A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation
Abstract
1. Introduction
2. Dark Fermentation Process
3. Process Parameters
3.1. Types of Substrate
3.2. Microorganism Type
3.3. Fermentation Process Parameters
| Bioreactor | Advantage | Disadvantage | Reference |
|---|---|---|---|
| CSTR |
|
| [23,48,97,98] |
| MBR |
|
| [23,59] |
| UASB |
|
| [100,101] |
| AFBR |
|
| [102,103] |
4. Modelling and Simulation
4.1. Process Description
4.2. Process Simulation
4.3. Simulation Results
5. Possible Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| AFBR | anaerobic fluidized bed reactors |
| ATP | adenosine triphosphate |
| CSTR | continuous stirred tank reactors |
| DF | dark fermentation |
| HRT | hydraulic retention time |
| LHV | lower heating value |
| MBR | membrane bioreactors |
| MEC | microbial electrolysis cells |
| NADH | nicotinamide adenine dinucleotide |
| PFL | pyruvate formate lyase |
| PFOR | pyruvate ferredoxin oxidoreductase |
| TS | total solid |
| TVS | total volatile solid |
| UASB | upflow anaerobic sludge blanket reactor |
References
- Singh, L.; Wahid, Z.A. Methods for enhancing bio-hydrogen production from biological process: A review. J. Ind. Eng. Chem. 2015, 21, 70–80. [Google Scholar] [CrossRef]
- Reungsang, A.; Sreela-or, C. Bio-hydrogen production from pineapple waste extract by anaerobic mixed cultures. Energies 2013, 6, 2175–2190. [Google Scholar] [CrossRef]
- Sinha, P.; Pandey, A. An evaluative report and challenges for fermentative biohydrogen production. Int. J. Hydrogen Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrogen Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling dark fermentation and microbial electrolysis cells for higher hydrogen yield: Technological competitiveness and challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Javed, M.A.; Zafar, A.M.; Hassan, A.A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The role of oxygen regulation and algal growth parameters in hydrogen production via biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Azwar, M.; Hussain, M.; Abdul-Wahab, A. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Müller, L.J.; Kätelhön, A.; Bringezu, S.; McCoy, S.; Suh, S.; Edwards, R.; Sick, V.; Kaiser, S.; Cuéllar-Franca, R.; El Khamlichi, A. The carbon footprint of the carbon feedstock CO2. Energy Environ. Sci. 2020, 13, 2979–2992. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Yogalakshmi, K.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef] [PubMed]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A. A combined system for asbestos-cement waste degradation by dark fermentation and resulting supernatant valorization in anaerobic digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Enhancement of dark fermentative H2 production by gas separation membranes: A review. Bioresour. Technol. 2020, 302, 122828. [Google Scholar] [CrossRef] [PubMed]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Chang, J.-S. Bioreactor and process design for biohydrogen production. Bioresour. Technol. 2011, 102, 8524–8533. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Guo, W.; Liu, B.; Cao, G.; Ding, J. Biological hydrogen production by dark fermentation: Challenges and prospects towards scaled-up production. Curr. Opin. Biotechnol. 2011, 22, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Li, C.; Fang, H.H. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit. Rev. Environ. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Show, K.; Lee, D.; Tay, J.; Lin, C.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen production from biomass and wastes via dark fermentation: A review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R. A review of dark fermentative hydrogen production from biodegradable municipal waste fractions. Waste Manag. 2013, 33, 1345–1361. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Mishra, P.; Roy, S.; Das, D. Comparative evaluation of the hydrogen production by mixed consortium, synthetic co-culture and pure culture using distillery effluent. Bioresour. Technol. 2015, 198, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.V.; Nespeca, M.G.; Sakamoto, I.K.; de Oliveira, J.E.; Varesche, M.B.A.; Maintinguer, S.I. Bioconversion of crude glycerol from waste cooking oils into hydrogen by sub-tropical mixed and pure cultures. Int. J. Hydrogen Energy 2019, 44, 144–154. [Google Scholar] [CrossRef]
- Mthethwa, N.P.; Nasr, M.; Kiambi, S.L.; Bux, F.; Kumari, S. Biohydrogen fermentation from Pistia stratiotes (aquatic weed) using mixed and pure bacterial cultures. Int. J. Hydrogen Energy 2019, 44, 17720–17731. [Google Scholar] [CrossRef]
- Rosa, D.; Medeiros, A.B.P.; Martinez-Burgos, W.J.; do Nascimento Junior, J.R.; de Carvalho, J.C.; Sydney, E.B.; Soccol, C.R. Biological hydrogen production from palm oil mill effluent (POME) by anaerobic consortia and Clostridium beijerinckii. J. Biotechnol. 2020, 323, 17–23. [Google Scholar] [CrossRef]
- Trancone, G.; Policastro, G.; Spasiano, D.; Race, M.; Parrino, F.; Fratino, U.; Fabbricino, M.; Pirozzi, F. Treatment of concrete waste from construction and demolition activities: Application of organic acids from continuous dark fermentation in moving bed biofilm reactors. Chem. Eng. J. 2025, 505, 159536. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Pengadeth, D. Mixed culture biotechnology and its versatility in dark fermentative hydrogen production. Bioresour. Technol. 2024, 394, 130286. [Google Scholar] [CrossRef]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: A long-term pilot scale experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Joseph, K.; Sukumaran, V. Bio hydrogen generation from kitchen waste in an inclined plug flow reactor. Int. J. Hydrogen Energy 2009, 34, 8854–8858. [Google Scholar] [CrossRef]
- Yahaya, E.; Lim, S.W.; Yeo, W.S.; Nandong, J. A review on process modeling and design of biohydrogen. Int. J. Hydrogen Energy 2022, 47, 30404–30427. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Factories 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.-H.; Chu, C.-Y.; Leu, H.-J.; Marone, A. Biohydrogen production by dark fermentation: Scaling-up and technologies integration for a sustainable system. Rev. Environ. Sci. Bio/Technol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Xu, R.-z.; Fang, S.; Zhang, L.; Huang, W.; Shao, Q.; Fang, F.; Feng, Q.; Cao, J.; Luo, J. Distribution patterns of functional microbial community in anaerobic digesters under different operational circumstances: A review. Bioresour. Technol. 2021, 341, 125823. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Wiktorowska-Sowa, E.; Piotrowski, J.; Salamon, A.; Kaźmierczak, W.; Błaszczyk, M.K.; Barberan, A.; Chen, Y. Dynamics and complexity of dark fermentation microbial communities producing hydrogen from sugar beet molasses in continuously operating packed bed reactors. Front. Microbiol. 2021, 11, 612344. [Google Scholar] [CrossRef]
- Navarro-Díaz, M.; Aparicio-Trejo, V.; Valdez-Vazquez, I.; Carrillo-Reyes, J.; Avitia, M.; Escalante, A.E. Levels of microbial diversity affect the stability and function of dark fermentation bioreactors. Front. Ind. Microbiol. 2024, 2, 1386726. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Improvement of fermentative hydrogen production: Various approaches. Appl. Microbiol. Biotechnol. 2004, 65, 520–529. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Microbial Technologies in Advanced Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Yokoi, H.; Ohkawara, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Characteristics of hydrogen production by aciduric Enterobacter aerogenes strain HO-39. J. Ferment. Bioeng. 1995, 80, 571–574. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process Biochem. 2000, 35, 589–593. [Google Scholar] [CrossRef]
- Segers, B.; Nimmegeers, P.; Spiller, M.; Tofani, G.; Grojzdek, E.J.; Dace, E.; Kikas, T.; Marchetti, J.M.; Rajić, M.; Yildiz, G. Lignocellulosic biomass valorisation: A review of feedstocks, processes and potential value chains, and their implications for the decision-making process. RSC Sustain. 2024, 2, 3730–3749. [Google Scholar] [CrossRef]
- Basera, P.; Chakraborty, S.; Sharma, N. Lignocellulosic biomass: Insights into enzymatic hydrolysis, influential factors, and economic viability. Discov. Sustain. 2024, 5, 311. [Google Scholar] [CrossRef]
- Mumtha, C.; Mahalingam, P.U. Biohydrogen production from co-substrates through dark fermentation by bacterial consortium. 3 Biotech 2024, 14, 281. [Google Scholar] [CrossRef]
- Romão, B.; Batista, F.; Ferreira, J.; Costa, H.; Resende, M.; Cardoso, V. Biohydrogen production through dark fermentation by a microbial consortium using whey permeate as substrate. Appl. Biochem. Biotechnol. 2014, 172, 3670–3685. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, C.; Chu, C.; Lee, D.; Chang, B.-V.; Liao, C. Producing hydrogen from wastewater sludge by Clostridium bifermentans. J. Biotechnol. 2003, 102, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef]
- Rawat, S.; Rautela, A.; Yadav, I.; Misra, S.; Kumar, S. A comprehensive review on enhanced biohydrogen production: Pretreatment, applied strategies, techno-economic assessment, and future perspective. BioEnergy Res. 2023, 16, 2131–2154. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Valdez-Vazquez, I.; Vital-Jácome, M.; Vargas, A.; Navarro-Díaz, M.; Cortez-Cervantes, J.; Chango-Cañola, A.P. Microbial Communities in Dark Fermentation, Analytical Tools to Elucidate Key Microorganisms and Metabolic Profiles. In Wastewater Exploitation: From Microbiological Activity to Energy; Springer: Berlin/Heidelberg, Germany, 2024; pp. 107–132. [Google Scholar]
- Oh, S.E.; Iyer, P.; Bruns, M.A.; Logan, B.E. Biological hydrogen production using a membrane bioreactor. Biotechnol. Bioeng. 2004, 87, 119–127. [Google Scholar] [CrossRef]
- Chu, C.-F.; Xu, K.-Q.; Li, Y.-Y.; Inamori, Y. Hydrogen and methane potential based on the nature of food waste materials in a two-stage thermophilic fermentation process. Int. J. Hydrogen Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Dong, C.; Miller, C.; Li, Y.; Wang, L.; Yao, W. Continuous biohydrogen production from liquid swine manure supplemented with glucose using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2009, 34, 6636–6645. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Z.; Fan, Y.; Hou, H. Biohydrogen production from dairy manures with acidification pretreatment by anaerobic fermentation. Environ. Sci. Pollut. Res. 2010, 17, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sheng, T.; Gao, L.; Zhao, L.; Liu, W.; Wang, A. Direct hydrogen production from lignocellulose by the newly isolated Thermoanaerobacterium thermosaccharolyticum strain DD32. RSC Adv. 2015, 5, 99781–99788. [Google Scholar] [CrossRef]
- Cui, M.; Shen, J. Effects of acid and alkaline pretreatments on the biohydrogen production from grass by anaerobic dark fermentation. Int. J. Hydrogen Energy 2012, 37, 1120–1124. [Google Scholar] [CrossRef]
- Ivanova, G.; Rákhely, G.; Kovács, K.L. Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int. J. Hydrogen Energy 2009, 34, 3659–3670. [Google Scholar] [CrossRef]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Reungsang, A.; Sittijunda, S.; Sompong, O. Bio-hydrogen production from glycerol by immobilized Enterobacter aerogenes ATCC 13048 on heat-treated UASB granules as affected by organic loading rate. Int. J. Hydrogen Energy 2013, 38, 6970–6979. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Tung, L.; Lin, C.-Y. Effect of substrate concentration and pH on biohydrogen production kinetics from food industry wastewater by mixed culture. Int. J. Hydrogen Energy 2013, 38, 15849–15855. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial production of hydrogen: An overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Vazquez, I.; Poggi-Varaldo, H.M. Hydrogen production by fermentative consortia. Renew. Sustain. Energy Rev. 2009, 13, 1000–1013. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrogen Energy 2008, 33, 2934–2941. [Google Scholar] [CrossRef]
- Kotay, S.M.; Das, D. Novel dark fermentation involving bioaugmentation with constructed bacterial consortium for enhanced biohydrogen production from pretreated sewage sludge. Int. J. Hydrogen Energy 2009, 34, 7489–7496. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Li, Y.-H.; Zheng, H.-Q.; Fan, Y.-T.; Hou, H.-W. Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain Clostridium sartagoforme FZ11. Bioresour. Technol. 2015, 192, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kogo, T.; Yoshida, Y.; Koganei, K.; Matsumoto, H.; Watanabe, T.; Ogihara, J.; Kasumi, T. Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Bioresour. Technol. 2017, 233, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Li, J.-Z.; Liu, F. Evaluation of different pretreatment methods for preparing hydrogen-producing seed inocula from waste activated sludge. Renew. Energy 2011, 36, 1517–1522. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mohan, S.V.; Babu, V.L.; Sarma, P. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Wang, A.; Chen, J. Chemical inhibitors of methanogenesis and putative applications. Appl. Microbiol. Biotechnol. 2011, 89, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, M.Z.; Mohee, R.; Hassan, M.A. Effects of pre-treatment technologies on dark fermentative biohydrogen production: A review. J. Environ. Manag. 2015, 157, 20–48. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Ríos-Leal, E.; Esparza-García, F.; Cecchi, F.; Poggi-Varaldo, H.M. Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: Mesophilic versus thermophilic regime. Int. J. Hydrogen Energy 2005, 30, 1383–1391. [Google Scholar] [CrossRef]
- Shin, H.-S.; Youn, J.-H.; Kim, S.-H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lin, P.-J.; Chang, J.-S. Temperature effects on biohydrogen production in a granular sludge bed induced by activated carbon carriers. Int. J. Hydrogen Energy 2006, 31, 465–472. [Google Scholar] [CrossRef]
- Foglia, D.; Wukovits, W.; Friedl, A.; de Vrije, T.; Claassen, P.A. Fermentative Hydrogen Production: Influence of Application of Mesophilic and Thermophilic Bacteria on Mass and Energy Balances. 2011. Available online: https://research.wur.nl/en/publications/fermentative-hydrogen-production-influence-of-application-of-meso (accessed on 21 February 2025).
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lay, C.-H.; Sen, B.; Chu, C.-Y.; Kumar, G.; Chen, C.-C.; Chang, J.-S. Fermentative hydrogen production from wastewaters: A review and prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Bowles, L.K.; Ellefson, W. Effects of butanol on Clostridium acetobutylicum. Appl. Environ. Microbiol. 1985, 50, 1165–1170. [Google Scholar] [CrossRef]
- Ginkel, S.V.; Sung, S.; Lay, J.-J. Biohydrogen production as a function of pH and substrate concentration. Environ. Sci. Technol. 2001, 35, 4726–4730. [Google Scholar] [CrossRef] [PubMed]
- Temudo, M.F.; Kleerebezem, R.; van Loosdrecht, M. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, H.-P.; Wu, J.-H.; Lin, C.-Y. Fermentative hydrogen production at high sulfate concentration. Int. J. Hydrogen Energy 2008, 33, 1573–1578. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, C.N.; Chang, J.S. Hydrogen production with immobilized sewage sludge in three-phase fluidized-bed bioreactors. Biotechnol. Prog. 2003, 19, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Venegas, E.; Ramirez, J.E.; Donoso-Bravo, A.; Jorquera, L.; Steyer, J.-P.; Ruiz-Filippi, G. Bio-hydrogen production during acidogenic fermentation in a multistage stirred tank reactor. Int. J. Hydrogen Energy 2013, 38, 2185–2190. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestóthy, N.; Simon, V.; Bélafi-Bakó, K. Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew. Sustain. Energy Rev. 2014, 40, 806–813. [Google Scholar] [CrossRef]
- Mandal, B.; Nath, K.; Das, D. Improvement of biohydrogen production under decreased partial pressure of H2 by Enterobacter cloacae. Biotechnol. Lett. 2006, 28, 831–835. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrogen Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen production from organic wastes by dark fermentation. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–144. [Google Scholar]
- Gavala, H.N.; Skiadas, I.V.; Ahring, B.K. Biological hydrogen production in suspended and attached growth anaerobic reactor systems. Int. J. Hydrogen Energy 2006, 31, 1164–1175. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lo, Y.-C.; Lin, P.-J.; Chang, J.-S. Improving biohydrogen production in a carrier-induced granular sludge bed by altering physical configuration and agitation pattern of the bioreactor. Int. J. Hydrogen Energy 2006, 31, 1648–1657. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Tay, J.-H.; Show, K.-Y.; Yan, R.; Liang, D.T.; Lee, D.-J.; Jiang, W.-J. Biohydrogen production in a granular activated carbon anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2007, 32, 185–191. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Show, K.-Y.; Tay, J.-H.; Liang, D.T.; Lee, D.-J. Biohydrogen production with anaerobic fluidized bed reactors—A comparison of biofilm-based and granule-based systems. Int. J. Hydrogen Energy 2008, 33, 1559–1564. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Chung, J. Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int. J. Hydrogen Energy 2010, 35, 11746–11755. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, S.-Y.; Lin, P.-J.; Chang, J.-S.; Hung, C.-H.; Lee, K.-S.; Lay, C.-H.; Chu, C.-Y.; Cheng, C.-H.; Chang, A.C. A pilot-scale high-rate biohydrogen production system with mixed microflora. Int. J. Hydrogen Energy 2011, 36, 8758–8764. [Google Scholar] [CrossRef]
- Mechery, J.; Thomas, D.M.; Kumar, C.P.; Joseph, L.; Sylas, V. Biohydrogen production from acidic and alkaline hydrolysates of paddy straw using locally isolated facultative bacteria through dark fermentation. Biomass Convers. Biorefinery 2021, 11, 1263–1272. [Google Scholar] [CrossRef]
- Kumar, K.; Roy, S.; Das, D. Continuous mode of carbon dioxide sequestration by C. sorokiniana and subsequent use of its biomass for hydrogen production by E. cloacae IIT-BT 08. Bioresour. Technol. 2013, 145, 116–122. [Google Scholar] [CrossRef]
- Niu, M.; Jin, B.; Huang, Y.; Wang, H.; Dong, Q.; Gu, H.; Yang, J. Co-gasification of high-ash sewage sludge and straw in a bubbling fluidized bed with oxygen-enriched air. Int. J. Chem. React. Eng. 2018, 16, 20170044. [Google Scholar] [CrossRef]
- Ruggeri, B.; Tommasi, T.; Sassi, G. Experimental kinetics and dynamics of hydrogen production on glucose by hydrogen forming bacteria (HFB) culture. Int. J. Hydrogen Energy 2009, 34, 753–763. [Google Scholar] [CrossRef]
- Fabiano, B.; Perego, P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy 2002, 27, 149–156. [Google Scholar] [CrossRef]
- Wong, Y.M.; Juan, J.C.; Ting, A.; Wu, T.Y. High efficiency bio-hydrogen production from glucose revealed in an inoculum of heat-pretreated landfill leachate sludge. Energy 2014, 72, 628–635. [Google Scholar] [CrossRef]
- Posten, C.H.; Cooney, C.L. Growth of microorganisms. Biotechnology 1993, 1, 111–162. [Google Scholar]
- Cecílio, D.M.; Gonçalves, J.R.M.; Correia, M.J.N.; Mateus, M.M. Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels 2023, 4, 221–242. [Google Scholar] [CrossRef]
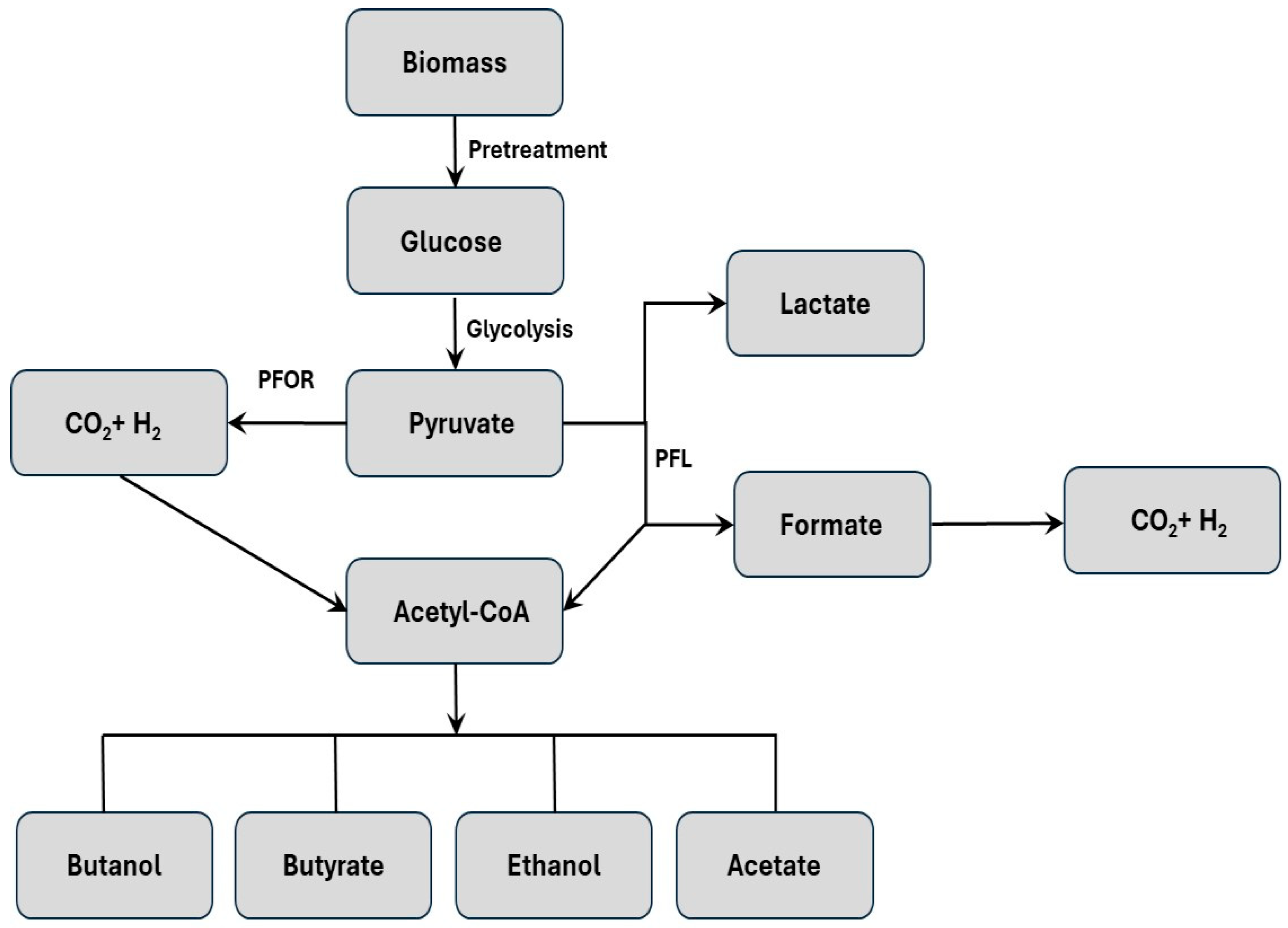
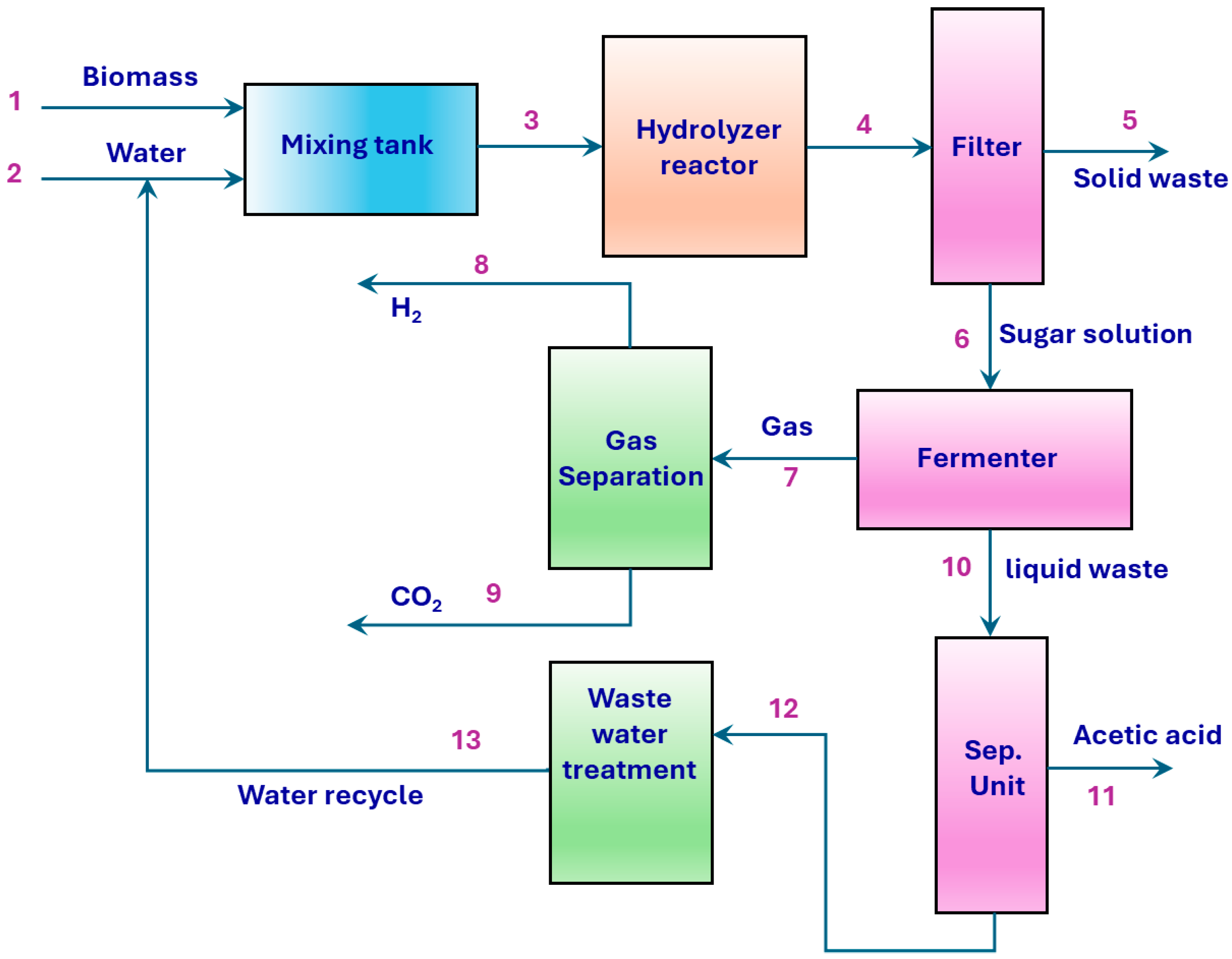
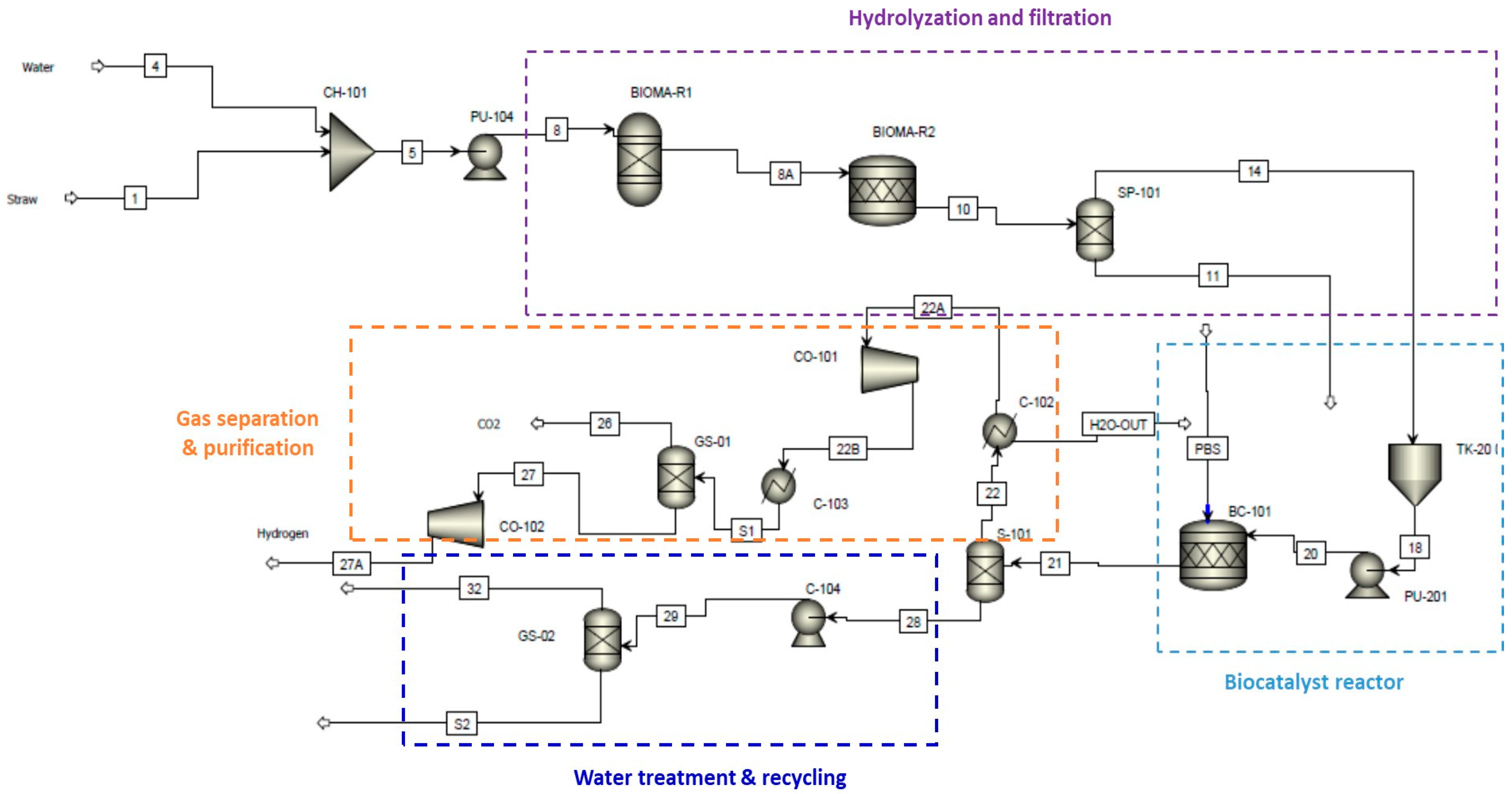
| Substrate | Example | Advantage | Disadvantage | Hydrogen Yield (Mole H2/Mole Substrate) | Reference |
|---|---|---|---|---|---|
| Monosaccharides | Glucose, D-Xylose | Biodegradable substrate, high yield | High cost for industrial level | 4, 0.95 | [59] |
| Disaccharides | Sucrose, lactose | Biodegradable substrate, high yield | High cost for industrial level | 6, 3 | [50] |
| Organic municipal waste | Kitchen waste, kitchen garbage | Low cost and availability | Pretreatment is needed | 72 cm3 H2/g VS, 66 cm3 H2/g VS | [37] [60] |
| Manure | Swine manure, dairy manure | Reduce the gas pollution, low cost | Wastewater treatment is needed | 1.63, 31.5 cm3 H2/g TVS | [61] [62] |
| Agriculture waste | Cornstalk, bagasse, wheat straw | Availability, low cost | Lignin present in waste resists on biodegradation | 6.38 mole/kg substrate 2.3, 3.8 | [63] [64] [65] |
| Industrial waste | Cheese whey, glycerol waste, brewery wastewater | Reduce the cost of waste treatment, low cost | Presence unwanted component inhibit the process | 0.78, 0.33, 2 | [66] [67] [68] |
| Pretreatment Method | Operating Condition | Advantage | Disadvantage | Comment | Reference |
|---|---|---|---|---|---|
| Heat shock | 80–121 °C | Increase cell permeability Simple and cost-effective | Cell damage Energy consumption | Treating the culture with a high temperature kills non-spore-forming bacteria. | [70,71] |
| Freezing and thawing | −25 to −10 °C freezing Thawing Incubating 20–30 °C | Cell disruption Low cost | Time-consuming Limited scalability | Inoculum is frozen and this is maintained, followed by thawing and the incubating | [72] |
| Aeration | Time: 2 hours–14 days | Enhance microbial growth Low cost Scaleable | Energy-intensive | use of the air to eliminate the bacteria sensitive to oxygen. | [73,74] |
| Acid and alkaline treatment | Acid: pH 2–4 Alkaline:pH 10–12 | Efficient biomass breakdown Widely used | Corrosive chemical disposal | Adjustment of the pH of the inoculum to a value that microorganisms cannot survive. | [75,76] |
| Chemical treatment | 2-bromoethane sulfonate (2-BES) 2-bromoethane sulfonic acid (2-BESA) | Selective breakdown High efficiency | Toxic byproduct High cost | Chemical compounds can block metabolic pathways of methanogenic bacteria selectively. | [77,78] |
| Microwave | Frequencies ranging from 300 MHz to 300 GHz | Rapid and efficient Scalable | Uneven heating Energy consumption | This applies for the pretreatment of lignocellulosic biomass for enhanced hydrolysis | [79] |
| Ultraviolet | Wavelengths varying from 10 nm to 400 nm | Effective for sterilization Environmental friendly | Limited penetration Cost | Ultraviolet lies between visible light and x-rays in the electromagnetic spectrum It applies for inactivation of bacterial population | [79] |
| Ultimate Analysis (wt%) | Proximate Analysis (wt%) | ||
|---|---|---|---|
| C | 42.5 | Moisture | 8.3 |
| H | 6.3 | Volatiles | 70.3 |
| N | 0.8 | Fixed carbon | 18.7 |
| O | 38.7 | Ash | 11 |
| S | 0.2 | ||
| Cl | 0.5 | ||
| LHV (MJ/kg) | 16.32 | ||
| Streams No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | 25 | 25 | 25 | 50 | 50 | 34 | 20 | 20 | 20 | 25 | 25 | 25 | 25 |
| P (Bar) | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 4 | 4 | 1 | 1 | 1 | 3 |
| F (kg/h) | 10,000 | 105,144 | 115,144 | 115,144 | 35,267 | 79,877 | 2733 | 275 | 2458 | 77,144 | 3984 | 73,160 | 65,144 |
| Phase | Liquid | Liquid | Liquid | Liquid | Liquid | Liquid | Vapor | Vapor | Vapor | Liquid | Liquid | Liquid | Liquid |
| mBiomass | 10,000 | 0 | 10,000 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mash | 0 | 0 | 0 | 1016 | 1016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mCO2 | 0 | 0 | 0 | 0 | 0 | 0 | 2504 | 50 | 2454 | 0 | 0 | 0 | 0 |
| mH2O | 0 | 105,144 | 105,144 | 105,912 | 31,773 | 74,139 | 0 | 0 | 0 | 73,114 | 731 | 72,381 | 65,144 |
| mH2 | 0 | 0 | 0 | 0 | 0 | 0 | 229 | 225 | 4 | 0 | 0 | 0 | 0 |
| msolid | 0 | 0 | 0 | 2433 | 2433 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mCH3COOH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3417 | 3246 | 171 | 0 |
| mglucose | 0 | 0 | 0 | 5694 | 0 | 5694 | 0 | 0 | 0 | 569 | 5 | 564 | 0 |
| mNH4Cl | 0 | 0 | 0 | 68 | 34 | 34 | 0 | 0 | 0 | 34 | 1 | 34 | 0 |
| msulphur | 0 | 0 | 0 | 21 | 11 | 10 | 0 | 0 | 0 | 10 | 1 | 10 | 0 |
| Streams No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | 25 | 25 | 25 | 50 | 50 | 34 | 20 | 20 | 20 | 25 | 25 | 25 | 25 |
| P (Bar) | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 4 | 4 | 1 | 1 | 1 | 3 |
| F (kg/h) | 10,000 | 106,358 | 116,358 | 116,358 | 35,632 | 80,727 | 2676 | 219 | 2457 | 78,051 | 3565 | 74,486 | 66,358 |
| Phase | Liquid | Liquid | Liquid | Liquid | Liquid | Liquid | Vapor | Vapor | Vapor | Liquid | Liquid | Liquid | Liquid |
| mBiomass | 10,000 | 0 | 10,000 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mash | 0 | 0 | 0 | 1016 | 1016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mCO2 | 0 | 0 | 0 | 0 | 0 | 0 | 2504 | 50 | 2454 | 0 | 0 | 0 | 0 |
| mH2O | 0 | 106,358 | 106,358 | 107,126 | 32,138 | 74,988 | 0 | 0 | 0 | 74,476 | 745 | 73,731 | 66,358 |
| mH2 | 0 | 0 | 0 | 0 | 0 | 0 | 172 | 169 | 3 | 0 | 0 | 0 | 0 |
| msolid | 0 | 0 | 0 | 2433 | 2433 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mCH3COOH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1708 | 1623 | 85 | 0 |
| mbutyrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1253 | 1190 | 63 | 0 |
| mglucose | 0 | 0 | 0 | 5694 | 0 | 5694 | 0 | 0 | 0 | 569 | 5 | 564 | 0 |
| mNH4Cl | 0 | 0 | 0 | 68 | 34 | 34 | 0 | 0 | 0 | 34 | 1 | 34 | 0 |
| msulphur | 0 | 0 | 0 | 21 | 11 | 10 | 0 | 0 | 0 | 10 | 1 | 10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtarani, B.; Zanganeh, J.; Moghtaderi, B. A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies 2025, 18, 1092. https://doi.org/10.3390/en18051092
Mokhtarani B, Zanganeh J, Moghtaderi B. A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies. 2025; 18(5):1092. https://doi.org/10.3390/en18051092
Chicago/Turabian StyleMokhtarani, Babak, Jafar Zanganeh, and Behdad Moghtaderi. 2025. "A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation" Energies 18, no. 5: 1092. https://doi.org/10.3390/en18051092
APA StyleMokhtarani, B., Zanganeh, J., & Moghtaderi, B. (2025). A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies, 18(5), 1092. https://doi.org/10.3390/en18051092





