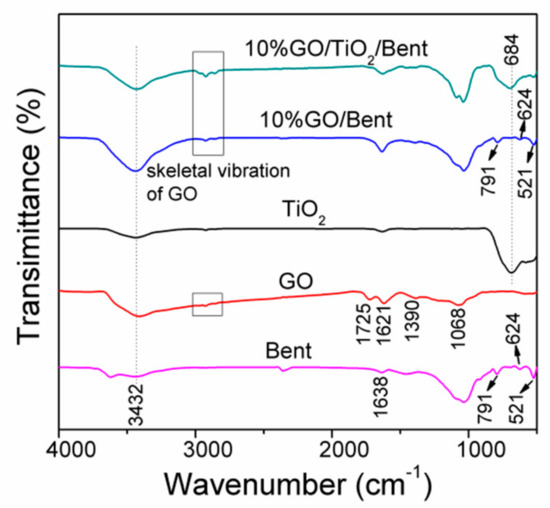
Figure 2.
FTIR spectra of Bent, GO, TiO2, 10% GO/Bent and 10% GO/TiO2/Bent sponges.
For GO, C=O stretching at 1725 cm−1, O–H bending and aromatic C=C stretching at 1621 cm−1, tertiary C–OH stretching at 1390 cm−1 and epoxy C–O stretching at 1068cm−1 are clearly observed, suggesting the presence of carboxyl, hydroxyl and oxygenation functional groups. For TiO2, the strong absorption bands observed at low frequency 684 cm−1 corresponds to the vibration of Ti–O–Ti bonds [40]. GO/Bent exhibits the characteristic peaks of skeleton vibration of GO, the deformation and bending vibration of Si–O bond at 791, 624 and 521 cm−1, respectively. For 10% GO/TiO2/Bent, the characteristic peaks of Si–O bond of Bent and Ti–O–Ti bond of TiO2as well as the characteristic peak of functional group of GO are found.
The characteristic skeletal vibration peaks of GO sponge marked by rectangle are also observed in FTIR spectra [25]. Due to the strong combination between TiO2 and Bent, the main skeletal vibration peaks of GO shift from 2928 cm−1 to 2924 cm−1 (See Supplementary Materials Figure S1). It is indicated that the chemical interaction between GO and TiO2/Bent.
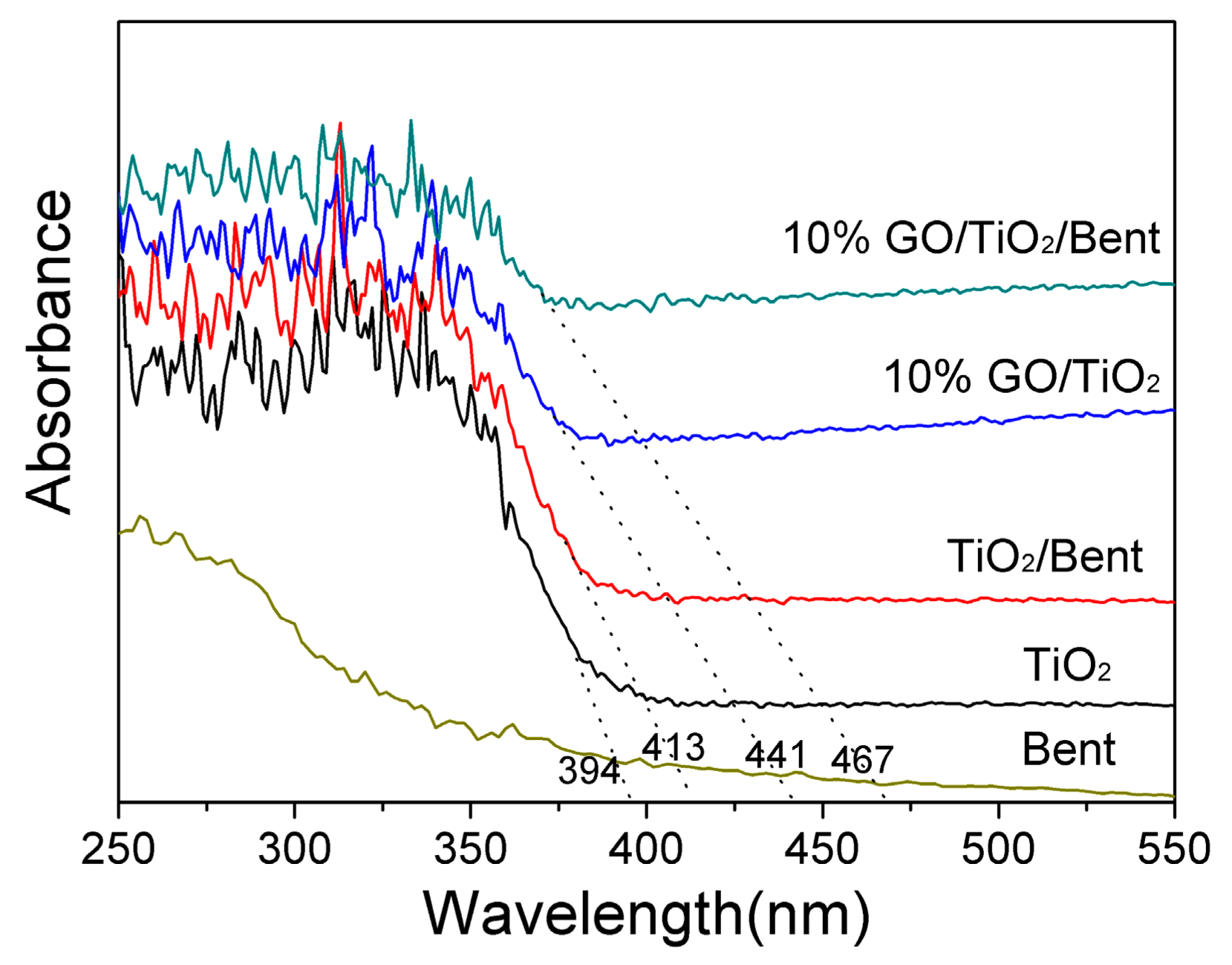
Diffuse reflectance spectra of TiO2, Bent, TiO2/Bent, 10% GO/TiO2 and 10% GO/TiO2/Bent are shown in Figure 3. As can be seen, Bent is almost transparent in the wavelength range longer than 350 nm [16]. TiO2/Bent displays an obvious red shift of about 19 nm in the absorption edge compared to TiO2. This may be attributed to the effects of Bent or some elements of Bent which may be doped into TiO2 [17]. Whereas the photocatalysts of TiO2, 10% GO/TiO2 and 10% GO/TiO2/Bent show the absorption edge at about 394, 441 and 467 nm, respectively. Furthermore, the figures indicate that the threshold of photocatalysts (10% GO/TiO2 and 10% GO/TiO2/Bent) display obvious red shifts to longer wavelength regions when compared with pure TiO2. The experimental results show that GO has a significant influence on the band gap of TiO2. As a result, solar light is utilized more efficiently by 10% GO/TiO2/Bent than other compared photocatalysts. This may be attributed to the formation of Ti–O–C chemical bonding in the composites [25,41,42].
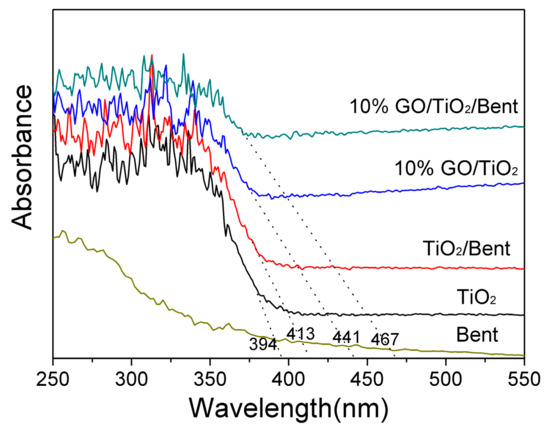
Figure 3.
Diffuse reflectance absorption spectra of Bent, TiO2, TiO2/Bent, 10% GO/TiO2 and 10% GO/TiO2/Bent sponge.
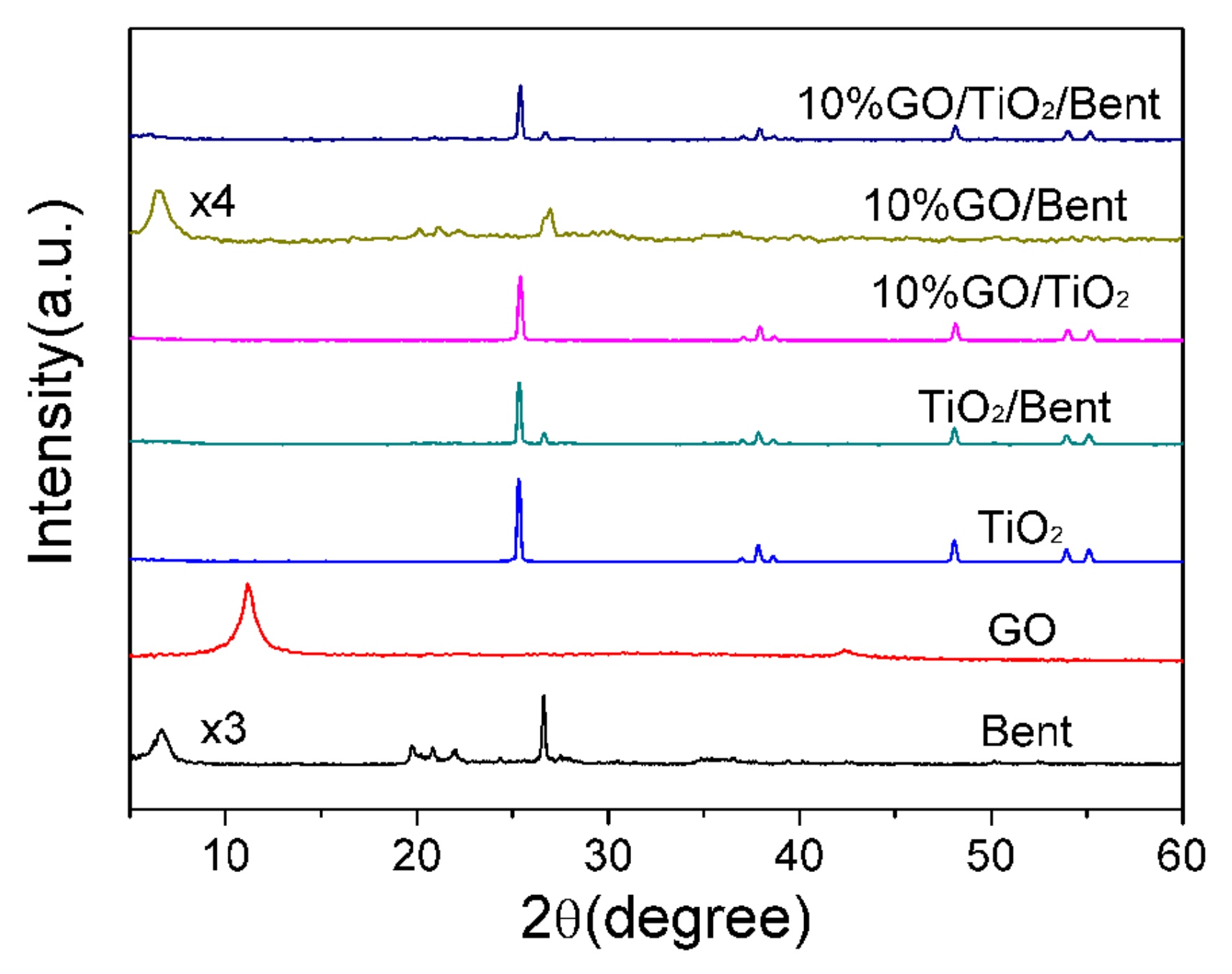
Figure 4 shows the XRD patterns of Bent, GO, TiO2, TiO2/Bent, 10% GO/Bent and 10% GO/TiO2/Bent. The peaks at 2θ = 11.16° and 42.51° are assigned to the (002) and (100) reflections of GO. Clearly, all the peaks for TiO2 are readily indexed to the anatase phase of TiO2 (JCPDS No. 21-1272). Furthermore, the absence of the typical diffraction of GO stacking layers in the composites (10% GO/Bent and 10% GO/TiO2/Bent) might be attributed to the disruption and well exfoliation of GO in the composite or its low diffraction intensity [41,43]. The acute diffraction peak at 2θ = 6.68° in Bent is the (001) reflection, which indicates the existence of a layered structure. However, the TiO2/Bent sample does not have a sufficiently ordered and oriented silicate layer structure to show the (001) peak, as does the sample of GO/TiO2 [44]. The typical characteristic peak of GO and Bent do not appear in the composite. This is because the diffraction peak of TiO2 is so strong that the diffraction peaks of GO and Bent are submerged [45].
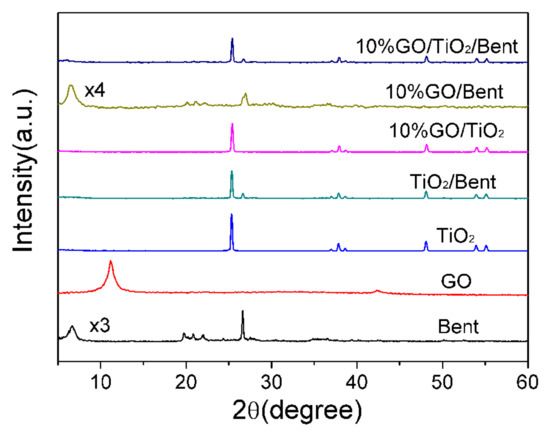
Figure 4.
XRD patterns of Bent, GO, TiO2, TiO2/Bent, 10% GO/TiO2, 10% GO/Bent and 10% GO/TiO2/Bent.
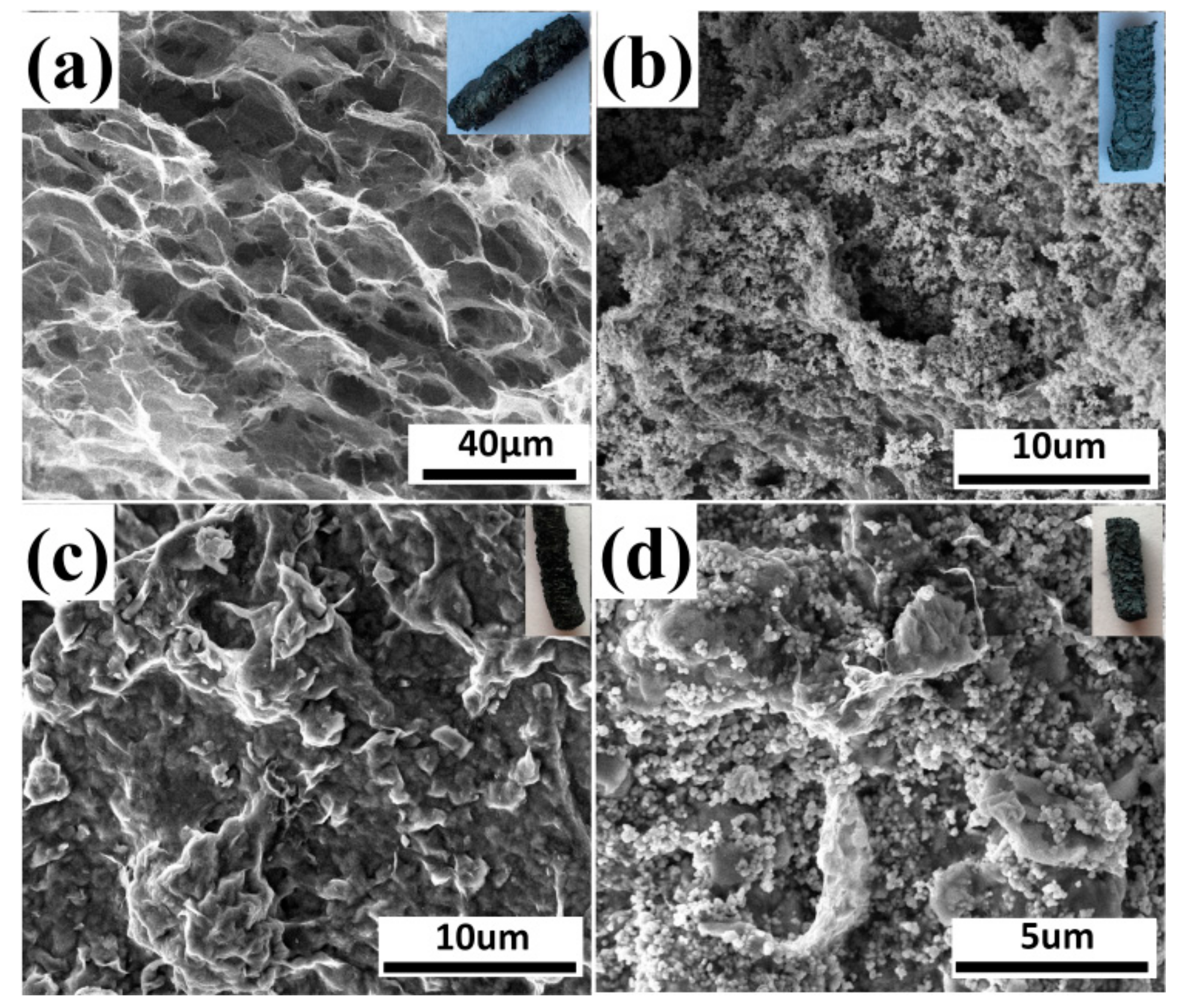
Figure 5 displays SEM images of GO, 10% GO/TiO2, 10% GO/Bent, and 10% GO/TiO2/Bent. The inset of each picture corresponds to the macroscopic columnar sponge. As can be seen from Figure 5a, GO appears as honeycomb-like sponge structure with large holes. After TiO2 is added, the surface of holes in GO sponge is covered by TiO2.The structure of 10% GO/TiO2/Bent is similar to that of 10% GO/Bent. The sponge-like structure of GO is beneficial for the transmitting of photo-generated electrons between GO layers and provides an ideal support for the deposition of TiO2 and Bent particles [46].
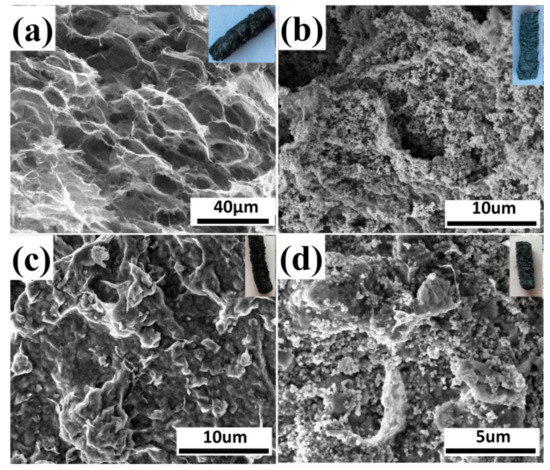
Figure 5.
SEM images of (a) GO, (b) 10% GO/TiO2, (c) 10% GO/Bent, and (d) 10% GO/TiO2/Bent. Insets are pictures of macroscopic columnar sponge.
3.2. Photocatalytic Degradation
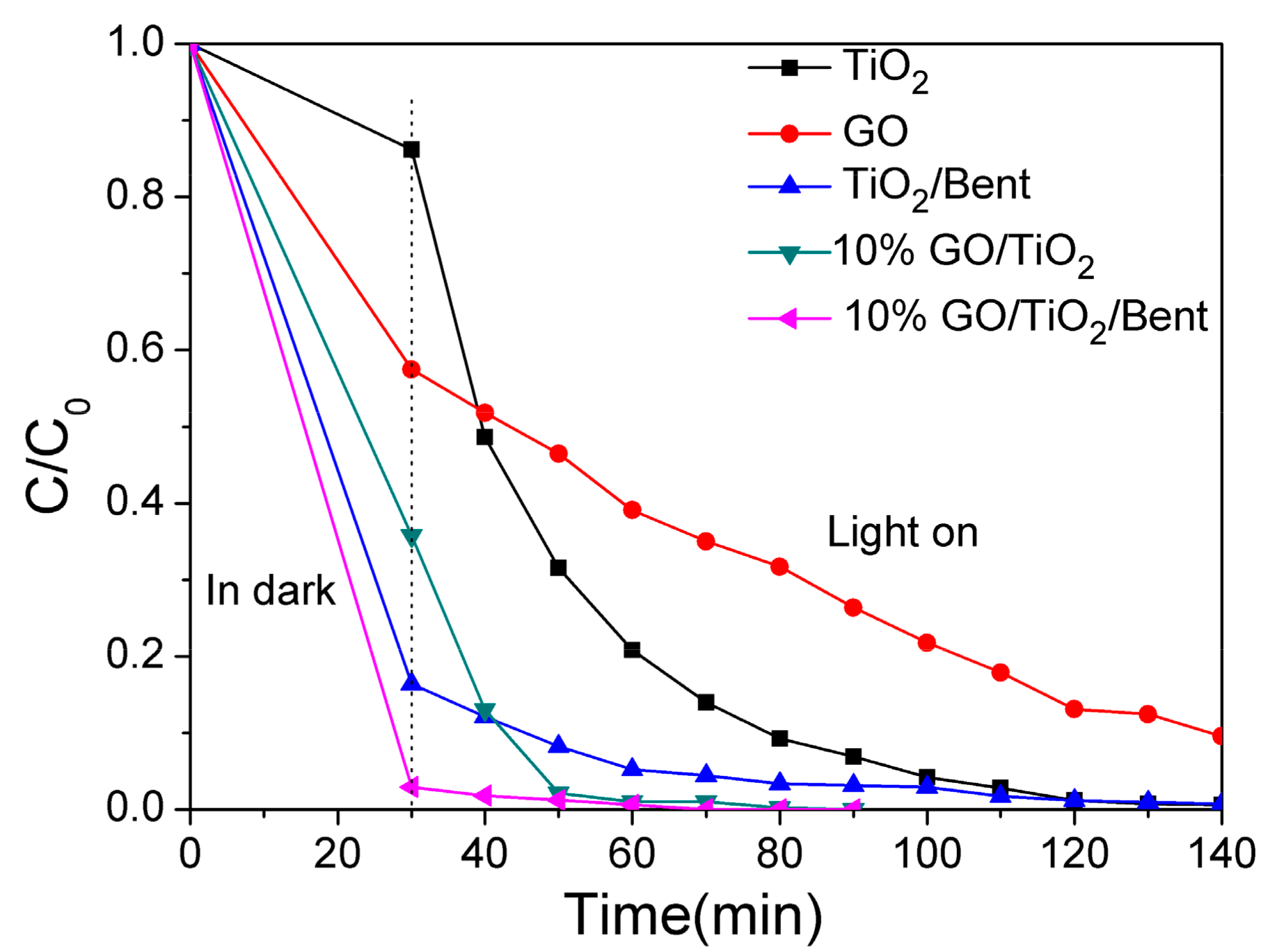
The removal rate of MB by GO/TiO2/Bent composite with different GO proportions was evaluated and the results were shown in Figure S2. It can be seen that the removal efficiency by 10% GO/TiO2/Bent is the best. Therefore, 10% GO/TiO2/Bent sponge is selected for the next experiments. In parallel experiments, the MB removal rates by TiO2, GO, TiO2/Bent, 10% GO/TiO2, 10% GO/TiO2/Bent, respectively, are obtained under simulated sunlight illumination after 30 min dark adsorption, and the results are shown in Figure 6. It can be seen that 10% GO/TiO2/Bent composite shows significant progress in the adsorption and degradation of MB. In the first 30 min (dark reaction), the removal rate of MB is about 99.7%, and subsequently increases to up to 100% at 70 min under sunlight. Additionally, in the first 30 min, TiO2/Bent exhibits a better adsorption capacity of MB than that of 10% GO/TiO2. However, with the increase of time, 10% GO/TiO2 shows good degradation ability. BET values of each catalyst are not related to the photocatalytic dye degradation activity (Figure S3 and Table S1).
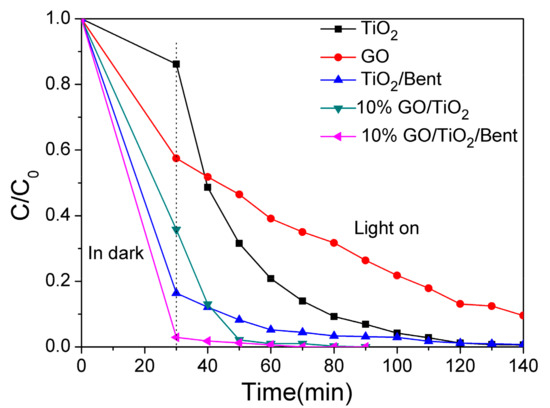
Figure 6.
Removal rates of MB by different materials of TiO2, GO, TiO2/Bent, 10% GO/TiO2 and 10% GO/TiO2/Bent sponge, respectively (Concentration of MB: 10 mg/L in 100mL MB solution; Mass of materials: 50 mg).
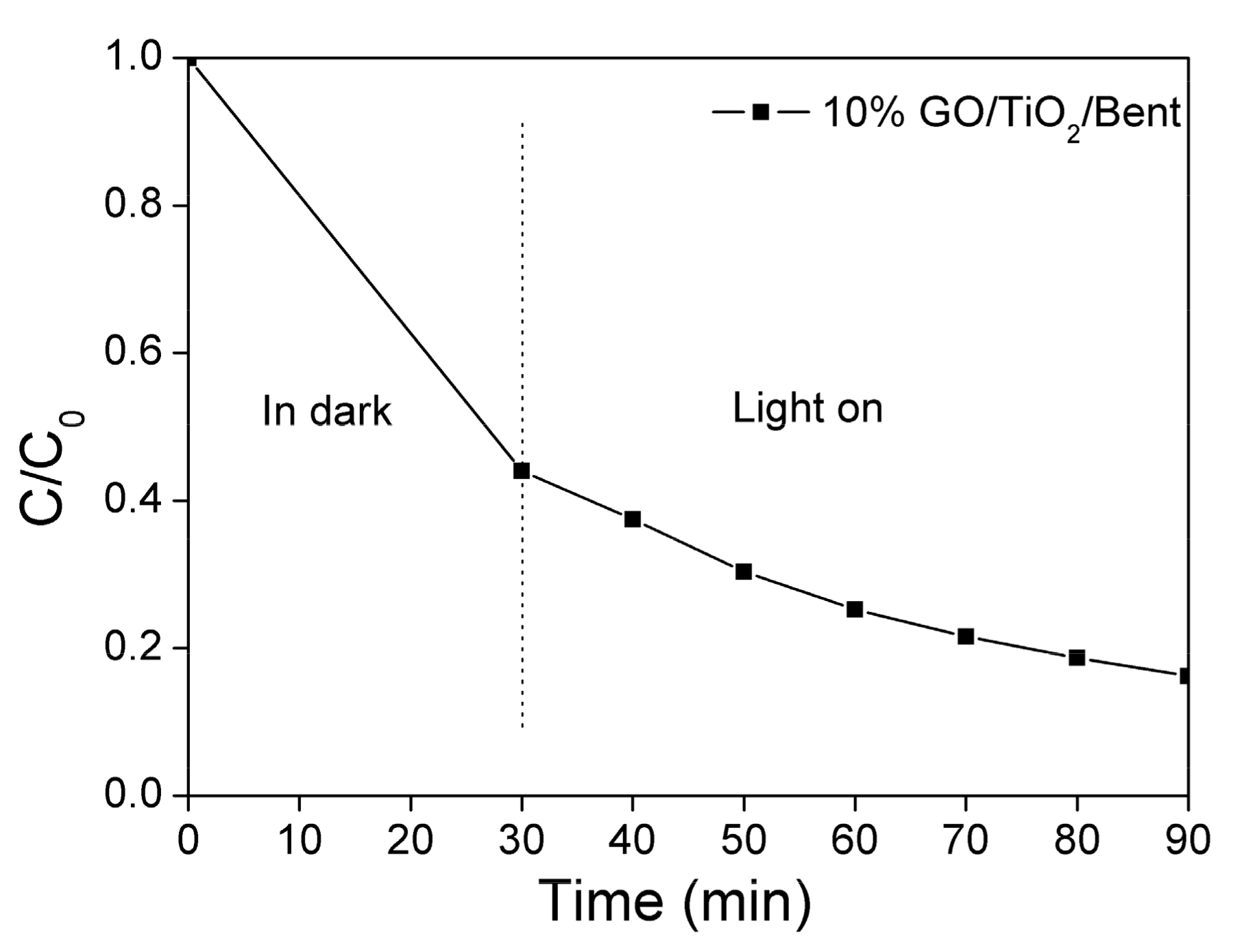
As mentioned above, in the first 30 min, the adsorption of MB by 10% GO/TiO2/Bent is particularly prominent, with a removal rate as high as 99.7%. Therefore, the photocatalytic ability of the sponge to MB under light is not well demonstrated. Here, to better present the effectiveness of photocatalysis, the concentration of MB is specifically increased to 100 mg/L (Figure 7), which is ten times the original MB concentration shown in Figure 6. It can be seen from Figure 7 that the adsorbent of 10% GO/TiO2/Bent sponge presents not only a high adsorption effect on MB, but also a highly photocatalytic efficiency under light. In general, 10% GO/TiO2/Bent sponge exhibits an excellent removal rate of MB, which is still as high as 80% in 90 min at so high a concentration.
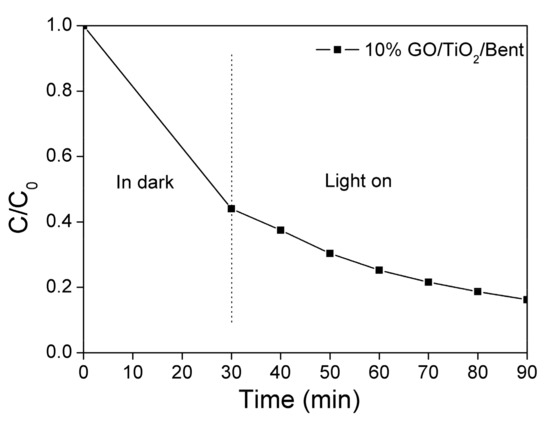
Figure 7.
Removal rate of MB in solution with a higher concentration by 10% GO/TiO2/Bent (Concentration of MB: 100 mg/L in100 mL MB solution; Mass of 10% GO/TiO2/Bent: 50 mg).
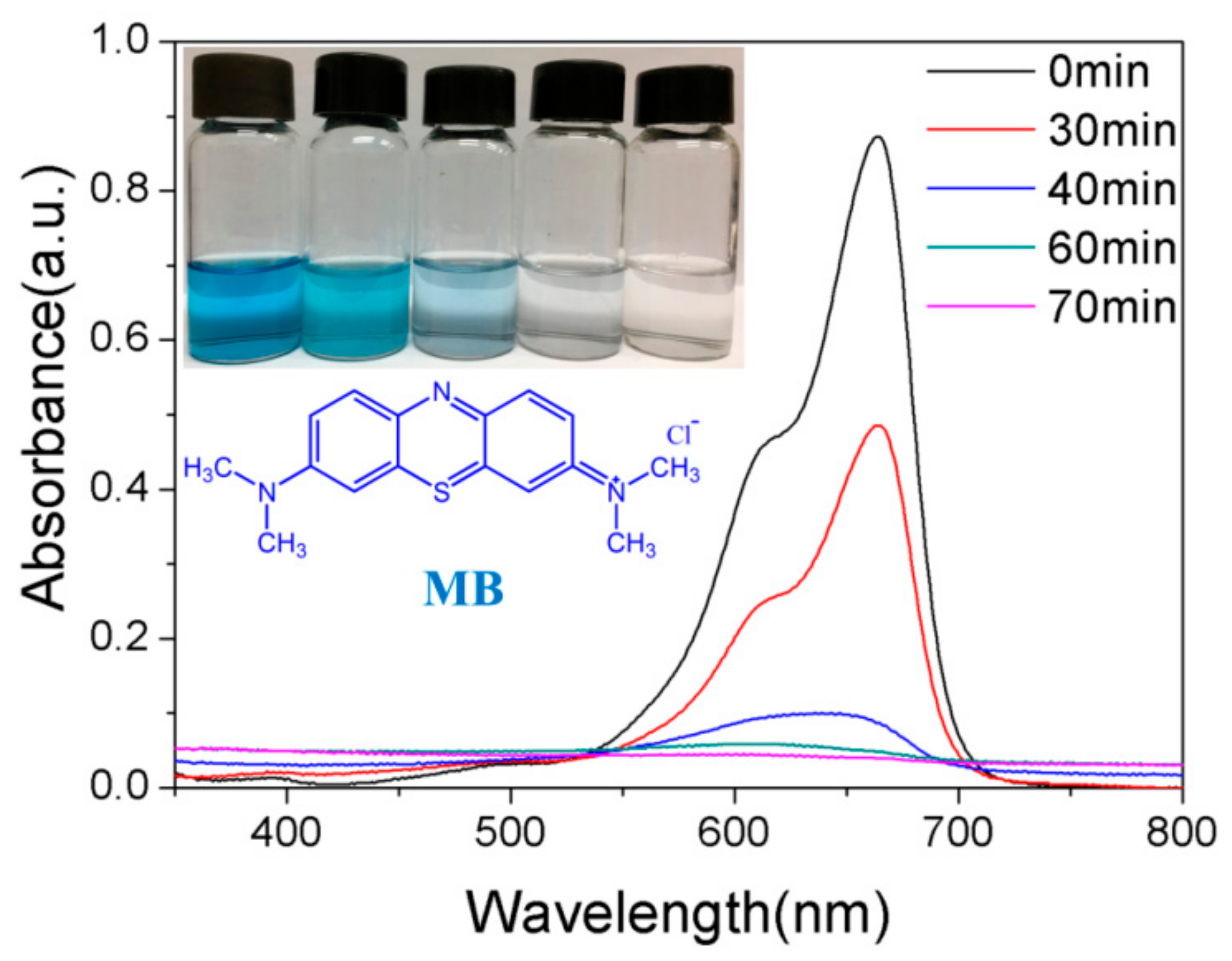
Figure 8 shows the evolutions of absorption spectra of MB solution in the presence of 10% GO/TiO2/Bent sponge under sunlight at different exposure time. It can be clearly seen that the characteristic absorption peak of MB solution at 664 nm is significantly decreased in intensity with the increasing irradiation time. In addition, the absorption peak disappears when the exposure time is increased to 70 min, along with the color fading of the solution, indicating that MB is completely removed, which is consistent with the result shown in Figure 6.
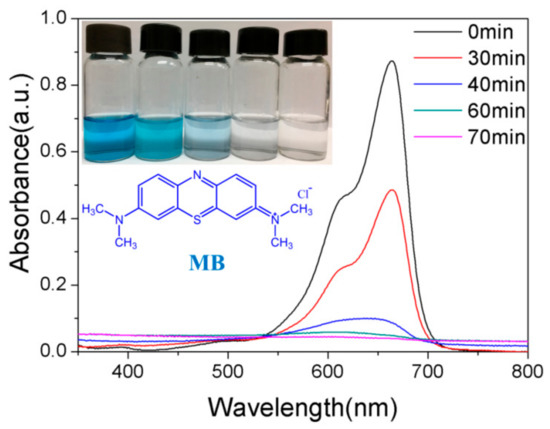
Figure 8.
The evolutions of absorption spectra of MB in the presence of 10% GO/TiO2/Bent sponge under sun light at different time (Content of MB:10 mg/L, 200 mL; Mass of 10% GO/TiO2/Bent: 100 mg).
Table 1 shows the removal rates of MB by different materials previously used. It can be observed that the removal rate of MB by 10% GO/TiO2/Bent is much higher than that of other materials listed, indicating that 10% GO/TiO2/Bent has great potential application in MB removal from water.

Table 1.
The removal rate of MB by various materials.
3.3. Photocatalytic Mechanism
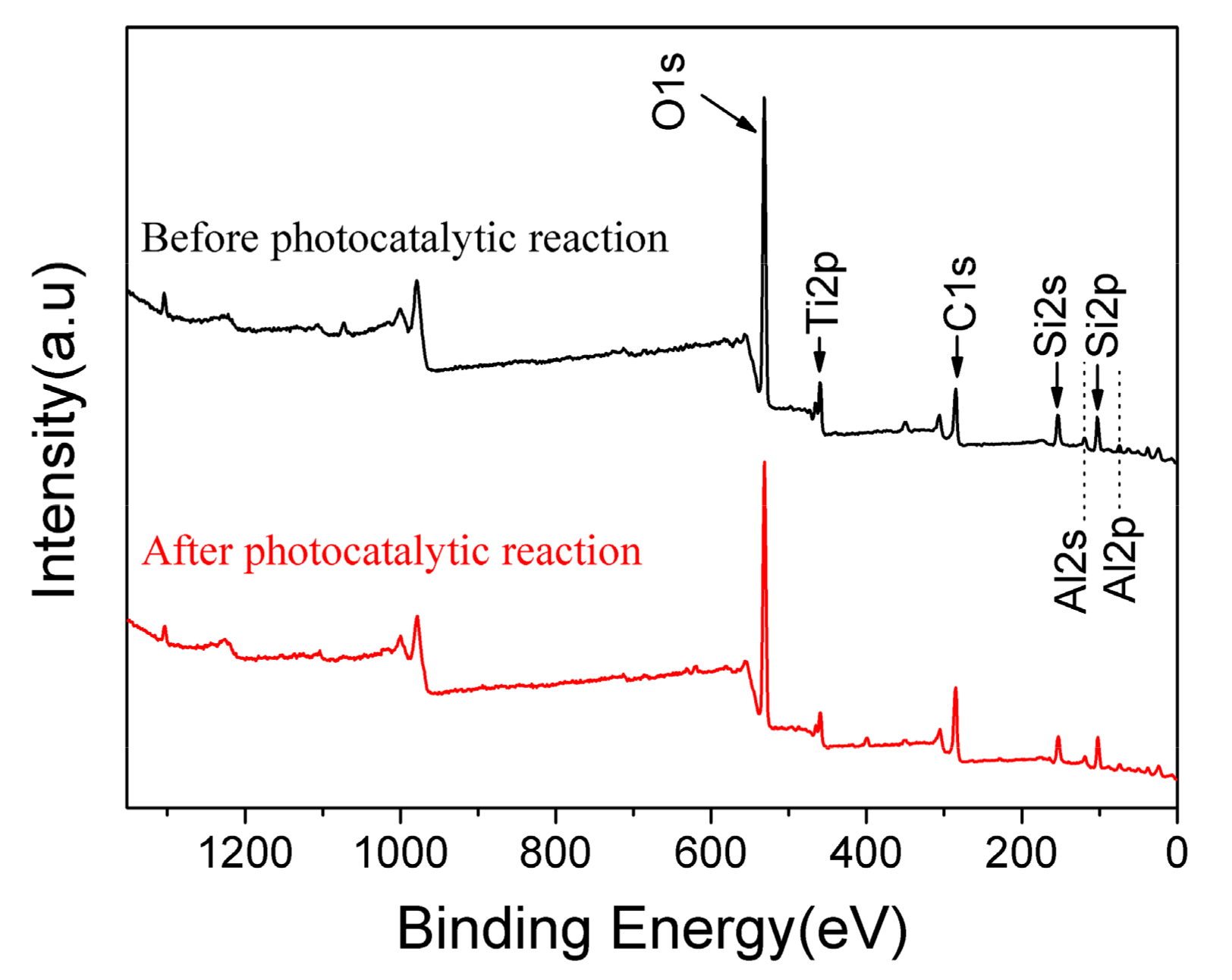
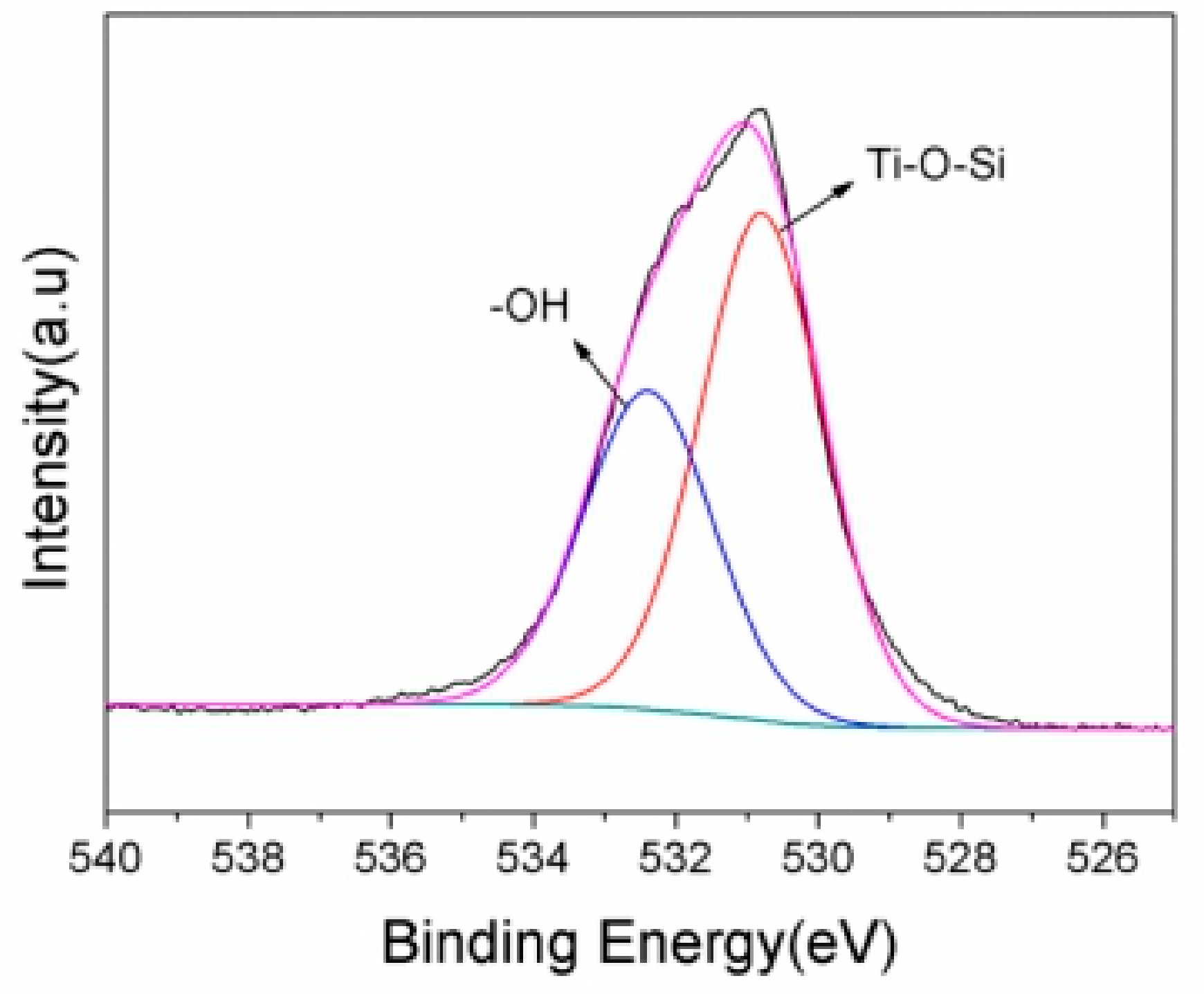
Based on the experimental data listed above, it is known that 10% GO/TiO2/Bent exhibits excellent removal efficiency of MB based on both adsorption and photocatalytic process. To explore the mechanism of photocatalysis and detect the surface elements of holes in sponge structure, XPS analysis is carried out on the GO/TiO2/Bent sample. Figure 9 is the full spectrum of XPS before and after photocatalytic reaction of the sample. After adsorption and photocatalysis, the surface elements of the composite change slightly. There is a small decrease of the content of Si (from 16.64% to 14.93%) and Al (from 5.2% to 4.6%) on the surface of the composite after reaction, due to the slight dissolution of Bent on the outer surface during the reaction. In fact, the reaction, which occurs on Bent surface by consuming the hydroxyl groups to produce •OH, will accelerate the dissolution of Bent on the outer surface. Figure 10 is the XPS spectrum of O element in 10% GO/TiO2/Bent. Binding energy 532.2 eV is the characteristic peak of –OH on the catalyst surface [62,63]. The binding energy of 529.8 eV is Ti–O–Si bond, indicating that Ti–O–Si bond is formed between TiO2 and Bent. It is the binding of chemical bond and strong loading of TiO2 on the surface of Bent, which is favorable for charge transfer [64,65].
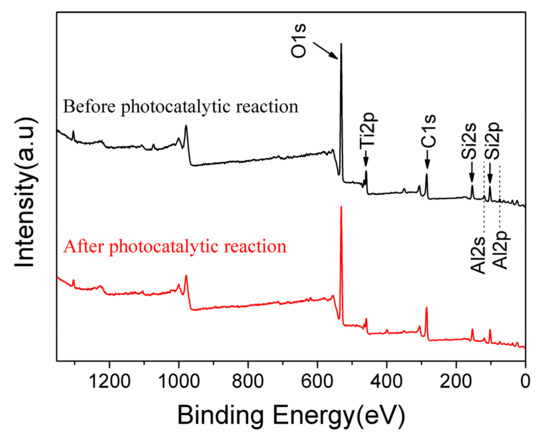
Figure 9.
XPS survey spectrum of 10% GO/TiO2/Bent sponge before and after photocatalytic reaction.
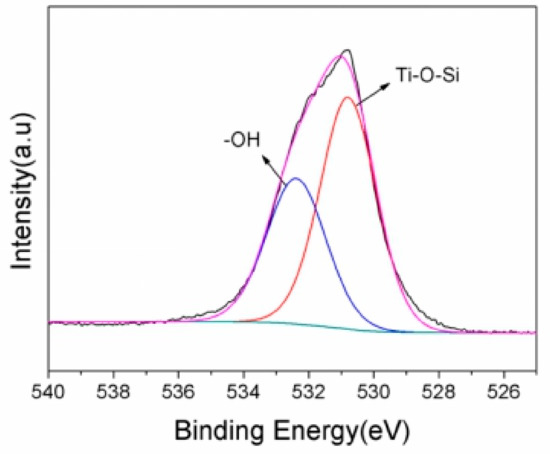
Figure 10.
XPS spectra of O element in 10% GO/TiO2/Bent sponge.
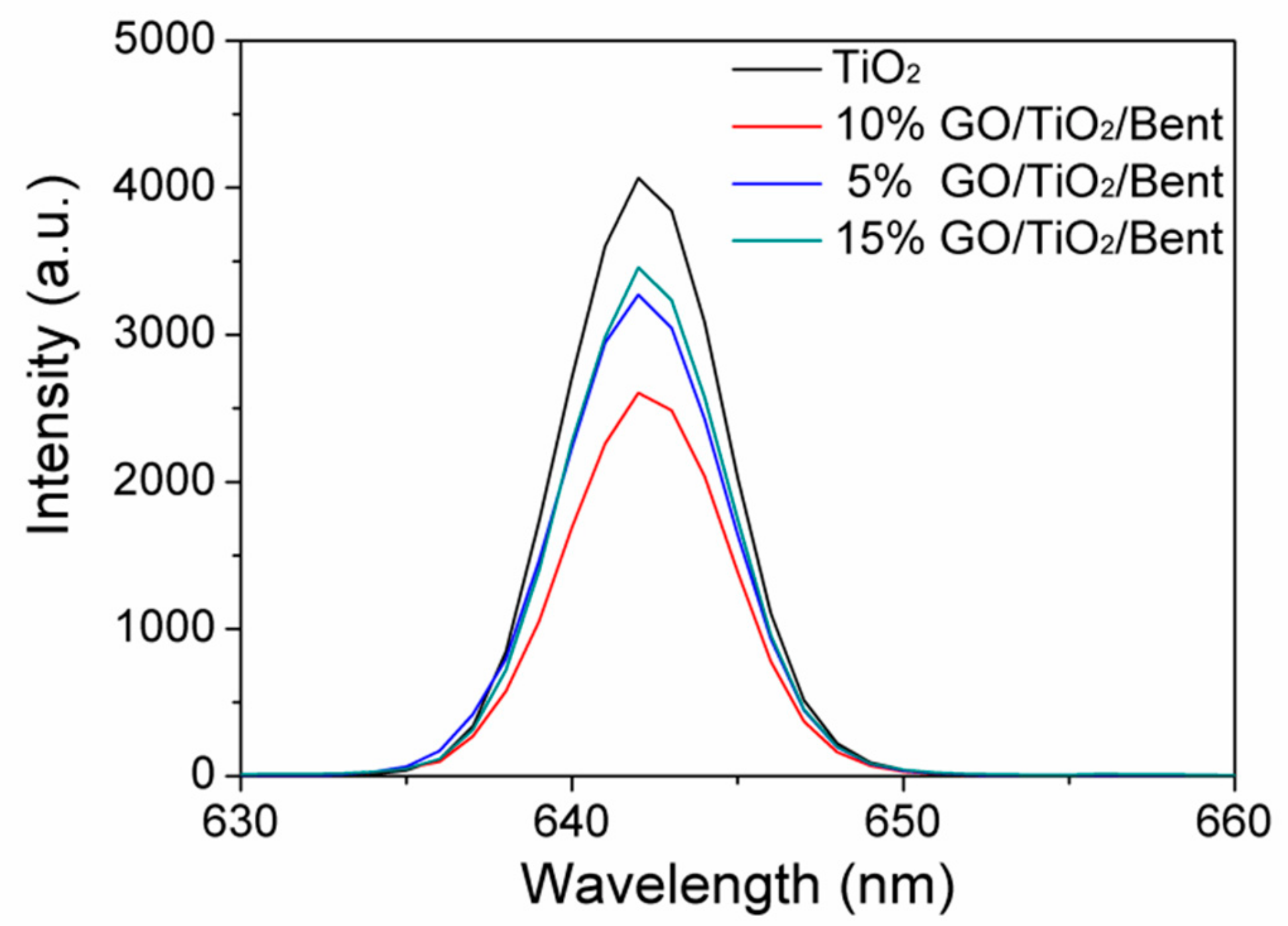
It is known that photoluminescence (PL) spectra are often employed to reveal the separation performances of electron–hole pairs in semiconductors [66]. Therefore, the separation properties of photo-generated electron–hole pairs of composites were further studied by PL. Figure 11 shows the PL emission spectra of TiO2 and GO/TiO2/Bent composites. It can be found that the spectra of GO/TiO2/Bent samples appear to be similar with that of TiO2, which means that GO and Bent have not induced new photoluminescence. In addition, the PL intensities of the composites are weakened in comparison to pure TiO2 and gradually decrease with the increasing of GO content. When GO content is less than 10%, the fluorescence intensity of GO/TiO2/Bent decreases with the increasing of GO content, which indicates that GO can rapidly accept electrons generated by photo excitation of TiO2, and effectively inhibit the recombination of electron–hole pairs. However, the PL intensity of 15% GO/TiO2/Bent is lower than that of TiO2, but higher than those of 5% and 10% GO/TiO2/Bent. When GO content is more than 10%, the fluorescence intensity of GO/TiO2/Bent increases with the increasing of GO content, indicating that excess GO has no positive effect on the light effect of composite. Moreover, excessive GO will cause the mask effect, which is not favorable for enhancing the photocatalytic activity of photocatalysts toward MB. To sum up, the photoluminescence (PL) spectra verify that GO/TiO2/Bent sponges exhibit excellent electron transport ability.
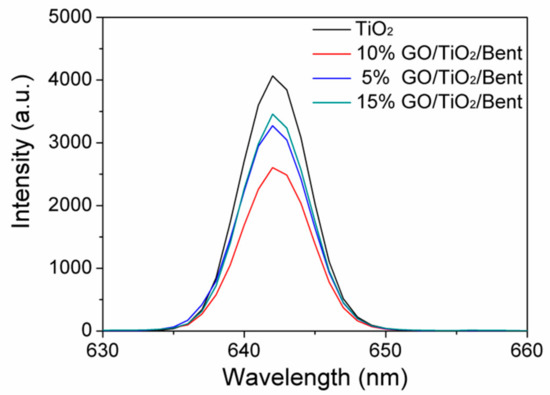
Figure 11.
Photoluminescence (PL) spectra of TiO2 and GO/TiO2/Bent sponges.
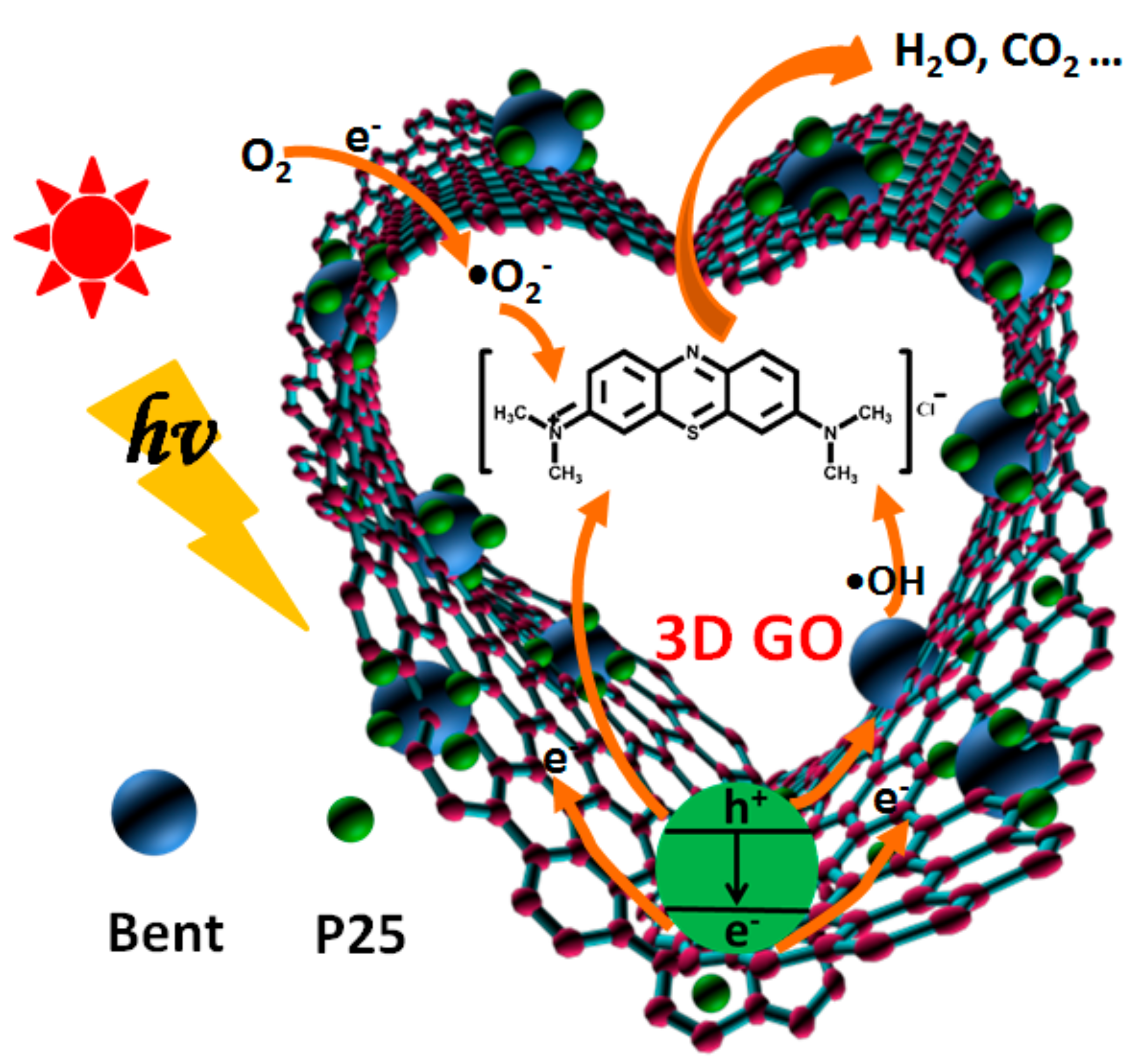
The whole mechanism of photocatalytic degradation is shown in Figure 12 and can be described by the following equations.
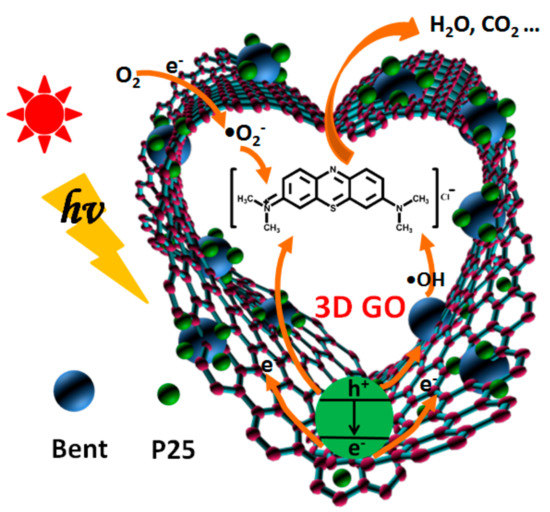
Figure 12.
The photocatalytic degradation mechanism of MB based on GO/TiO2/Bent sponge.
The organic pollutant MB molecule is first adsorbed to the surface of GO/TiO2/Bent and then decomposed in situ from the surface of the composite. The three-dimensional GO sponge has excellent electronic transmission ability, which can effectively prevent the electron–hole pair recombination of TiO2 and improve the photocatalytic efficiency. Bentonite not only presents strong adsorption ability, but also provides a large number of hydroxyl groups on its surface, which can react with the holes (Equation (3)) to increase the production of •OH. These •OH can effectively attack the organic dyes adsorbed on the composite surface. Hydroxyl radical has strong oxidation ability and can degrade organic pollutants into CO2 and H2O, which plays an important role in photocatalytic reaction [67,68]. Therefore, Equation (3) based on Bent is especially crucial in the mechanism.
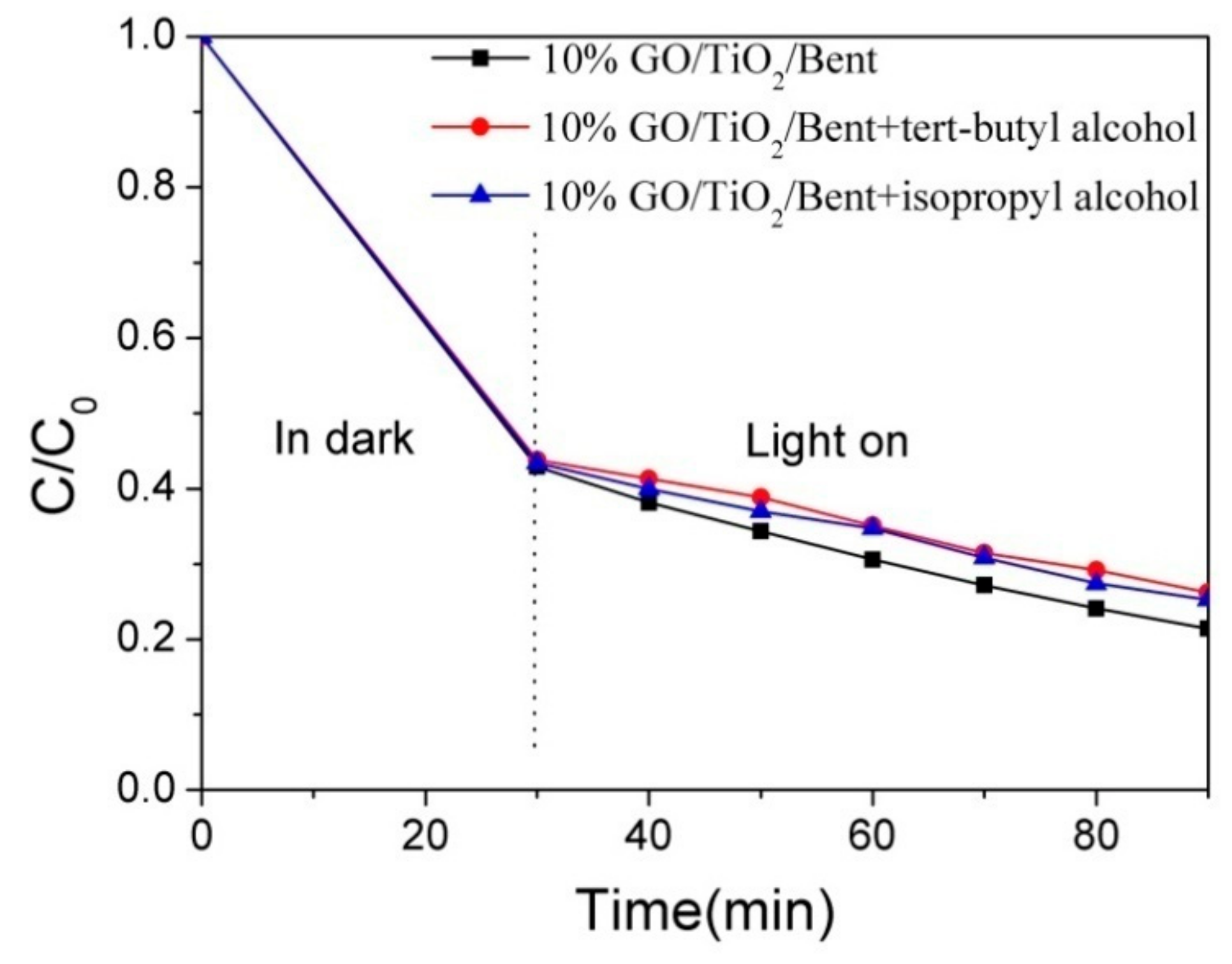
To verify whether 10% GO/TiO2/Bent produces •OH in photocatalytic reaction to improve photocatalytic efficiency, tert-butanol and isopropanol, which can selectively quench hydroxyl radical, were added to MB solution in this experiment to analyze the photolysis behavior under xenon lamp irradiation. To study the photo-degradation reaction after reaching adsorption equilibrium, the concentration of MB was selected as 100 mg/L, and the control experiment was carried out under the same conditions. The first group of MB solution did not contain any reagents and was used as blank control group; the second group of MB solution was combined with tert-butanol (40 mmol/L); the third group of MB solution was combined with isopropanol (40 mmol/L). The experimental results are shown in Figure 13. It can be seen that the degradation rate of MB is reduced by adding tert-butanol and isopropanol, proving that •OH produced by 10% GO/TiO2/Bent participates in the degradation of MB under light irradiation.
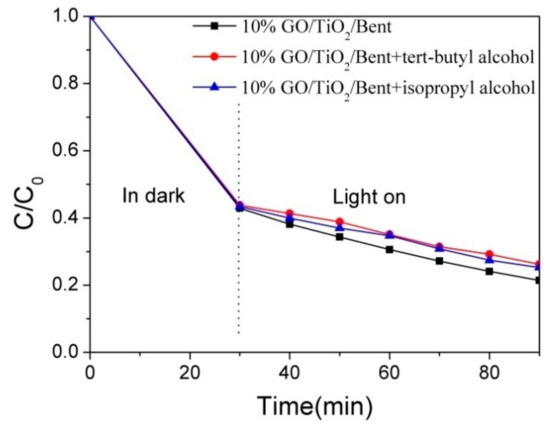
Figure 13.
Effect of radical quenching agents on MB degradation by 10% GO/TiO2/Bent (Concentration of MB: 100 mg/L).
4. Conclusions
In this work, the novel GO/TiO2/Bent sponge was synthesized successfully by a facile hydrothermal method. It was found that the removal rate of 10% GO/TiO2/Bent is better than many other materials reported, and MB can be completely removed within 70 min. It is remarkable that even at a high MB concentration of ten times the original one, the sponge still exhibits an excellent removal rate of 80% within 90 min. Both Bent and GO sponge play an important role in the adsorption and degradation of the dyes. The surface of Bent is rich in hydroxyl groups, promoting the formation of •OH and enhancing the ability to degrade organic pollutants through photo-generated hole. Also Bent can immobilize nano-TiO2 and prevent its agglomeration. Three-dimensional GO sponge exhibits excellent electron transport ability, which can effectively prevent the combination of TiO2 electron–hole pairs and improve the photocatalytic efficiency. Significantly, its sponge-like structure not only provides a large specific surface area and more adsorption sites for TiO2/Bent, but also benefits the recycle of the composite. Because as a whole sponge it can be taken out of the solution, avoid the loss of TiO2 and Bent and then be easily reused. The results show that the removal rate is better than that of single-component and bi-component systems, which indicates that the enhanced removal performance presented by 10% GO/TiO2/Bent is not a simple superposition of the performance of each component, but a result of synergistic action among three components. In summary, the synthesized GO/TiO2/Bent sponge has great application prospects for the removal of pollutants from water.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/4/824/s1, Figure S1: FTIR spectra of GO, 10% GO/Bent and 10% GO/TiO2/Bent sponges; Figure S2: Photodegradation of MB by GO/TiO2/Bent sponge with different GO proportions under simulated sunlight irradiation after 30 min dark adsorption; Figure S3: The N2 adsorption-desorption isotherms of TiO2, GO, TiO2/Bent, 10% GO/TiO2 and 10% GO/TiO2/Bent; Table S1: The BET surface area of GO, TiO2, TiO2/Bent, 10% GO/TiO2 and 10% GO/TiO2/Bent.
Author Contributions
Conceptualization, L.W. and M.P.; experiments and data analysis, Y.L., L.W. and N.X.; writing—original draft preparation, Y.L., N.X. and P.W.; writing—review and editing, L.W. and W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (Grant Nos. 51672106 and 21802052) and Shandong Provincial Natural Science Foundation (Grant No. ZR2019MB066).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferreira Neto, E.; Ullah, S.; Simões, M.; Perissinotto, A.; De Vicente, F.; Noeske, P.L.; Ribeiro, S.; Rodrigues Filho, U. Solvent-controlled deposition of titania on silica spheres for the preparation of SiO2@TiO2 core@shell nanoparticles with enhanced photocatalytic activity. Colloid. Surf. A 2019, 570, 293–305. [Google Scholar] [CrossRef]
- Gan, W.; Shang, X.; Li, X.H.; Zhang, J.; Fu, X. Achieving high adsorption capacity and ultrafast removal of methylene blue and Pb2+ by graphene-like TiO2@C. Colloid. Surf. A 2018, 561, 218–225. [Google Scholar] [CrossRef]
- Field, R. Surface science: Separation by reconfiguration. Nature 2012, 489, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2014, 54, 2328–2338. [Google Scholar] [CrossRef]
- Mathieu Denoncourt, J.; Martyniuk, C.; De Solla, S.; Balakrishnan, V.; Langlois, V. Sediment contaminated with the azo dye disperse yellow 7 alters cellular stress and androgen-related transcription in silurana tropicalis larvae. Environ. Sci. Technol. 2014, 48, 2952–2961. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Bai, N.; Dong, H.; Mao, D. One-step synthesis of cationic hydrogel for efficient dye adsorption and its second use for emulsified oil separation. ACS Sustain. Chem. Eng. 2017, 5, 5598–5607. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, S.; Li, G.; Yang, J. Synergetic Effect of ultrasound, the heterogeneous fenton reaction and photocatalysis by TiO2 loaded on nickel foam on the degradation of pollutants. Materials 2016, 9, 457. [Google Scholar] [CrossRef]
- Wang, K.; Endo Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Bouclé, J.; Ohtani, B.; Kowalska, E.; Herlin Boime, N. Carbon/Graphene-modified titania with enhanced photocatalytic activity under uv and vis irradiation. Materials 2019, 12, 4158. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.S.; Sorcar, S.; Razzaq, A.; Grimes, C.A.; In, S.I. Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. J. Photochem. Photobiol. A Chem. 2018, 358, 432–440. [Google Scholar] [CrossRef]
- Saurav, S.; Thompson, J.F.; Hwang, Y.; Park, Y.H.; Majima, T.; Grimes, C.A.; Durrant, J.R.; In, S. High-rate solar-light photo conversion of CO2 to fuel: Controllable transformation from C1 to C2 products. Energy Environ. Sci. 2018, 11, 3183–3193. [Google Scholar]
- Malato, S.; Fernandez Ibanez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Ullah, R.; Sun, H.; Wang, S.; Ang, H.; Tadé, M. Wet-Chemical synthesis of InTaO4 for photocatalytic decomposition of organic contaminants in air and water with UV-vis light. Ind. Eng. Chem. Res. 2012, 51, 1563–1569. [Google Scholar] [CrossRef]
- Yu, J.; Ho, W.; Yu, J.; Yip, H.; Wong, P.K.; Zhao, J. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 2005, 39, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Wang, L.; Qu, J.; Wang, A. Visible-light-induced photocatalytic degradation of azodyes in aqueous AgI/TiO2 dispersion. Environ. Sci. Technol. 2006, 40, 7903–7907. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, W. Visible-light-induced photocatalytic degradation of 4-chlorophenol and phenolic compounds in aqueous suspension of pure titania: Demonstrating the existence of a surface-complex-mediated path. J. Phys. Chem. B 2005, 109, 5143–5149. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Li, X.; Deng, L.; Fan, G.; He, Y. A novel nano-sized MoS2 decorated Bi2O3 heterojunction with enhanced photocatalytic performance for methylene blue and tetracycline degradation. Ceram. Int. 2019, 45, 15824–15833. [Google Scholar] [CrossRef]
- Carreño Lizcano, M.I.; Gualdrón Reyes, A.F.; Rodríguez González, V.; Pedraza Avella, J.A.; Niño Gómez, M.E. Photoelectrocatalytic phenol oxidation employing nitrogen doped TiO2-rGO films as photoanodes. Catal. Today 2020, 341, 96–103. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, X.; He, F.; Yu, X.; Zhou, J.; Hu, Y.; Xie, J. Low-temperature synthesis and photocatalytic activity of TiO2 pillared montmorillonite. Langmuir 2008, 24, 1026–1030. [Google Scholar] [CrossRef]
- Ma, Z.; Zeng, C.; Hu, L.; Zhao, Q.; Yang, Q.; Niu, J.; Yao, B.; He, Y. A high-performance photocatalyst of ZnTCPP sensitized porous graphitic carbon nitride for antibiotic degradation under visible light irradiation. Appl. Surf. Sci. 2019, 484, 489–500. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Rao, T.N.; Sathish, M.; Rangappa, D.; Honma, I.; Miyauchi, M. Superhydrophilic graphene-loaded TiO2 thin film for self-cleaning applications. ACS Appl. Mater. Interfaces 2013, 5, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pastrana Martínez, L.M.; Morales Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide-TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, K. Reduced graphene oxide-TiO2 nanocomposite with high photocatalystic activity for the degradation of rhodamine B. J. Mol. Catal. A Chem. 2011, 345, 101–107. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Liu, S.; Wang, S. Graphene facilitated visible light photodegradation of methylene blue over titanium dioxide photocatalysts. Chem. Eng. J. 2013, 214, 298–303. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef]
- Liu, M.; Yang, H. Large surface area mesoporous Al2O3 from kaolin: Methodology and characterization. Appl. Clay Sci. 2010, 50, 554–559. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Liang, C.H.; Xie, Q.; Wang, Y.N.; Zhang, S.; Qiao, X.; Li, X. Quantum chemical investigation on the mechanism and kinetics of PBDE photo oxidation by center dot oh: A case study for bde-15. Environ. Sci. Technol. 2011, 45, 4839–4845. [Google Scholar] [CrossRef]
- Paul, B.; Martens, W.N.; Frost, R.L. Immobilised anatase on clay mineral particles as a photocatalyst for herbicides degradation. Appl. Clay Sci. 2012, 57, 49–54. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Bujdák, J. Effect of the layer charge of clay minerals on optical properties of organic dyes. A review. Appl. Clay Sci. 2006, 34, 58–73. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Ng, S.; Plank, J. Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem. Concr. Res. 2012, 42, 847–854. [Google Scholar] [CrossRef]
- Mohsin, N.; Waheed, M.; Jiseon, J.; Dae, S. One-step hydrothermal synthesis of porous 3D reduced graphene oxide/TiO2 aerogel for carbamazepine photodegradation in aqueous solution. Appl. Catal. B Environ. 2017, 203, 85–89. [Google Scholar]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol a over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Xue, N.; Wang, L.; Pei, M.; He, Y.; Du, Y.; Guo, W. Preparation and characterization of sodium polyacrylate-grafted bentonite and its performance removing Pb2+ from aqueous solutions. RSC Adv. 2016, 6, 98945–98951. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Ding, M.; Pei, M.; Guo, W. Ultrasensitive electrochemical detection of ochratoxin A based on signal amplification by one-pot synthesized flower-like PEDOT-AuNFs supported on graphene oxide sponge. Analyst 2019, 144, 5866–5874. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Synthesis and Characterization of amidoximated polyacrylonitrile/organobentonite composite for Cu(II), Zn(II), and Cd(II) adsorption from aqueous solutions and industry wastewaters. Ind. Eng. Chem. Res. 2008, 47, 6175–6184. [Google Scholar] [CrossRef]
- Nguyen Phan, T.D.; Pham, V.H.; Shin, E.W.; Pham, H.D.; Kim, S.; Chung, J.S.; Kim, E.J.; Hur, S.H. The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhuang, Y.; Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Magalhães, P.; Ângelo, J.; Sousa, V.M.; Nunes, O.C.; Andrade, L.; Mendes, A. Synthesis and assessment of a graphene-based composite photocatalyst. Biochem. Eng. J. 2015, 104, 20–26. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Y.; Yu, L.; Li, F.; Yang, Z.; Hou, T.; Hu, D.; Xia, M. Enhanced photocatalytic activity of microwave treated TiO2 pillared montmorillonite. Mater. Chem. Phys. 2006, 98, 377–381. [Google Scholar] [CrossRef]
- Miao, S.; Liu, Z.; Han, B.; Zhang, J.; Yu, X.; Du, J.; Sun, Z. Synthesis and characterization of TiO2-montmorillonite nano composites and their application for removal of methylene blue. J. Mater. Chem. 2006, 16, 579–584. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J.; Yang, K.; Feng, S.; Tan, W.; Tao, Y.; Mao, H.; Kong, Y. Preparation of S-doped TiO2-three dimensional graphene aerogels as a highly efficient photocatalyst. Synth. Met. 2017, 231, 51–57. [Google Scholar] [CrossRef]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant soot for pollutant dye degradation: Soluble graphene nanosheets for visible light induced photodegradation of methylene blue. ACS Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, A.; Wei, Z.; Liu, S. Construction of three-dimensional hemin-functionalized graphene hydrogel with high mechanical stability and adsorption capacity for enhancing photodegradation of methylene blue. ACS Appl. Mater. Interfaces 2017, 9, 4006–4014. [Google Scholar] [CrossRef]
- Breault, T.M.; Bartlett, B.M. Lowering the band gap of anatase-structured TiO2 by coalloying with nb and n: Electronic structure and photocatalytic degradation of methylene blue dye. J. Phys. Chem. C 2012, 116, 5986–5994. [Google Scholar] [CrossRef]
- DePuccio, D.P.; Botella, P.; O’Rourke, B.; Landry, C.C. Degradation of methylene blue using porous WO3, SiO2-WO3, and their au-loaded analogs: Adsorption and photocatalytic studies. ACS Appl. Mater. Interfaces 2015, 7, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Xu, L.; Shang, S.; Zhou, X.; Meng, L. Visible light induced methylene blue dye degradation photo-catalyzed by WO3/graphene nanocomposites and the mechanism. Ceram. Int. 2016, 42, 15235–15241. [Google Scholar] [CrossRef]
- Shanmugam, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced photocatalytic performance of the graphene-V2O5 nanocomposite in the degradation of methylene blue dye under direct sunlight. ACS Appl. Mater. Interfaces 2015, 7, 14905–14911. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Wu, X.; Meng, M.; Zhu, X.; Yang, L.; Chu, P. Photothermal contribution to enhanced photocatalytic performance of graphene-based nanocomposites. ACS Nano 2014, 8, 9304–9310. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shi, L.; He, C.; Gao, F.; Li, B.; Zhou, Q.; Hu, H.; Shao, G.; Wang, X.; Qiu, J. Highly efficient synthesis of graphene/MnO2 hybrids and their application for ultrafast oxidative decomposition of methylene blue. Carbon 2014, 66, 485–492. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Liu, S. Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methyl blue under visible-light irradiation. J. Mater. Chem. A 2015, 3, 8552–8558. [Google Scholar] [CrossRef]
- Guo, R.; Yin, G.; Sha, X.; Wei, L.; Zhao, Q. Effect of surface modification on the adhesion enhancement of electrolessly deposited Ag-PTFE antibacterial composite coatings to polymer substrates. Mater. Lett. 2015, 143, 256–260. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Wen, H.; Tang, Z.; Zhao, H.; Li, Y.; Wang, D. Photocatalytic properties of graphdiyne and graphene modified TiO2: From theory to experiment. ACS Nano 2013, 7, 1504–1512. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Vossoughi, M.; Zakeri, S.M.E.; Rahimi, M. Enhancement of efficient Ag-S/TiO2 nanophotocatalyst for photocatalytic degradation under visible light. Ind. Eng. Chem. Res. 2014, 53, 9578–9586. [Google Scholar] [CrossRef]
- Khare, P.; Singh, A.; Verma, S.; Bhati, A.; Sonker, A.; Tripathi, K.; Sonkar, S. Sunlight-induced selective photocatalytic degradation of methylene blue in bacterial culture by pollutant soot derived nontoxic graphene nanosheets. ACS. Sustain. Chem. Eng. 2018, 6, 579–589. [Google Scholar] [CrossRef]
- Lee, C.G.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P. Porous Electrospun fibers embedding TiO2 for Adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Vyas, R.K.; Dalai, A.K. Thermodynamic and kinetic studies of methylene blue degradation using reactive adsorption and its comparison with adsorption. J. Chem. Eng. Data 2017, 62, 3651–3662. [Google Scholar] [CrossRef]
- Yu, C.; Wei, L.; Chen, J.; Xie, Y.; Zhou, W.; Fan, Q. Enhancing the photocatalytic performance of commercial TiO2 crystals by coupling with trace narrow-band-gap Ag2CO3. Ind. Eng. Chem. Res. 2014, 53, 5759–5766. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Liu, G.; Li, M.; Liu, J.; Sun, W.; Ye, J.; Lin, J. Efficient organic degradation under visible light by α-Bi2O3 with a CuOx-assistant electron transfer process. Appl. Catal. B Environ. 2015, 163, 267–276. [Google Scholar] [CrossRef]
- López, T.; Bosch, P.; Tzompantzi, F.; Gómez, R.; Navarrete, J.; López Salinas, E.; Llanos, M.E. Effect of sulfation methods on TiO2-SiO2 sol-gel catalyst acidity. Appl. Catal. A Gen. 2000, 197, 107–117. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, T.J.; Jin, Y. Surface characteristics of hydrous silica-coated TiO2 particles. Powder Technol. 2002, 123, 194–198. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Gao, Q.; Yuan, J.; Wen, J.; Fang, Y.; Liu, W.; Zhang, S.; Liu, Y. Metal-free carbon nanotube-SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catal. Sci. Technol. 2015, 5, 2798–2806. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).