Strategies for Improving Antimicrobial Properties of Stainless Steel
Abstract
1. Introduction
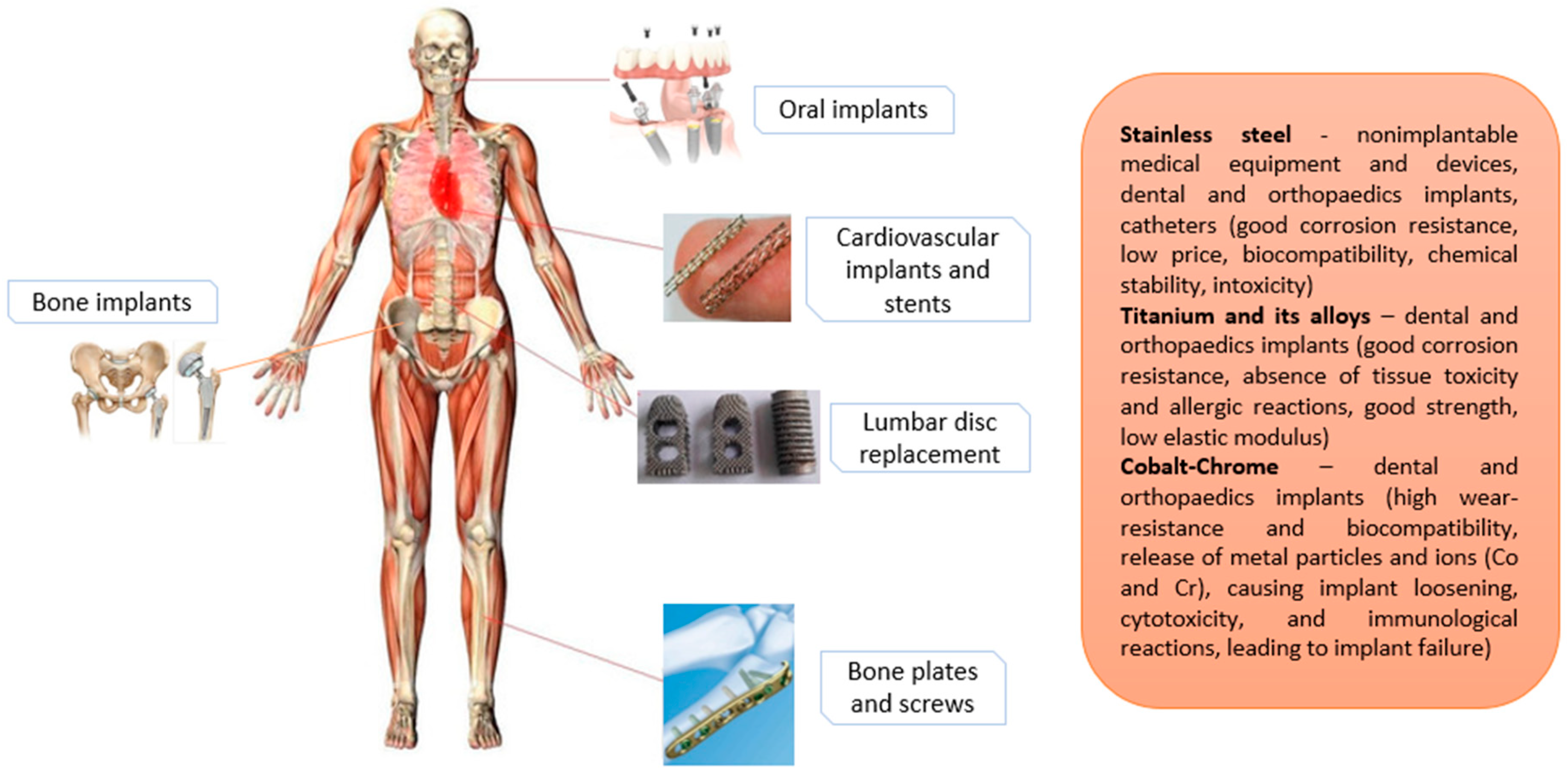
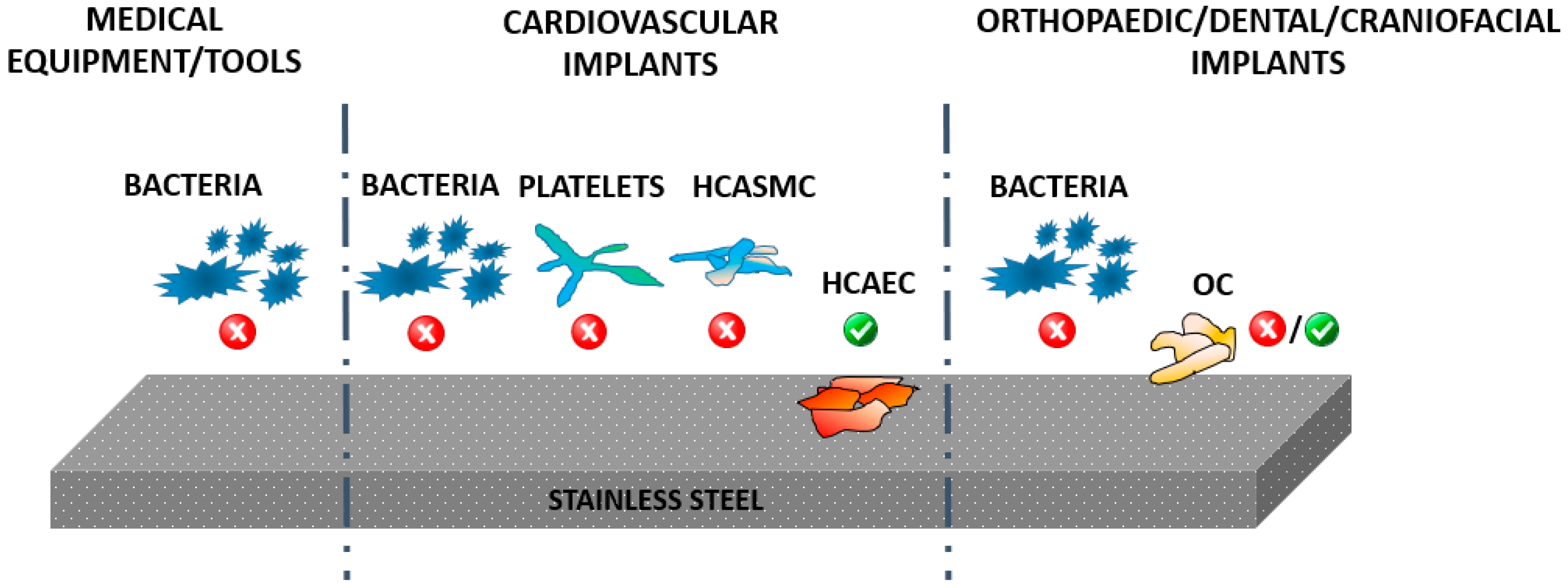
2. The Use of SS in the Biomedical Field
- Martensitic (the hardest crystal structure, magnetic, contains Cr (12–14%), Mo (0.2–1%), 0.1–1% C and no Ni. Extremely strong and tough, it can be hardened by heat treatment and is highly machinable. It is not as corrosion resistant as other grades) [26].
- Ferritic (overall it contains Cr (10.5–27%) and a low amount of Ni, if any).
- Austenitic (max. of 0.15% C, min. of 16% Cr and sufficient Ni and/or Mn to retain an austenitic structure at all temperatures. Better resistance to stress-corrosion cracking with a higher Ni content and nitrogen additions).
- Duplex (has two microstructure phases—ferrite and austenite, with high Cr (19–28%) and Mo (up to 5%) and lower Ni contents than austenitic SS. Alloys have higher strength and greater resistance to localized corrosion, particularly pitting, crevice corrosion and stress corrosion cracking due to chlorides).
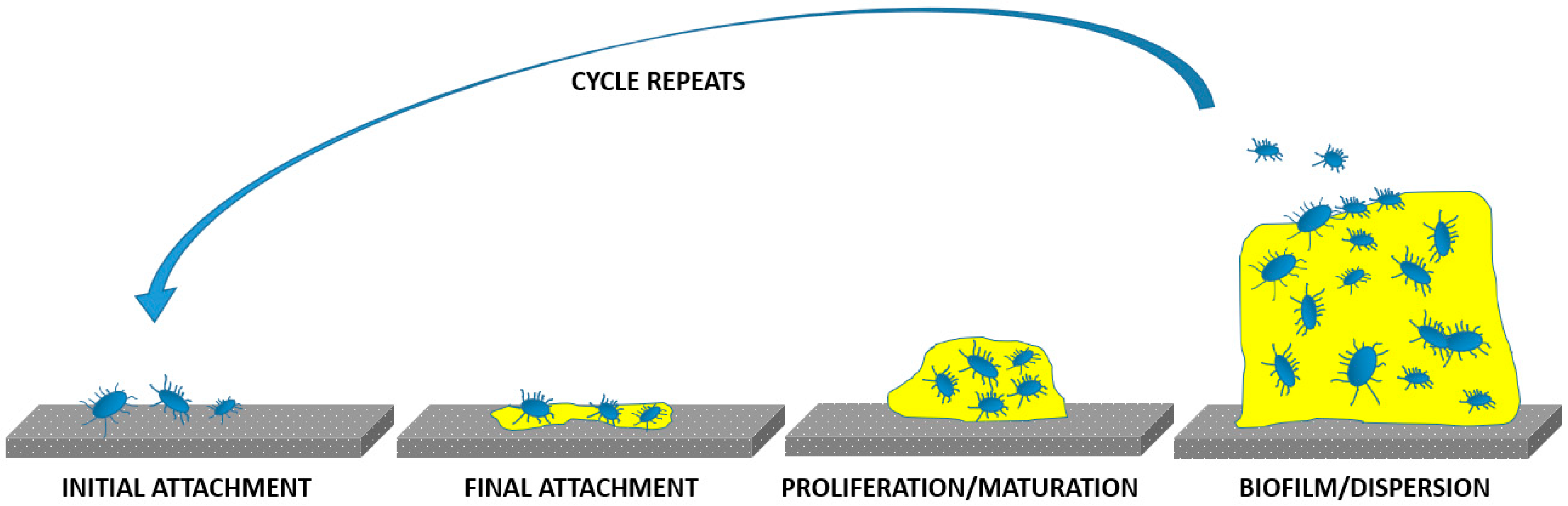
3. Bacterial Infections
4. Common Strategies Used for Improving the Antimicrobial Properties of SS
4.1. Surface Chemistry
4.2. Nanostructure
4.3. Wettability and Surface Energy
5. Use of Plasma Technologies for Antimicrobial Properties of SS
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakterij, A.J.M.T. An overview of the influence of stainless-steel surface properties on bacterial adhesion. Mater. Tehnol. 2014, 48, 609–617. [Google Scholar]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R. Metals for biomedical applications. Biomed. Eng. Theory Appl. 2011, 2011, 411–430. [Google Scholar]
- Beaupré, G.S.; Csongradi, J.J. Refracture risk after plate removal in the forearm. J. Orthop. Trauma 1996, 10, 87–92. [Google Scholar] [CrossRef]
- Boyd, A.H.; Hylwa, S.A. Nickel release from surgical instruments and operating room equipment. Dermatol. Online J. 2018, 24. [Google Scholar]
- Neupane, M.P.; Park, I.S.; Lee, S.J.; Kim, K.A.; Lee, M.H.; Bae, T.S. Study of anodic oxide films of titanium fabricated by voltammetric technique in phosphate buffer media. Int. J. Electrochem. Sci 2009, 4, 197–207. [Google Scholar]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. Journal of tissue engineering 2018, 9, 2041731418789838. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Santos, M.; Filipe, E.C.; Michael, P.L.; Hung, J.; Wise, S.G.; Bilek, M.M. Mechanically robust plasma-activated interfaces optimized for vascular stent applications. ACS Appl. Mater. Interfaces 2016, 8, 9635–9650. [Google Scholar] [CrossRef]
- Benčina, M.; Mavrič, T.; Junkar, I.; Bajt, A.; Krajnović, A.; Lakota, K.; Žigon, P.; Sodin-Šemrl, S.; Kralj-Iglič, V.; Iglič, A. The Importance of Antibacterial Surfaces in Biomedical Applications. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–165. [Google Scholar]
- Milosev, L.; Antolic, V.; Minovic, A.; Cor, A.; Herman, S.; Pavlovcic, V.; Campbell, P. Extensive metallosis and necrosis in failed prostheses with cemented titanium-alloy stems and ceramic heads. J. Bone Jt. Surg. Br. Vol. 2000, 82, 352–357. [Google Scholar] [CrossRef]
- Villapún, V.M.; Dover, L.G.; Cross, A.; González, S. Antibacterial metallic touch surfaces. Materials 2016, 9, 736. [Google Scholar] [CrossRef]
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Sroka-Oleksiak, A.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antimicrobial properties of selected copper alloys on Staphylococcus Aureus and Escherichia Coli in different simulations of environmental conditions: With vs. without organic contamination. Int. J. Environ. Res. Public Health 2017, 14, 813. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Drašler, B.; Rugelj, N.; Mazare, A.; Flašker, A.; Drobne, D.; Humpolíček, P.; Resnik, M.; Schmuki, P. Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef]
- Kopf, B.S.; Schipanski, A.; Rottmar, M.; Berner, S.; Maniura-Weber, K. Enhanced differentiation of human osteoblasts on Ti surfaces pre-treated with human whole blood. Acta Biomater. 2015, 19, 180–190. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Theodossiades, G.; Mourouzis, C.; Evangelou, A. Effect of titanium maxillofacial implants and osteosynthesis materials on platelet function. Br. J. Oral Maxillofac. Surg. 2011, 49, 538–541. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Nune, K.; Somani, M.; Spencer, C.; Misra, R. Cellular response of Staphylococcus aureus to nanostructured metallic biomedical devices: Surface binding and mechanism of disruption of colonization. Mater. Technol. 2017, 32, 22–31. [Google Scholar] [CrossRef]
- Harris, L.G.; Meredith, D.O.; Eschbach, L.; Richards, R.G. Staphylococcus aureus adhesion to standard micro-rough and electropolished implant materials. J. Mater. Sci. Mater. Med. 2007, 18, 1151–1156. [Google Scholar] [CrossRef]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; Van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801. [Google Scholar]
- Junkar, I.; Kulkarni, M.; Benčina, M.; Kovač, J.; Mrak-Poljšak, K.a.; Lakota, K.; Sodin-Šemrl, S.n.; Mozetič, M.; Iglič, A. Titanium Dioxide Nanotube Arrays for Cardiovascular Stent Applications. ACS Omega 2020, 5, 7280–7289. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglič, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, Z.; Sun, D.; Yang, C.; Zhang, X.; Huang, N.; Jiang, X.; Yang, K. Antibacterial durability and biocompatibility of antibacterial-passivated 316L stainless steel in simulated physiological environment. Mater. Sci. Eng. C 2019, 100, 396–410. [Google Scholar] [CrossRef]
- Jana, S. Effect of heat input on the HAZ properties of two duplex stainless steels. J. Mater. Process. Technol. 1992, 33, 247–261. [Google Scholar] [CrossRef]
- Sedriks, A.J. Corrosion of Stainless Steel, 2nd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Tanzi, M.C.; Farè, S.; Candiani, G. Foundations of Biomaterials Engineering; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Manam, N.; Harun, W.; Shri, D.; Ghani, S.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Ojeil, M.; Jermann, C.; Holah, J.; Denyer, S.P.; Maillard, J.-Y. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J. Hosp. Infect. 2013, 85, 274–281. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Summary of the latest data on antibiotic resistance in the European Union 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/EAAD%20EARS-Net%20summary.pdf (accessed on 28 June 2020).
- Chen, J.; Howell, C.; Haller, C.A.; Patel, M.S.; Ayala, P.; Moravec, K.A.; Dai, E.; Liu, L.; Sotiri, I.; Aizenberg, M. An immobilized liquid interface prevents device associated bacterial infection in vivo. Biomaterials 2017, 113, 80–92. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.-W.; Kim, J.; Song, E.-H.; Jung, H.-D.; Park, J.-U.; Kim, H.-E.; Kim, S.; Jang, T.-S. Reduced fibrous capsule formation at nano-engineered silicone surfaces via tantalum ion implantation. Biomater. Sci. 2019, 7, 2907–2919. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Seebach, E.; Kubatzky, K.F. Chronic implant-related bone infections–can immune modulation be a therapeutic strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro-and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Colligon, J.; Verran, J. Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surf. B Biointerfaces 2005, 41, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Saraeva, I.N.; Tolordava, E.R.; Nastulyavichus, A.A.; Ivanova, A.K.; Kudryashov, S.I.; Rudenko, A.A.; Melnik, N.N.; Zayarny, D.A.; Ionin, A.A.; Romanova, Y.M. A bacterial misericorde: Laser-generated silicon nanorazors with embedded biotoxic nanoparticles combat the formation of durable biofilms. Laser Phys. Lett. 2020, 17, 025601. [Google Scholar] [CrossRef]
- Krylach, I.; Kudryashov, S.; Olekhnovich, R.; Moskvin, M.; Uspenskaya, M. Tuning water wetting angle of a steel surface via nanosecond laser ablative nano/microtexturing for chemical and biomedical microfluidic applications. Laser Phys. Lett. 2019, 16, 105602. [Google Scholar] [CrossRef]
- Saltuganov, P.; Ionin, A.; Kudryashov, S.; Rukhadze, A.; Gavrilov, A.; Makarov, S.; Rudenko, A.; Zayarny, D. Fabrication of superhydrophobic coating on stainless steel surface by femtosecond laser texturing and chemisorption of an hydrophobic agent. J. Russ. Laser Res. 2015, 36, 81–85. [Google Scholar] [CrossRef]
- Thomas, V.; Dean, D.R.; Vohra, Y.K. Nanostructured biomaterials for regenerative medicine. Curr. Nanosci. 2006, 2, 155–177. [Google Scholar] [CrossRef]
- Kargar, M.; Wang, J.; Nain, A.S.; Behkam, B. Controlling bacterial adhesion to surfaces using topographical cues: A study of the interaction of Pseudomonas aeruginosa with nanofiber-textured surfaces. Soft Matter 2012, 8, 10254–10259. [Google Scholar] [CrossRef]
- Slama, T.G. Gram-negative antibiotic resistance: There is a price to pay. Crit. Care 2008, 12, S4. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Barnacle cement as surface anchor for “clicking” of antifouling and antimicrobial polymer brushes on stainless steel. Biomacromolecules 2013, 14, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Cécius, M.; Jérôme, C. A fully aqueous sustainable process for strongly adhering antimicrobial coatings on stainless steel. Prog. Org. Coat. 2011, 70, 220–223. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef]
- Pradhan, D. Sol-Gel Prepared Niobium Oxide and Silicon Oxide Coatings on 316L Stainless Steel for Biomedical Applications. Ph.D. Thesis, Alfred University, Alfred, NY, USA, 2016. [Google Scholar]
- Ciacotich, N.; Din, R.U.; Sloth, J.J.; Møller, P.; Gram, L. An electroplated copper–silver alloy as antibacterial coating on stainless steel. Surf. Coat. Technol. 2018, 345, 96–104. [Google Scholar] [CrossRef]
- Hayes, J.S.; Richards, R.G. Surfaces to control tissue adhesion for osteosynthesis with metal implants: In vitro and in vivo studies to bring solutions to the patient. Expert Rev. Med. Devices 2010, 7, 131–142. [Google Scholar] [CrossRef]
- Koniari, I.; Kounis, N.G.; Hahalis, G. In-stent restenosis and thrombosis due to metal hypersensitivity: Implications for Kounis syndrome. J. Thorac. Dis. 2016, 8, 3056. [Google Scholar] [CrossRef]
- Hayes, J.; Klöppel, H.; Wieling, R.; Sprecher, C.; Richards, R. Influence of steel implant surface microtopography on soft and hard tissue integration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 705–715. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Wu, C.; Zhou, H.; Zreiqat, H. Biological response of human bone cells to zinc-modified Ca–Si-based ceramics. Acta Biomater. 2008, 4, 1487–1497. [Google Scholar] [CrossRef]
- Ducheyne, P.; Van Raemdonck, W.; Heughebaert, J.; Heughebaert, M. Structural analysis of hydroxyapatite coatings on titanium. Biomaterials 1986, 7, 97–103. [Google Scholar] [CrossRef]
- Bagherpour, I. Fabrication of hardystonite nano-bioceramic coating on 306L stainless steel substrate using electrophoretic method and evaluation of its corrosion resistance to improve medical performance. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Henry B. González Convention Center, San Antonio, TX, USA, 10–14 March 2019. [Google Scholar]
- Bhat, A.; Smith, B.; Dinu, C.-Z.; Guiseppi-Elie, A. Molecular engineering of poly (HEMA-co-PEGMA)-based hydrogels: Role of minor AEMA and DMAEMA inclusion. Mater. Sci. Eng. C 2019, 98, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Aggas, J.R.; Bhat, A.; Walther, B.K.; Guiseppi-Elie, A. Nano-Pt ennobling of stainless steel for biomedical applications. Electrochim. Acta 2019, 301, 153–161. [Google Scholar] [CrossRef]
- Cowley, A. A healthy future: Platinum in medical applications. Platin. Met. Rev. 2011, 55, 98–107. [Google Scholar] [CrossRef]
- Khalili, E.; Sarafbidabad, M. Combination of laser patterning and nano PTFE sputtering for the creation a super-hydrophobic surface on 304 stainless steel in medical applications. Surf. Interfaces 2017, 8, 219–224. [Google Scholar] [CrossRef]
- Antonov, E.; Bagratashvili, V.; Popov, V.; Ball, M.; Grant, D.; Howdle, S.; Scotchford, C. Properties of calcium phosphate coatings deposited and modified with lasers. J. Mater. Sci. Mater. Med. 2003, 14, 151–155. [Google Scholar] [CrossRef]
- Kurella, A.; Dahotre, N.B. Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J. Albumin and fibronectin protein adsorption on CO2-laser-modified biograde stainless steel. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 47–55. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dental Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef]
- Jang, Y.; Choi, W.T.; Johnson, C.T.; García, A.J.; Singh, P.M.; Breedveld, V.; Hess, D.W.; Champion, J.A. Inhibition of bacterial adhesion on nanotextured stainless steel 316L by electrochemical etching. ACS Biomater. Sci. Eng. 2017, 4, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, A.; Bello, D.G.; Flynn, S.; Variola, F.; Wuest, J.D.; Nanci, A. Chemical nanocavitation of surfaces to enhance the utility of stainless steel as a medical material. Colloids Surf. B Biointerfaces 2018, 161, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Nastulyavichus, A.; Kudryashov, S.; Saraeva, I.; Smirnov, N.; Rudenko, A.; Tolordava, E.; Zayarny, D.; Gonchukov, S.; Ionin, A. Nanostructured steel for antibacterial applications. Laser Phys. Lett. 2019, 17, 016003. [Google Scholar] [CrossRef]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Kathiresan, S.; Mohan, B. In-vitro bacterial adhesion study on stainless steel 316L subjected to magneto rheological abrasive flow finishing. Biomed. Res. 2017, 28, 55434624. [Google Scholar]
- Derrien, T.-Y.; Torres, R.; Sarnet, T.; Sentis, M.; Itina, T.E. Formation of femtosecond laser induced surface structures on silicon: Insights from numerical modeling and single pulse experiments. Appl. Surf. Sci. 2012, 258, 9487–9490. [Google Scholar] [CrossRef]
- Eichstädt, J.; Römer, G.; Huis, A. Towards friction control using laser-induced periodic surface structures. Phys. Procedia 2011, 12, 7–15. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Niu, J.; Chen, Y. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 2013, 29, 4647–4651. [Google Scholar] [CrossRef]
- Kumar, H.; Rani, R.; Salar, R. Reverse micellar synthesis, characterization & antibacterial study of nickel nanoparticles. Adv. Control Chem. Eng. Civil Eng. Mech. Eng. 2010, 2010, 88–94. [Google Scholar]
- Chaudhary, R.G.; Tanna, J.A.; Gandhare, N.V.; Rai, A.R.; Juneja, H.D. Synthesis of nickel nanoparticles: Microscopic investigation, an efficient catalyst and effective antibacterial activity. Adv. Mater. Lett 2015, 6, 990–998. [Google Scholar] [CrossRef]
- Kokkoris, M.; Trapalis, C.; Kossionides, S.; Vlastou, R.; Nsouli, B.; Grötzschel, R.; Spartalis, S.; Kordas, G.; Paradellis, T. RBS and HIRBS studies of nanostructured AgSiO2 sol–gel thin coatings. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 188, 67–72. [Google Scholar] [CrossRef]
- Yu, B.; Lesiuk, A.; Davis, E.; Irvin, R.T.; Li, D. Surface nanocrystallization for bacterial control. Langmuir 2010, 26, 10930–10934. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Guo, Z. Bioinspired silica-based superhydrophobic materials. Appl. Surf. Sci. 2017, 426, 1–18. [Google Scholar] [CrossRef]
- Reiner, Ž.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.-R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure–A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Hasan, J.; Raj, S.; Yadav, L.; Chatterjee, K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv. 2015, 5, 44953–44959. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef]
- Miola, M.; Ferraris, S.; Di Nunzio, S.; Robotti, P.; Bianchi, G.; Fucale, G.; Maina, G.; Cannas, M.; Gatti, S.; Massé, A. Surface silver-doping of biocompatible glasses to induce antibacterial properties. Part II: Plasma sprayed glass-coatings. J. Mater. Sci. Mater. Med. 2009, 20, 741. [Google Scholar] [CrossRef]
- Emori, T.G.; Banerjee, S.N.; Culver, D.H.; Gaynes, R.P.; Horan, T.C.; Edwards, J.R.; Jarvis, W.R.; Tolson, J.S.; Henderson, T.S.; Martone, W.J. Nosocomial infections in elderly patients in the United States, 1986–1990. Am. J. Med. 1991, 91, S289–S293. [Google Scholar] [CrossRef]
- Gould, I. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int. J. Antimicrob. Agents 2006, 28, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-y.; Huang, X.-B.; Jiang, L.; Ma, Y.; Fan, A.-L.; Tang, B. Antibacterial property of Cu modified stainless steel by plasma surface alloying. J. Iron Steel Res. Int. 2012, 19, 75–79. [Google Scholar] [CrossRef]
- Allion-Maurer, A.; Saulou-Berion, C.; Briandet, R.; Zanna, S.; Lebleu, N.; Marcus, P.; Raynaud, P.; Despax, B.; Mercier-Bonin, M. Plasma-deposited nanocomposite polymer-silver coating against Escherichia coli and Staphylococcus aureus: Antibacterial properties and ageing. Surf. Coat. Technol. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Tian, L.; Bell, T.; Sammons, R.; Dong, H. Towards long-lasting antibacterial stainless steel surfaces by combining double glow plasma silvering with active screen plasma nitriding. Acta Biomater. 2011, 7, 447–457. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y.; Nakashima, J.; Sawase, T.; Morimura, T. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Sarghini, S.; Paulussen, S.; Terryn, H. Atmospheric pressure plasma technology: A straightforward deposition of antibacterial coatings. Plasma Process Polym. 2011, 8, 59–69. [Google Scholar] [CrossRef]
- Ibis, F.; Oflaz, H.; Ercan, U.K. Biofilm Inactivation and prevention on common implant material surfaces by nonthermal DBD plasma treatment. Plasma Med. 2016, 6, 33–45. [Google Scholar] [CrossRef]
- Rocha, J.d.L.; Pereira, R.d.S.; de Oliveira, M.C.L.; Antunes, R.A. Investigation on the relationship between the surface chemistry and the corrosion resistance of Electrochemically Nitrided AISI 304 stainless steel. Int. J. Corros. 2019, 2019. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Simmen, H.P.; Filgueira, L. Bio-corrosion of stainless steel by osteoclasts—in vitro evidence. J. Orthop. Res. 2009, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-W.; Chen, T.-E.; Lo, K.-Y.; Lee, Y.-L. Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium. Materials 2019, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Caramazza, R.; Pizzoferrato, A. In vitro adhesion of Staphylococcus epidermidis on heparin-surface-modified intraocular lenses. J. Cataract Refract. Surg. 1994, 20, 158–161. [Google Scholar] [CrossRef]
- Taglietti, A.; Dacarro, G.; Barbieri, D.; Cucca, L.; Grisoli, P.; Patrini, M.; Arciola, C.R.; Pallavicini, P. High bactericidal self-assembled nano-monolayer of silver sulfadiazine on hydroxylated material Surfaces. Materials 2019, 12, 2761. [Google Scholar] [CrossRef]
- Sabino, R.M.; Kauk, K.; Madruga, L.Y.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Enhanced hemocompatibility and antibacterial activity on titania nanotubes with tanfloc/heparin polyelectrolyte multilayers. J. Biomed. Mater. Res. Part A 2020, 108, 992–1005. [Google Scholar] [CrossRef]

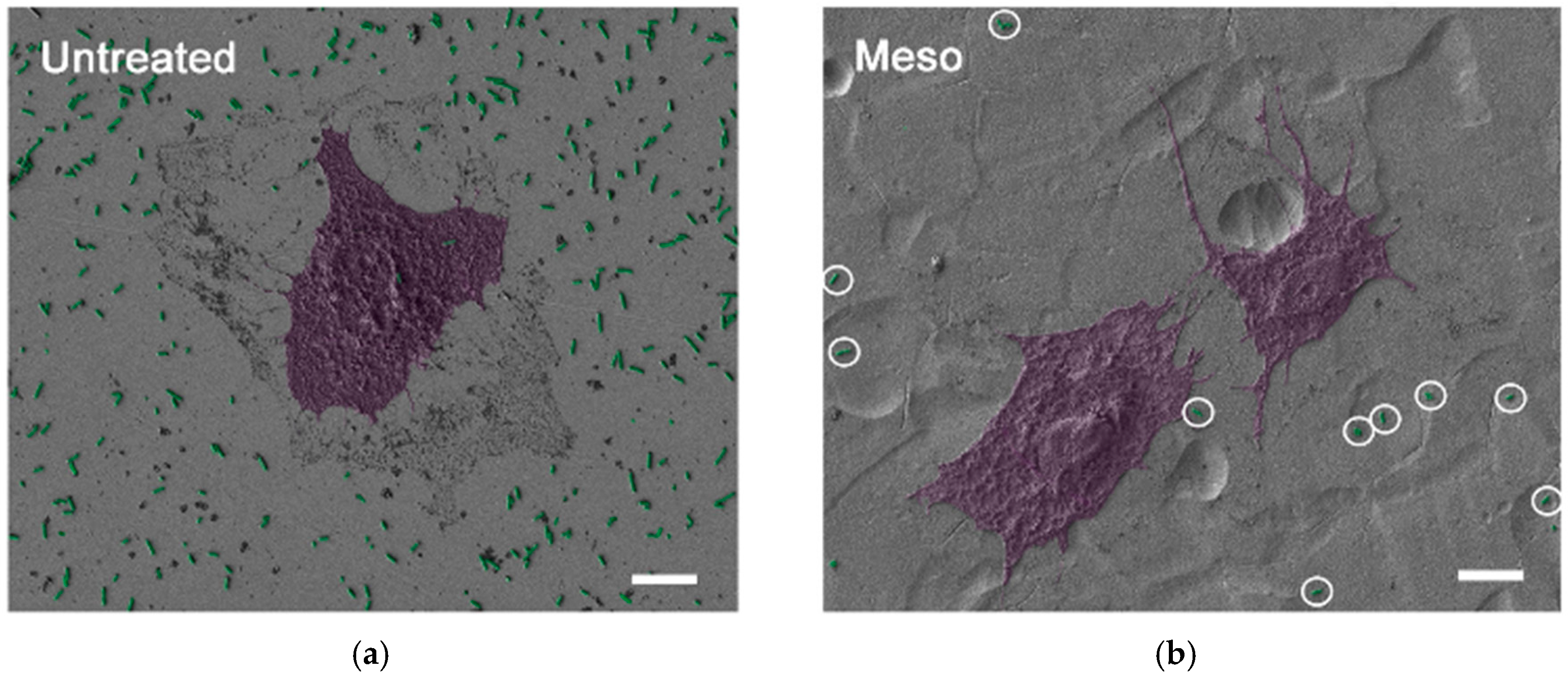
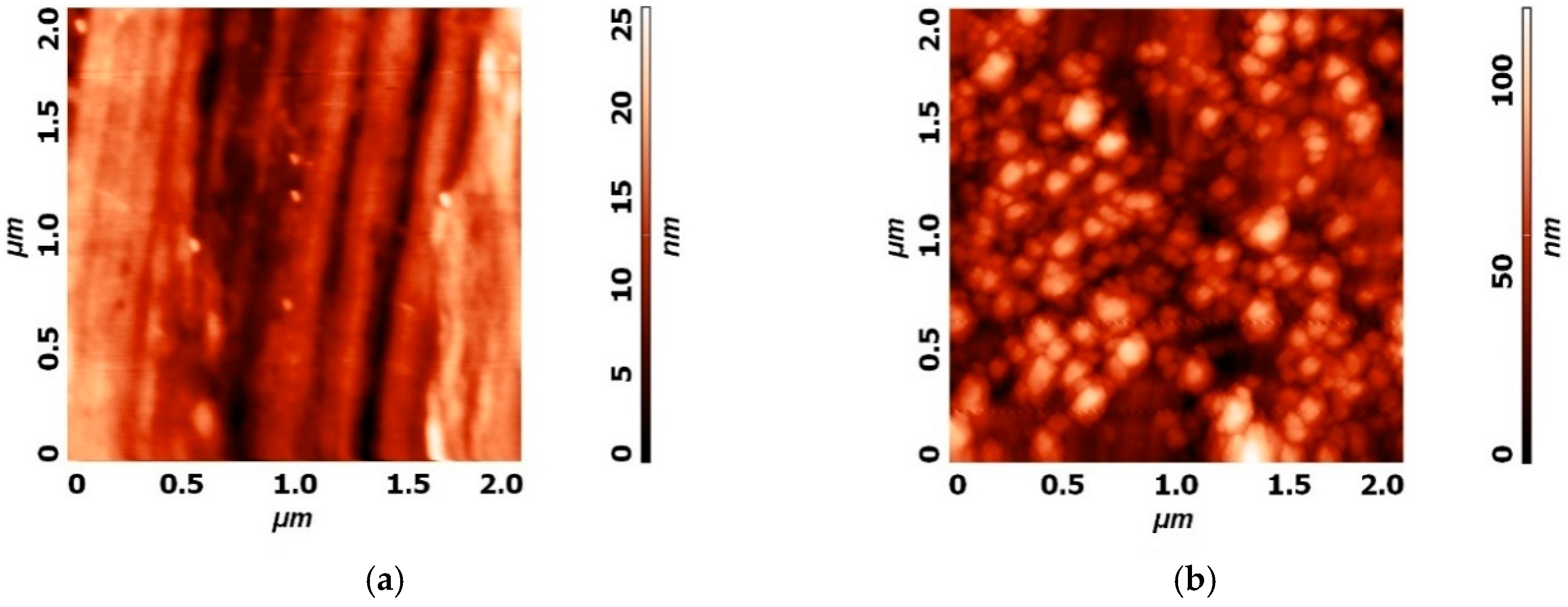
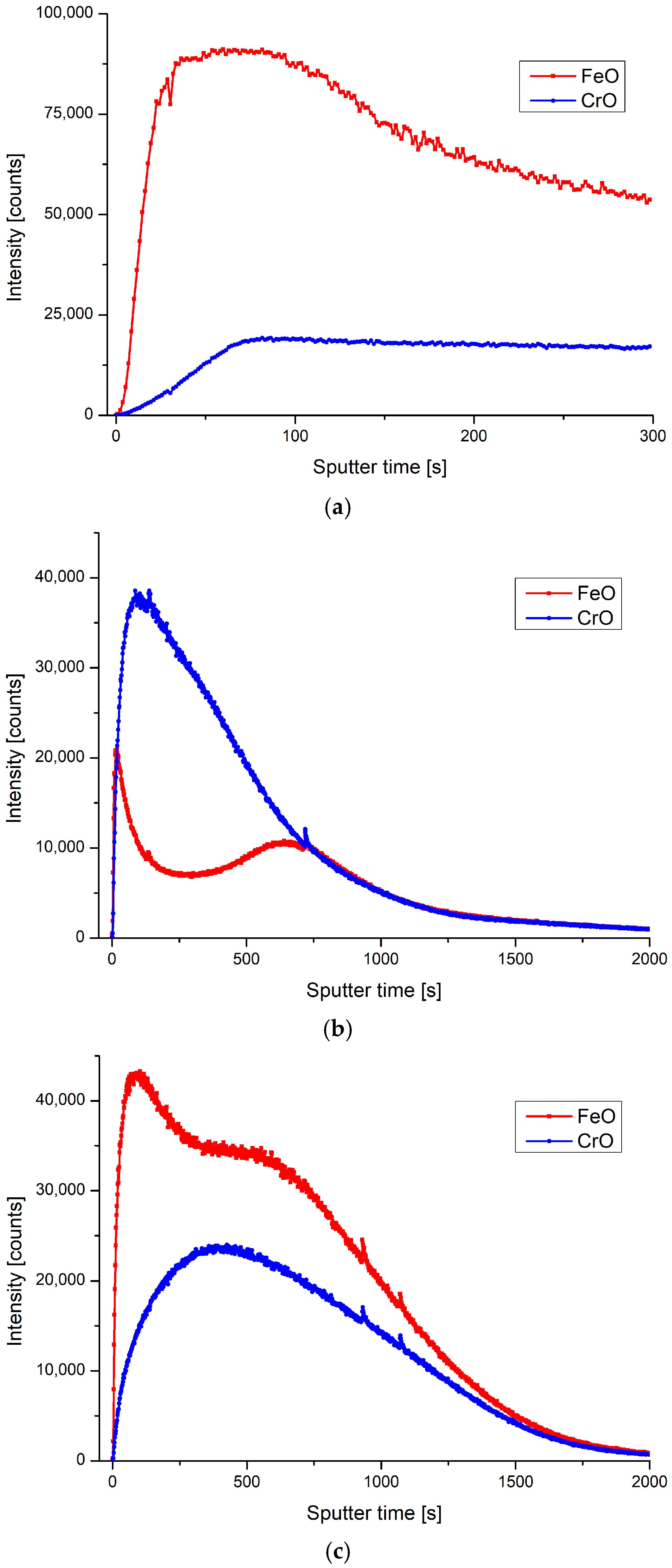

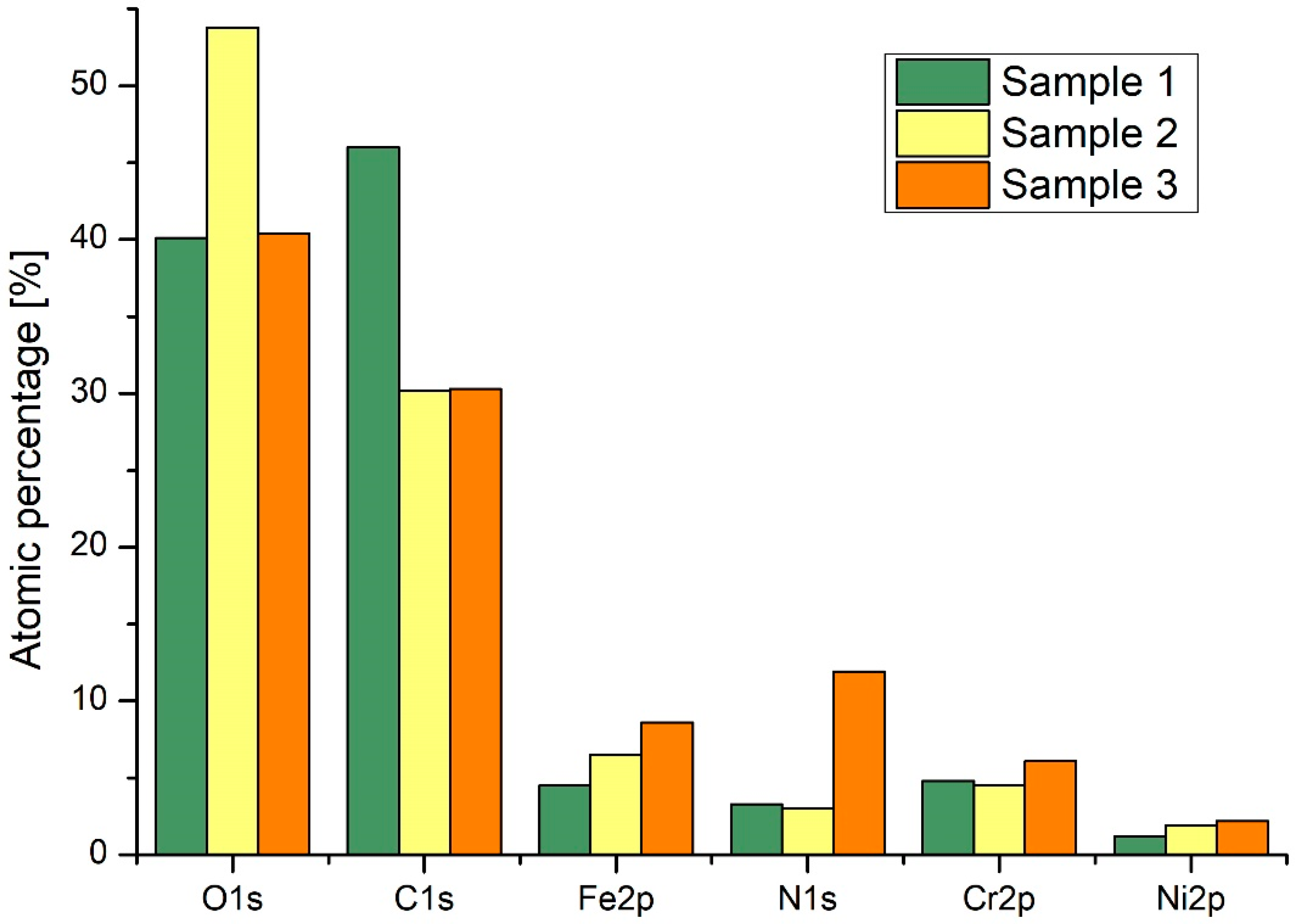
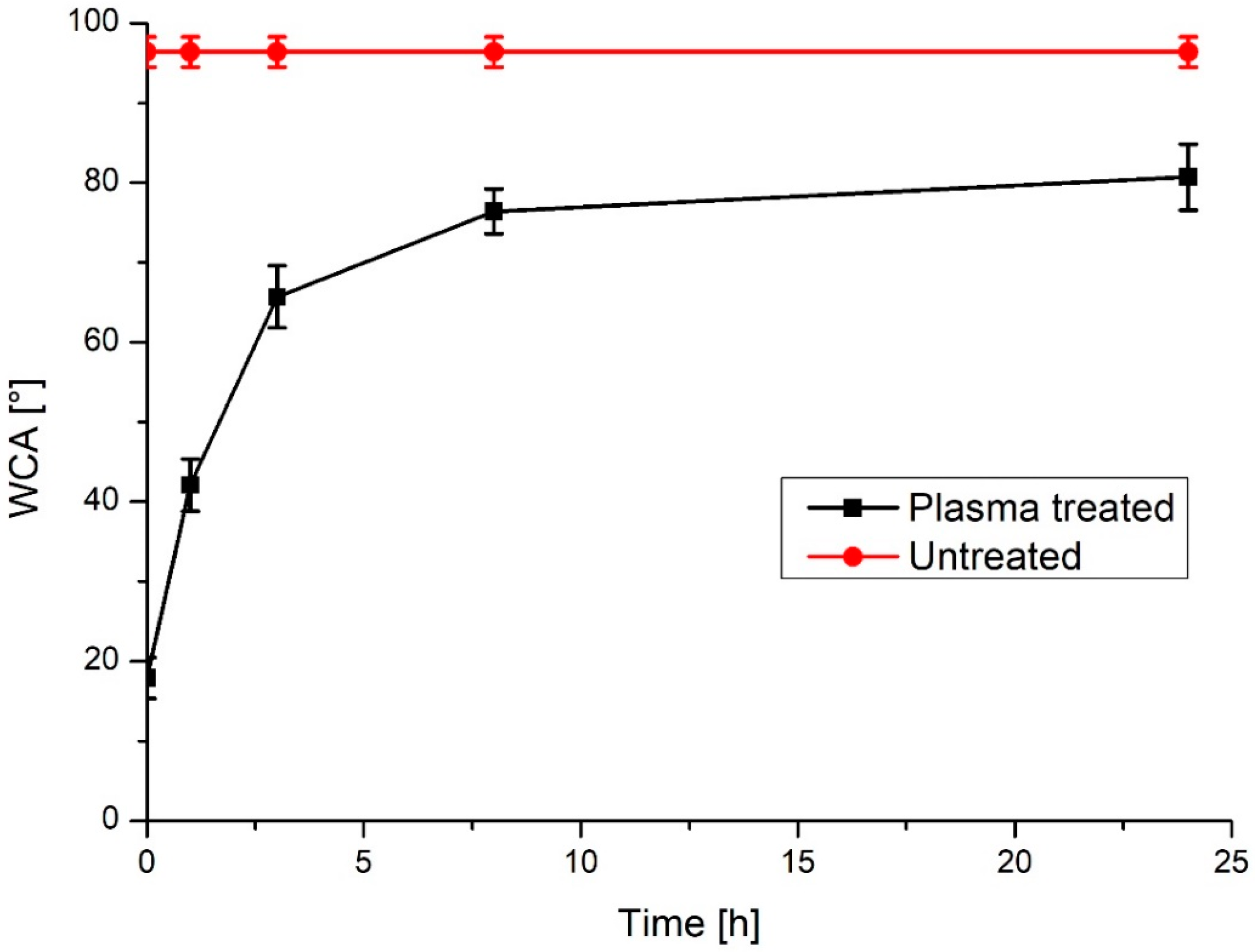
| Sample | Ra (nm) | Stdandard Deviation (nm) |
|---|---|---|
| Untreated | 132 | 6 |
| Plasma 1 (CrO) | 157 | 8 |
| Plasma 2 (FeO) | 146 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resnik, M.; Benčina, M.; Levičnik, E.; Rawat, N.; Iglič, A.; Junkar, I. Strategies for Improving Antimicrobial Properties of Stainless Steel. Materials 2020, 13, 2944. https://doi.org/10.3390/ma13132944
Resnik M, Benčina M, Levičnik E, Rawat N, Iglič A, Junkar I. Strategies for Improving Antimicrobial Properties of Stainless Steel. Materials. 2020; 13(13):2944. https://doi.org/10.3390/ma13132944
Chicago/Turabian StyleResnik, Matic, Metka Benčina, Eva Levičnik, Niharika Rawat, Aleš Iglič, and Ita Junkar. 2020. "Strategies for Improving Antimicrobial Properties of Stainless Steel" Materials 13, no. 13: 2944. https://doi.org/10.3390/ma13132944
APA StyleResnik, M., Benčina, M., Levičnik, E., Rawat, N., Iglič, A., & Junkar, I. (2020). Strategies for Improving Antimicrobial Properties of Stainless Steel. Materials, 13(13), 2944. https://doi.org/10.3390/ma13132944








