Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
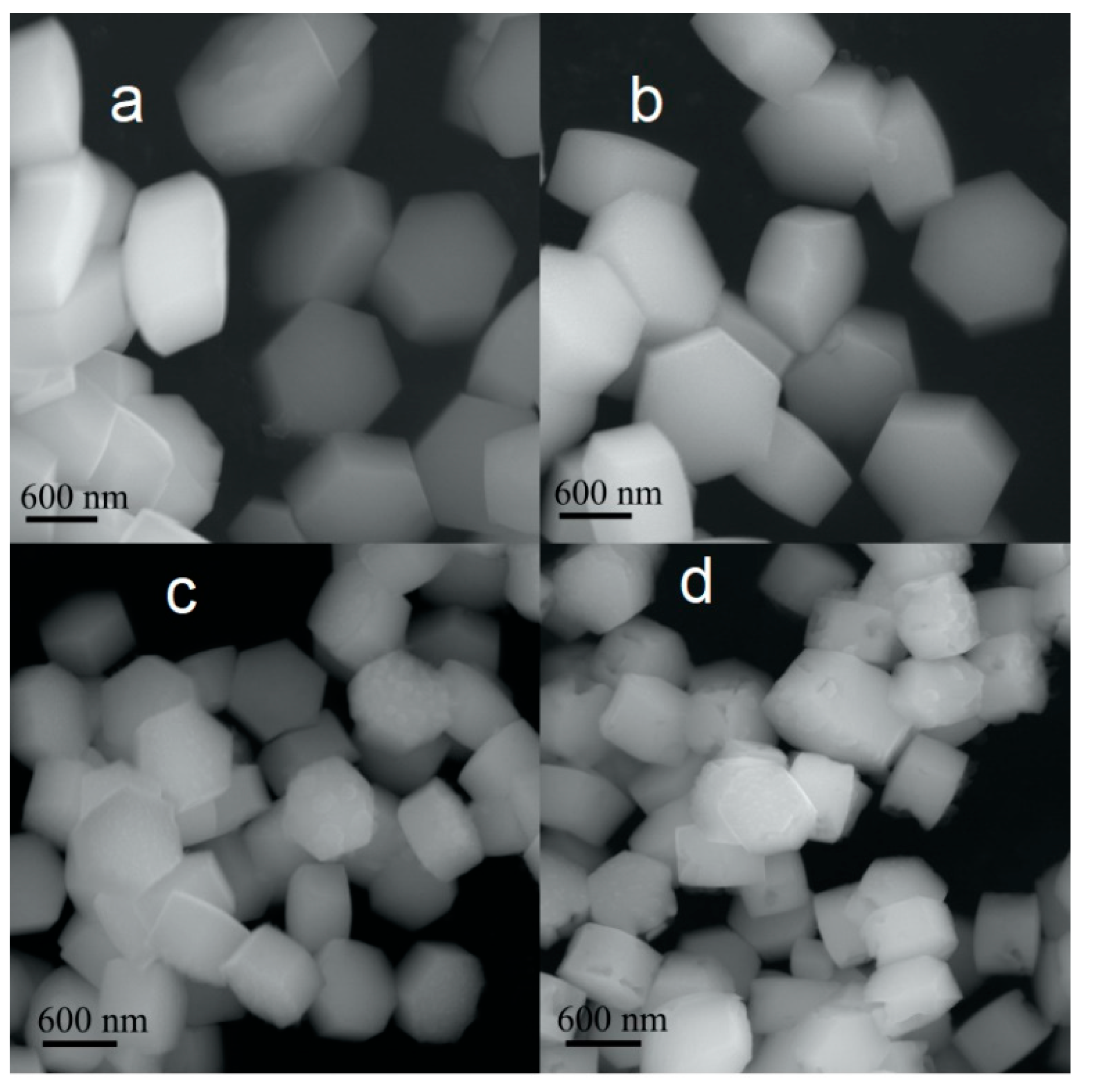
3.1. Crystal Structure and Morfology
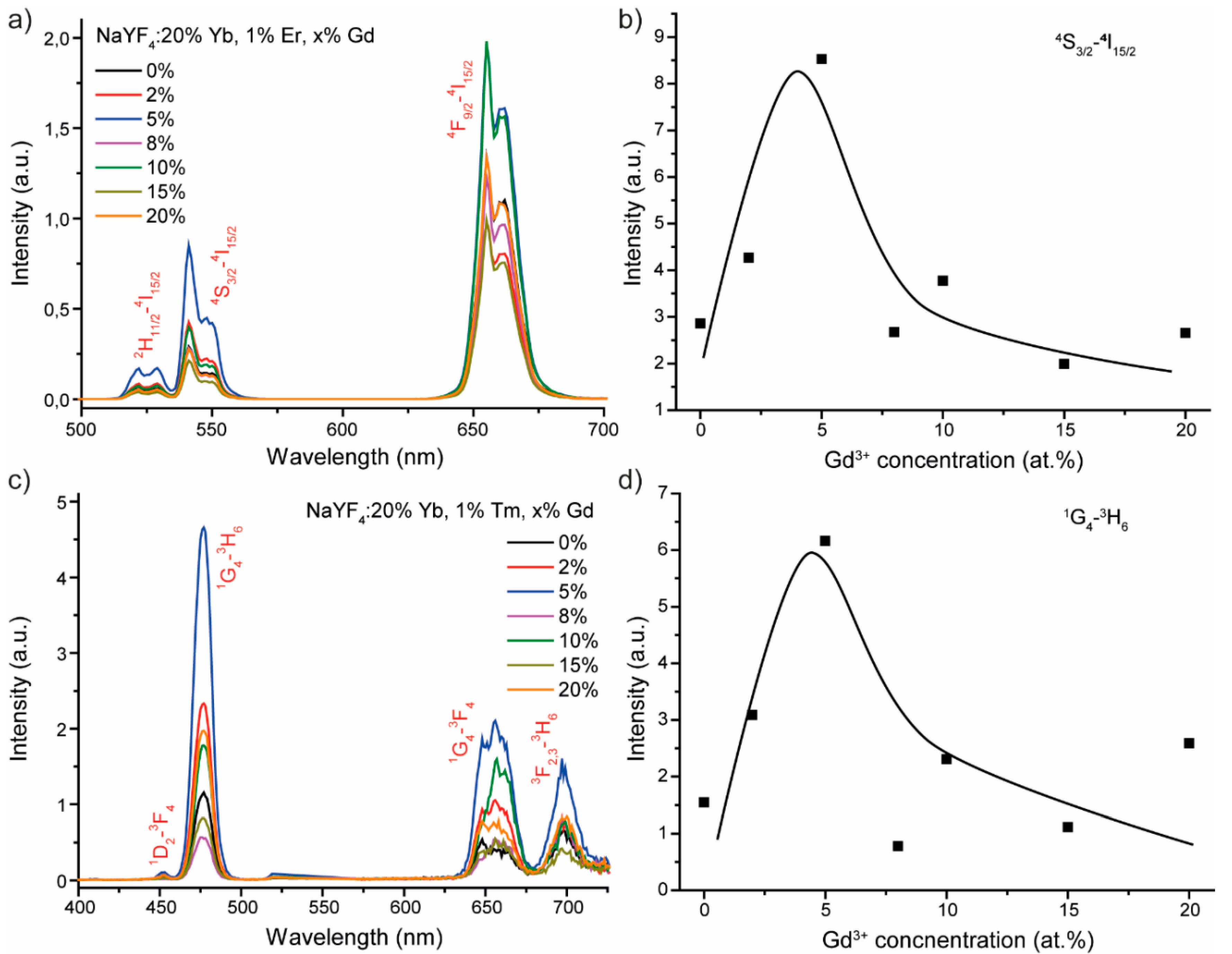
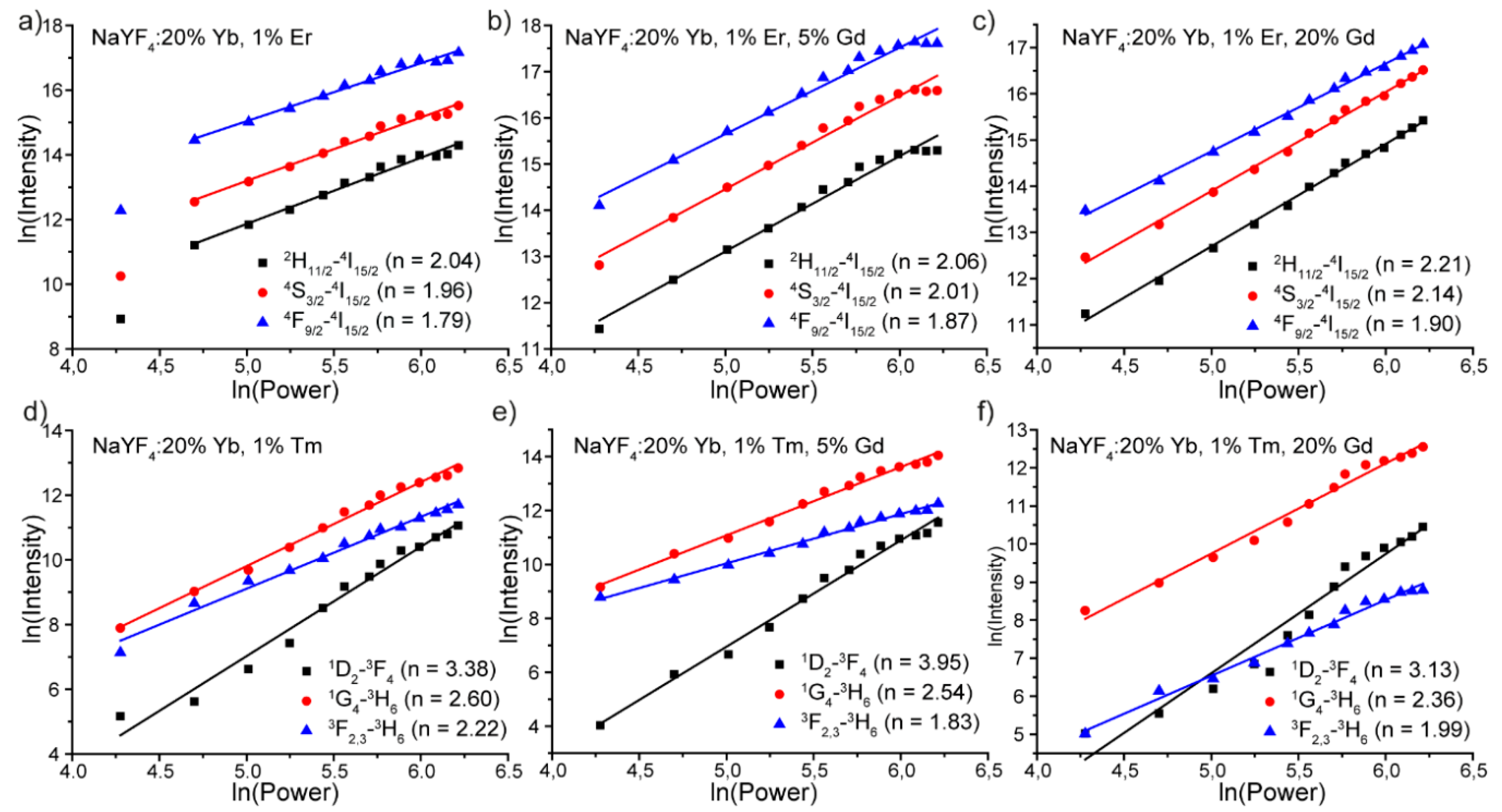
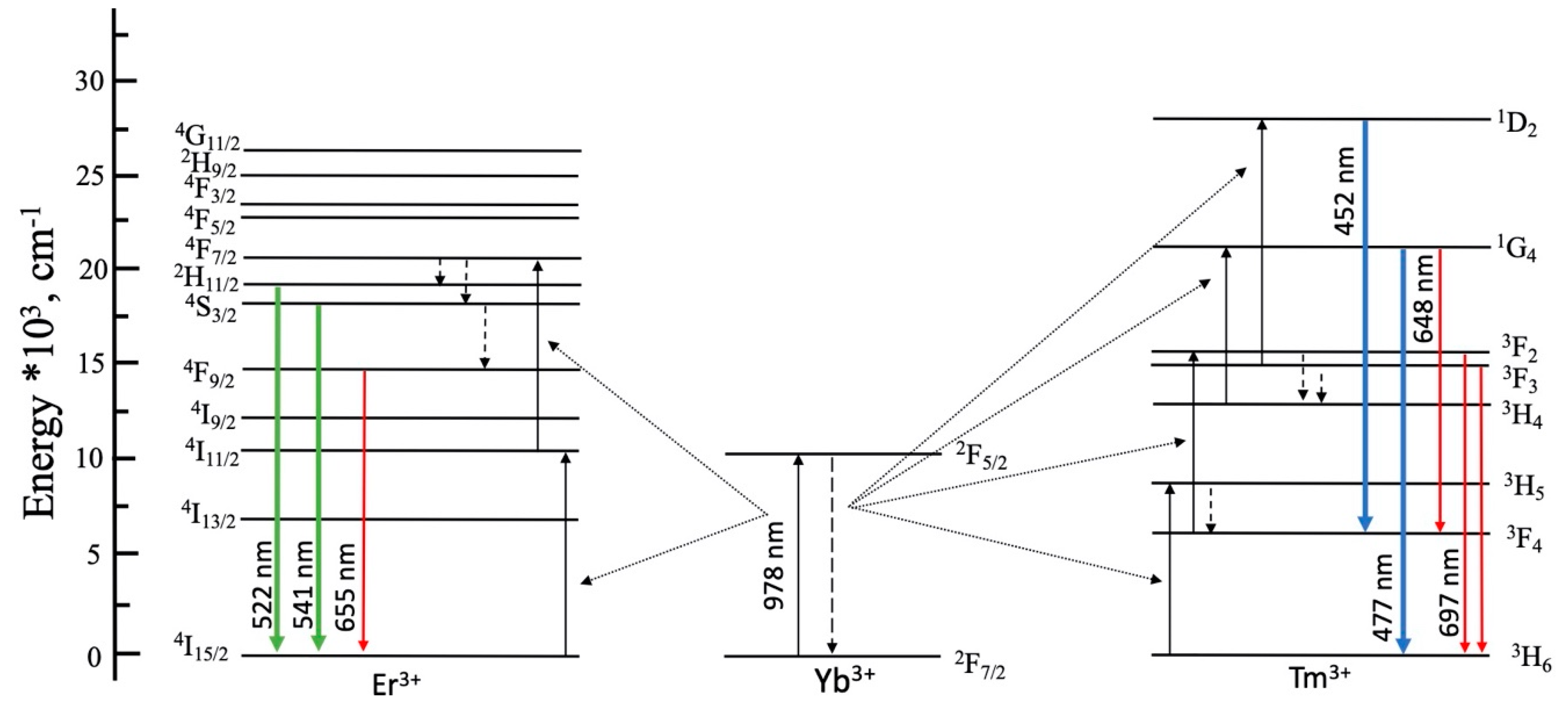
3.2. Luminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 4, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wu, H.; Hu, H.; Wu, M.; Su, Q. Enhanced blue and green upconversion in hydrothermally synthesized hexagonal NaY1−xYbxF4: Ln3+(Ln3+ = Er3+ or Tm3+). J. Alloys Compd. 2004, 368, 94–100. [Google Scholar] [CrossRef]
- Page, R.H.; Schaffers, K.I.; Waide, P.A.; Tassano, J.B.; Payne, S.A.; Krupke, W.K. Upconversion-pumped luminescence efficiency of rare-earth-doped hosts sensitized with trivalent ytterbium. J. Opt. Soc. Am. B 1998, 15, 996–1008. [Google Scholar] [CrossRef]
- Kumar, B.R.; Nyk, M.; Ohulchanskyy, T.Y.; Flask, C.A.; Prasad, P.N. Combined Optical and MR Bioimaging Using Rare Earth Ion Doped NaYF4 Nanocrystals. Adv. Funct. Mater. 2009, 19, 853–859. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, F.; Yao, L.; Cai, W.; Li, X. Selected synthesis of cubic and hexagonal NaYF4 crystals via a complex-assisted hydrothermal route. J. Cryst. Growth 2006, 290, 296–300. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z.; Jiang, M.; Que, D.; Shi, S.; Zheng, N. Preparation and photodynamic therapy application of NaYF4: Yb, Tm/NaYF4: Yb, Er multifunctional upconverting nanoparticles. New J. Chem. 2013, 37, 1782–1788. [Google Scholar] [CrossRef]
- Liu, X.; Qian, H.; Ji, Y.; Li, Z.; Shao, Y.; Hu, Y.; Tong, G.; Li, L.; Guo, W.; Guo, H. Mesoporous silica-coated NaYF4 nanocrystals: Facile synthesis, in vitro bioimaging and photodynamic therapy of cancer cells. RSC Adv. 2012, 2, 12263–12268. [Google Scholar] [CrossRef]
- Miteva, T.; Yakutkin, V.; Nelles, G.; Baluschev, S. Annihilation assisted upconversion: All-organic, flexible and transparent multicolour display. New J. Phys. 2008, 10, 103002. [Google Scholar] [CrossRef]
- Martin, N.; Boutinaud, P.; Malinowski, M.; Mahiou, R.; Cousseins, J.C. Optical spectra and analysis of Pr3+ in b-NaYF4. J. Alloys Compd. 1998, 277, 304–306. [Google Scholar] [CrossRef]
- Tropper, A.C.; Carter, J.N.; Lauder, R.D.T.; Hanna, D.C. Analysis of blue and red laser performance of the infrared-pumped praseodymium-doped fluoride fiber laser. J. Opt. Soc. Am. B 1994, 11, 886–893. [Google Scholar] [CrossRef]
- Sandrock, T.; Scheife, H.; Heumann, E.; Huber, G. High-power continuous-wave upconversion fiber laser at room temperature. Opt. Let. 1997, 22, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, M.; Xie, M.; Chen, L.; Li, Y.; Wang, Q. Dopant-Controlled Synthesis of Water-Soluble Hexagonal NaYF4 Nanorods with Efficient Upconversion Fluorescence for Multicolor Bioimaging. Nano Res. 2010, 3, 51–60. [Google Scholar] [CrossRef]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Jin, S.; Yin, W. TWEEN coated NaYF4: Yb, Er/NaYF4 core/shell upconversion nanoparicles for bioimaging and drug delivery. RSC Adv. 2012, 2, 7037–7041. [Google Scholar] [CrossRef]
- Das, P.; Sedighi, A.; Krull, U.J. Cancer biomarker determination by resonance energy transfer using functional fluorescent nanoprobes. Anal. Chim. Acta. 2018, 1041, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, W.; Ni, Y.; Lu, C.; Xu, Z. Different Upconversion Properties of β-NaYF4: Yb3+, Tm3+/Er3+ in Affecting the Near-Infrared-Driven Photocatalytic Activity of High-Reactive TiO2. ACS Appl. Mater. Interfaces 2014, 6, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qin, W.; Zhou, J. Hydrothermal synthesis and aspect ratio dependent upconversion luminescence of NaYF4: Yb3+/Er3+ microcrystals. J. Nanosci. Nanotechnol. 2016, 16, 3806–3810. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion Luminescence Properties of NaYF4:Yb:Er Nanoparticles Co-doped with Gd3+. Opt. Mater. 2015, 90, 200–207. [Google Scholar] [CrossRef]
- He, E.; Zheng, H.; Gao, W.; Tu, Y.; Lu, Y.; Li, G. Investigation of upconversion and downconversion fluorescence emissions from β-NaLn1F4: Yb3+, Ln23+ (Ln1 = Y, Lu; Ln2 = Er, Ho, Tm, Eu) hexagonal disk system. Mater. Res. Bull. 2013, 48, 3505–3512. [Google Scholar] [CrossRef]
- Ding, M.; Lu, C.; Cao, L.; Ni, Y.; Xu, Z. Controllable synthesis, formation mechanism and upconversion luminescence of β-NaYF4: Yb3+/Er3+ microcrystals by hydrothermal process. Cryst. Eng. Comm. 2013, 15, 8366–8373. [Google Scholar] [CrossRef]
- Vukovic, M.; Mancic, L.; Dinic, I.; Vulic, P.; Nikolic, M.; Tan, Z.; Milosevic, O. The gadolinium effect on crystallization behavior and luminescence of β-NaYF4: Yb, Er phase. Int. J. Appl. Ceram. Technol. 2019, 17, 1445–1452. [Google Scholar] [CrossRef]
- Pires, A.M.; Heer, S.; Güdel, H.U.; Serra, O.A. Er, Yb Doped Yttrium Based Nanosized Phosphors: Particle Size, “Host Lattice” and Doping Ion Concentration Effects on Upconversion Efficiency. J. Fluoresc. 2006, 16, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Riesen, H. Mechanochemical preparation of nanocrystalline NaYF4: Gd3+/Yb3+/Tm3+: An efficient upconversion phosphor. Chem. Phys. Lett. 2015, 641, 1–4. [Google Scholar] [CrossRef]
- Yi, G.D.; Chow, G.M. Synthesis of Hexagonal-Phase NaYF4: Yb, Er and NaYF4: Yb, Tm Nanocrystals with Efficient Up-Conversion Fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Sun, Y.; Song, K.; Yu, Y.; Du, C.; Kong, X.; Zhang, H. Ionothermal synthesis of hexagonal-phase NaYF4:Yb3+, Er3+/Tm3+ upconversion nanophosphors. Chem. Comm. 2009, 43, 6628–6630. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, K.; Wei, X.; Jiang, G.; Duan, C.; Chen, Y.; Yin, M. Upconversion luminescence of NaYF4: Yb3+, Er3+ for temperature sensing. Opt. Commun. 2013, 291, 138–142. [Google Scholar] [CrossRef]
- Liang, B.X.; Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. Synthesis of NaYF4 Nanocrystals with Predictable Phase and Shape. Adv. Funct. Mater. 2007, 17, 2757–2765. [Google Scholar] [CrossRef]
- Sui, Y.; Tao, K.; Tian, Q.; Sun, K. Interaction Between Y3+ and Oleate Ions for the Cubic-to-Hexagonal Phase Transformation of NaYF4 Nanocrystals. J. Phys. Chem. 2012, 116, 1732–1739. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y. Synthesis of Hexagonal-Phase Core-Shell NaYF4 Nanocrystals with Tunable Upconversion Fluorescence. Langmuir 2008, 24, 12123–12125. [Google Scholar] [CrossRef]
- Tong, L.; Li, X.; Hua, R.; Li, X.; Zheng, H.; Sun, J.; Zhang, J.; Cheng, L.; Chen, B. Comparative study on upconversion luminescence and temperature sensing of α- and β- NaYF4: Yb3+/Er3+ nano-/micro-crystals derived from a microwave-assisted hydrothermal route. J. Lumin. 2015, 167, 386–390. [Google Scholar] [CrossRef]
- Yu, S.; Gao, X.; Jing, H.; Zhao, J.; Su, H. A synthesis and up-conversional photoluminescence study of hexagonal phase NaYF4:Yb,Er nanoparticles. Cryst. Eng. Comm. 2013, 15, 10100–10106. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Damasco, J.A.; Chen, G.; Shao, W.; Agren, H.; Huang, H.; Song, W.; Lovell, J.F.; Prasad, P.N. Size-Tunable and Monodisperse Tm3+/Gd3+-Doped Hexagonal NaYF4 Nanoparticles with Engineered Efficient Near Infrared-to-Near Infrared Upconversion for in Vivo Imaging. ACS Appl. Mater. Interfaces 2014, 6, 13884–13893. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, A.A.; Kurochkin, M.A.; Kolomytsev, A.Y.; Khasbieva, R.S.; Kolesnikov, E.Y.; Lähderanta, E.; Kolesnikov, I.E. Yb3+/Er3+-codoped GeO2-PbO-PbF2 glass ceramics for ratiometric upconversion temperature sensing based on thermally and non-thermally coupled levels. Opt. Mater. 2019, 90, 200–207. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Kurochkin, M.A.; Kalinichev, A.A.; Kolesnikov, E.Y.; Lähderanta, E. Optical temperature sensing in Tm3+/Yb3+-doped GeO2-PbO-PbF2 glass ceramics based on ratiometric and spectral line position approaches. Sens. Actuators A 2018, 284, 251–259. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, S.; Shao, W.; Zhang, X.; Xu, J.; Yu, L.; Chen, K. Enhanced up-conversion luminescence from NaYF4:Yb,Er nanocrystals be Gd3+ ions induced phase transformation and plasmonic Au nonsphere arrays. RSC Adv. 2016, 6, 102869–102874. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, Y. Sub-10 nm and monodisperse b-NaYF4: Yb, Tm, Gd nanocrystals with intense ultraviolet upconversion luminescence. J. Mater. Chem. 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Galleani, G.; Santagneli, S.H.; Lendemi, Y.; Messaddeq, Y. Ultraviolet Upconversion Luminescence in a Highly Transparent Triply-Doped Gd3+-Tm3+-Yb3+ Fluoride-Phosphate Glasses. J. Phys. Chem. 2018, 122, 2275–2284. [Google Scholar] [CrossRef]
- Zheng, K.; Zhao, D.; Zhang, D.; Liu, N.; Shi, F.; Qin, W.J. Sensitized high-order ultraviolet upconversion emissions of Gd3+ by Er3+ in NaYF4 microcrystals. J. Alloys Compd. 2011, 509, 5848–5852. [Google Scholar] [CrossRef]
- Li, C.; Quan, Z.; Yang, J.; Yang, P.; Lin, J. Highly Uniform and Monodisperse β-NaYF4: Ln3+ (Ln = Eu, Tb, Yb/Er, and Yb/Tm) Hexagonal Microprism Crystals: Hydrothermal Synthesis and Luminescent Properties. Inorg. Chem. 2007, 46, 6329–6337. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, S.; Resch-Genger, U.; Schäferling, M.; Soukka, T. Quenching of the upconversion luminescence of NaYF4:Yb3+, Er3+ and NaYF4: Yb3+, Tm3+ nanophosphors by water: The role of the sensitizer Yb3+ in non-radiative relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef]
- Shi, F.; Wang, J.; Zhang, D.; Qin, G.; Qin, W. Greatly enhanced size-tunable ultraviolet upconversion luminescence of monodisperse β-NaYF4: Yb, Tm nanocrystals. J. Mater.Chem. 2011, 21, 13413–13421. [Google Scholar] [CrossRef]



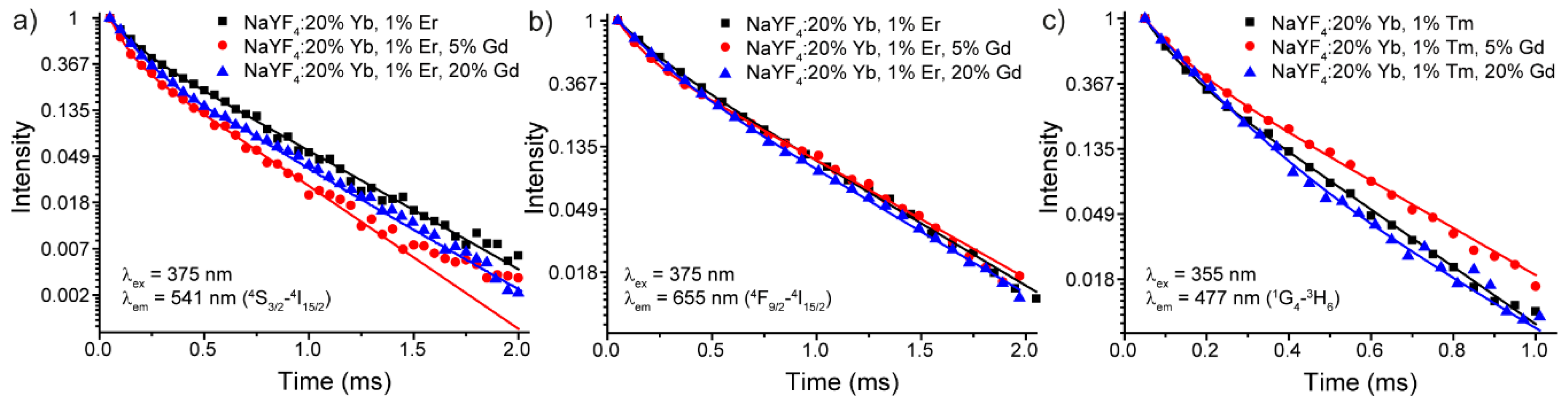
| Sample | Transition | τav, ms |
|---|---|---|
| NaYF4: 20% Yb, 1% Er | 4S3/2–4I15/2 (541 nm) | 0.34 |
| NaYF4: 20% Yb, 1% Er, 5% Gd | 0.25 | |
| NaYF4: 20% Yb, 1% Er, 20% Gd | 0.33 | |
| NaYF4: 20% Yb, 1% Er | 4F9/2–4I15/2 (655 nm) | 0.46 |
| NaYF4: 20% Yb, 1% Er, 5% Gd | 0.48 | |
| NaYF4: 20% Yb, 1% Er, 20% Gd | 0.44 | |
| NaYF4: 20% Yb, 1% Tm | 1G4–3H6 (477 nm) | 0.19 |
| NaYF4: 20% Yb, 1% Tm, 5% Gd | 0.23 | |
| NaYF4: 20% Yb, 1% Tm, 20%Gd | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidyakina, A.A.; Kolesnikov, I.E.; Bogachev, N.A.; Skripkin, M.Y.; Tumkin, I.I.; Lähderanta, E.; Mereshchenko, A.S. Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials 2020, 13, 3397. https://doi.org/10.3390/ma13153397
Vidyakina AA, Kolesnikov IE, Bogachev NA, Skripkin MY, Tumkin II, Lähderanta E, Mereshchenko AS. Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials. 2020; 13(15):3397. https://doi.org/10.3390/ma13153397
Chicago/Turabian StyleVidyakina, Aleksandra A., Ilya E. Kolesnikov, Nikita A. Bogachev, Mikhail Y. Skripkin, Ilya I. Tumkin, Erkki Lähderanta, and Andrey S. Mereshchenko. 2020. "Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles" Materials 13, no. 15: 3397. https://doi.org/10.3390/ma13153397
APA StyleVidyakina, A. A., Kolesnikov, I. E., Bogachev, N. A., Skripkin, M. Y., Tumkin, I. I., Lähderanta, E., & Mereshchenko, A. S. (2020). Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials, 13(15), 3397. https://doi.org/10.3390/ma13153397









