Abstract
This paper presents the effectiveness of representing the process of creating and burning a combustible mixture in vibroacoustic parameters of a compression ignition engine. Empirical engine tests allowed us to conduct analyses in terms of the operating conditions, fuel parameters, and fuel type. The influence of dimethyl ether on combustion efficiency was quantified using performance indicators, emission parameters, and vibration estimates (compared to diesel fuel). Mathematical models of combustion and its variability were created using the mean, peak-to-peak amplitude, root mean square error, and peak amplitudes of vibration accelerations, which were also represented using vibration graphics. Dimethyl ether positively influenced engine performance, emissions, and vibration reduction. The proposed method can predict combustion irregularities and detect their sources in engine designs with high kinetic energy, hybrid combustion modeling, and fuel composition identification. Dimethyl ether reduced hydrocarbons by 96–99%, particulate matter by 37–60%, and carbon monoxide by 2.5–19.5%, whereas nitrogen oxides increased by 1–8% (relative to diesel fuel). Emission models were created with accuracies of 0.88–0.96 (hydrocarbons), 0.80–0.98 (particulate matter), 0.95–0.99 (carbon monoxide), and 0.97–0.99 (nitrogen oxides). Dimethyl ether application reduced the mean amplitude of the vibrations in the range of 5.7–60.6% and the peak-to-peak amplitude in the range of 18.2–72.4%. The standard deviation of combustion was decreased by 8.8–49.1% (mean) and by 28.8–39.5% (peak-to-peak). The vibroacoustic models’ accuracy scores were 0.90–0.99 (diesel fuel) and 0.72–0.75 (dimethyl ether).
1. Introduction
The area, purpose, and scope of research on mechanical systems in which fast-changing processes are implemented depend on many factors related to the object itself, the working conditions, the operational purpose, and the external environment. The common denominator in terms of these activities is learning about the observed object sufficiently from the point of view of the main processes and accompanying processes implemented by it. Thanks to this approach, it is possible to assess an object’s operational efficiency according to a specific set of criteria and limiting conditions [1,2]. Consequently, such an assessment enables further optimization of its operations through specific structural or operational modifications. The increasingly high requirements that analyzed mechanical objects have to meet (in regard to the applicable set of multidimensional operational characteristics and their variability) make it necessary to search for increasingly sophisticated methods and models of analysis based on both classical methods and artificial intelligence models [3]. This assessment is all the more difficult because it takes into account requirements of different origins, e.g., technical, economic, social, environmental, biomedical, or hybrid sets of requirements (of a different nature, time, and space, as well as impact values). However, the pursuit of the highest possible overall efficiency of object operations is the first in a set of determinants of the criteria for designing and operating a modern mechanical object, in which the energy contained in a specific fuel is rapidly converted into its equivalent mechanical work. It is expected that the object will maintain this indicator of efficiency at the highest level and in the widest possible range. This should be achieved in terms of the object’s operation period, as well as a wide set of operating parameters pertaining to the object that are mapped to the traction operating conditions of the power receiver [1,2,3,4,5]. Fulfilling this condition requires that both the design of a mechanical system and the management of its operation are improved, as well as the application of accurate methods for monitoring and diagnosing problems in real time (early detection of the first symptoms of process irregularities and structural failures). A multi-criteria evaluation of the system in real time was carried out thanks to the multidimensional set of parameters (combining the main and accompanying processes). Contemporary requirements to minimize the negative environmental impact [4,5] of a system have accelerated the development of modern solutions in this area in order to meet acceptable levels of emissions of harmful and toxic exhaust components and vibration processes generated [1,2,3]. The study of vibration emissions and processes and their application in complex diagnostic evaluations is an important step in the search for more accurate methods of multidimensional analysis in order to manage the efficiency and operational reliability of thermodynamic mechanical systems more effectively [1,2,3].
The development of modern mechanical systems for energy generation and transformation is focused on finding a source and method of energy conversion with the highest possible total efficiency [3]. Such an assessment should be conducted from the beginning of the system’s acquisition to its operation for a specific task, also taking into account the recycling process of the system and its energy consumption. Along with the application of this idea in the design and production of high-efficiency electric cells (including fuel cells) used in vehicles, modern research and development works are also focused on the composition of various alternative fuels (either as a single type of fuel or in combination with conventional fuel). The abovementioned research direction is extremely important from the point of view of the global and rational management of limited natural resources and their physicochemical processing [1,2,3,4,5]. The abovementioned action is all the more justified when one notices the still relatively low total efficiency of electric drives and their defects, which have been revealed through variable operating conditions of the mechanical objects and variable thermal conditions of the electric cell operation (also affecting the total economic and environmental balance of the system’s operation). The energy stability of a cell and its high rate of energy conversion and accumulation are still subjects of scientific research. The application of the appropriate type of energy generation and conversion in a cell is one of the solutions in this area, which is also relevant in modern mechanical systems that convert chemical energy from fuel into a specific brake power. Therefore, the process of looking for the right type and composition of fuel that might be widely used in the future due to its favorable balance of energy, materials, and toxic exhaust emissions is very important. In this context, two important issues are noted: the selection of the optimal fuel composition (the type and shares) and the proper management of the process regarding its preparation and conversion (depending on the specified operating conditions and purpose).
Based on the above-formulated criteria of the energy conversion efficiency throughout the entire period of system operation, it is necessary to conduct appropriate research in which a set of real functional relations and discrete data will be obtained that capture the quality of the fuel composition quantitatively. The efficiency of the creation and combustion of the air–fuel mixture in a wide range of the object’s field of operation is its measurable reference. Therefore, the authors of this paper monitored the most important parameters and time indicators of the system’s operation, supplementing it with a package of on-line data obtained from vibration processes generated in the engine (as a result of the observed combustion process of a specific fuel composition).
The above-defined fidelity of the reproduction and quantification of the source process and its appropriate modification (in the primary phase of the energy source composition and its conditions) is an indispensable base for a correct methodical approach to conduct modeling and empirical verification of the combustion process of a specific fuel structure (in kinetic expansion and its development). Recognizing the need for a faithful representation of the signal form of these dynamic changes (due to complex chemical reactions), the application of accurate methods to obtain information on the sources and the temporal, spatial, and parametric form of the dynamic characteristics of the observed combustion process (cyclically or continuously) is undoubtedly of great importance. Looking for an unambiguous relationship between the main process and its signal representation is an indispensable condition for knowing more about the kinetic representation of the oxidation reaction characteristics for the specific fuel composition and its further quantification. As a result, the designated models faithfully reproduce the behavior of the mechanical object in terms of process evaluation. This enables the proper diagnostic evaluation and real-time analysis of the indicators that show how correct this energy conversion and their operational utility are. The above action enables the creation of ready-made tools used for the autonomous supervision of the correctness of the dynamic process for a specific evaluation criterion and limiting conditions (regarding an object’s structure and external factors). As a result, the early evaluation of the first symptoms of irregularities, which have a positive effect on maintaining a specific level of the object’s efficiency and reliability, might be identified.
This scientific paper is important from the point of view of current global research trends in this scientific area. The quantitative assessment of the diagnostic efficiency of vibration processes and their specific transformation and parameterization during alternative fuel combustion (in the example of a dimethyl ether) in relation to conventional fuel is the basis for parametric analyses in this area. The aim of empirical studies was to obtain reliable signal forms from measurement points with the highest information value for the observed combustion process. Detailed studies on mathematical representations of both types of processes were carried out, taking into account the operating parameters of the mechanical system and the parallel recording of vibration accelerations by the transducer (for three perpendicular measurement directions). A comparative analysis, presented in a later part of the paper, became the basis for finding the most effective vibration diagnostic parameter to verify its unambiguity and fidelity in the obtained changes of combustion possible to be used in wide operation conditions (also possible to be used in non-stationary conditions).
Thanks to this, it will be possible to determine the specific characteristics of various aspects (physicochemical, mechanical, thermodynamic, and tribological). These are necessary in the processes of designing and managing a system in which high-combustion kinetics takes place (particularly important in high-speed systems, e.g., turbojet engines). The quantitative effects of the verification of the formulated detailed research goals are presented in the above paper, ranking them for a specific set of variables of the system operation (thermodynamic and operational). The above reliability for new measures of the vibration process (for a particular analysis: time and process value) was referred to as the amplitude measures (determined previously), showing the differences between them and the nature of changes in each parameter (together with its mathematical equation and the degree of mapping its real form). The sensitivity of a given fuel composition to the dispersion of empirical data around their reference representations was also indicated, obtaining information on the determinism and statistical reliability of each elementary time representation of combustion processes.
The main objectives of the work (motivation and research gaps) are to assess the following:
- (a)
- The effectiveness of mapping the course of creation and kinetic combustion of alternative fuel (diesel fuel + DME) in characteristics and vibration parameters (impulsive and energetic) for compression ignition (CI) engines. This study contributes to the development of the knowledge necessary for the application of continuous monitoring and diagnosis of the combustion process (the entire period of operation and taking into account tribological changes in the system), assessment of the correlation between the type of fuel composition and its conversion efficiency, and identification of sources of irregularities (this work will provide graphics and reference parameters for a given fuel, changes between them, and deviations from their values);
- (b)
- Assessment of the quality of the fuel composition determined in relation to emission parameters (CO, HC, NOx, and PM), engine performance indicators, and measures of the vibration process (graphic and parametric representations of this variability). This research complements knowledge in the field of real correlation between the emissions of new fuel and variables affecting them, along with an empirical correlation of emissions with operational and vibration indicators;
- (c)
- Mathematical equations describing the above relationships (emission, vibrations, and indication) in relation to combustion (diesel fuel+DME). This study creates new value in the scope of the mathematical modeling of combustion of new fuels (and hybrid methods), the creation of a database (classical and AI models), and STD (together with the assessment of the uniformity of cyclical combustion kinetics).
The theoretical part of the above paper included the following issues: an introduction to the research topic, an analysis of the purpose and scope of the work, and a study of scientific achievements in the area of the undertaken research topic. The research part of this work was divided into the following parts: a description of the research methodology (taking into account the research stand and its particular components and measurement equipment), an analysis of the fuel specifications together with the mathematical description of their combustion and sources that influence its conversion, empirical research conditions, a description of research results and their analysis, and the analysis and formulation of mathematical models for vibration relationships that represent thermodynamic processes in a mechanical system.
2. Analysis of the Knowledge State in Research Topics
Contemporary achievements in science and engineering falling within the research problems discussed here concern the following categories of innovative issues:
- Composition and combustion of DME [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]:
- (a)
- An experimental and chemical kinetic study on the effects of H2-DME [6] and atomic insights into the combustion mechanism of DME/NH3 mixtures [7];
- (b)
- The combustion behavior and emission of biodiesel and DME [8] and a study of ammonia blending with DME steam reformed and cracked gases under a lean-burn condition [9];
- (c)
- The flame instability of NH3/DME blends with H2 addition [10] and swirl premixed dimethyl ether/methane flame stability and combustion characteristics in an industrial gas turbine combustor [11];
- (d)
- A study on NOx reduction from NH3/DME/air flame using a fuel staging method [12] and energy utilization of the methanol/dimethyl ether dual-fuel engine under the methanol-reforming strategy [13];
- (e)
- The hydrogen and EGR on energy efficiency improvement with ultra-low emissions in a common rail direct injection compression ignition engine fueled with dimethyl ether [14] and application of DME as a transitional solvent for enhanced oil recovery [15];
- (f)
- NH3 and NH3/DME laminar flame propagation in an O2/CO2 atmosphere [16] and ignition and cool flame interactions of DME/H2/air blends [17];
- (g)
- The thermodynamic and exergoeconomic analysis of DME as a dual expansion and triple recuperation cycle fuel with a high hydrocarbon ratio and self-oxygenating properties [18] and flame-dual field overpressure coupling evolution characteristics of LPG/DME blended gas explosion venting [19];
- (h)
- The emission characteristics of fully premixed combustion of dimethyl ether and hydrogen based on flame ions [20] and a chemical kinetics analysis of ammonia/dimethyl ether combustion under water addition conditions [21];
- (i)
- DME synthesis technologies for the energy transition [22] and dimensionless vent ratio on the flame-shock wave evolution dynamics of blended LPG/DME gas explosion venting [23];
- (j)
- Non-monotonic liftoff height behaviors in laminar nonpremixed coflow jet flames of DME with an ambient temperature variation [24] and combustion and emission characteristics of diesel polyoxymethylene dimethyl ethers blend fuels with exhaust gas recirculation and a double injection strategy [25];
- (k)
- The optimization of a theoretical pressure prediction model for the confined explosion of low-carbon fuels [26] and the effect of a pre-blended section ratio and a blended hydrogen ratio on the explosion dynamics of the blended dimethyl ether–hydrogen gas [27];
- (l)
- The effects of alternative fuels on diesel engine performance combustion and exhaust emissions [28] and steam dilution on the ignition delay time of dimethyl ether/air mixtures at a high pressure measured behind reflected shock waves [29];
- (m)
- The pre-ignition of DME involving complex interactions of physical and chemical processes [30] and the influence of LPG–DME mixture on the dynamic characteristics of a hybrid vehicle [31];
- (n)
- The dimethyl ether combustion performance and low NOx emissions of a compression ignition engine at a high injection pressure and a high EGR rate [32] and DME with diesel fuel mixture preliminary injection characterization of the injection rate and the quantity [33], as well as the effects of high EGR rates on DME fuel combustion performance, exhaust emissions, and particle emission characteristics in a small direct injection diesel engine under various injection timings [34].
- Conclusion:
- There is currently widespread interest in science and engineering in various application areas in the use of DME as a natural fuel within its composition with other alternative fuels. The aim of this research is to search for effective fuel that allows for obtaining the highest possible performance indicators of a mechanical object in stationary and non-stationary conditions of its operation, as well as its reliability and the lowest possible emission of harmful exhaust components (meeting future emission regulation requirements). The assessment of knowledge in this area reveals a lack of detailed empirical studies on the quantitative assessment of the combustion kinetics of a specific DME composition with diesel fuel together with its hybrid quantification, taking into account the indicated parameters and exhaust emissions, as well as their connection with vibration processes and their impulse and energy characteristics.
- Vibration diagnostics of the combustion process in the context of DME applications [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]:
- (a)
- The diagnostics of methanol active thermal atmosphere combustion in a compression ignition engine using an optical method [35] and the study of internal and external EGR combined with oxygenated n-butanol, polyoxymethylene dimethyl ethers, and dimethyl carbonate fuels to optimize the combustion process of Fischer–Tropsch synthetic diesel [36];
- (b)
- Optical studies on spray combustion characteristics for three different alternative fuels in combustion engines [37] and chemically sensitive diagnostics that are indispensable to unravel reactive processes in combustion [38];
- (c)
- The theoretical analysis of the displacement characteristics of moving components in a solenoid injector [39] and the combustion stability and flame development of ammonia/n-heptane dual fuel using multiple optical diagnostics and chemical kinetic analyses [40];
- (d)
- The evolution of turbulent boundary layers during flame–wall interaction investigated using highly resolved laser diagnostics [41] and the diagnosis of fuel volatility on the combustion characteristics of spray flame and wall-impinging flame [42];
- (e)
- The environmental qualities of diesel engines and their efficiency when a portion of their cylinders are deactivated in small-load modes [43] and structure-borne noise of marine diesel engines [44];
- (f)
- An online condition monitoring and maintenance strategy for cylinder liner-piston rings of diesel engines [45] and vibration analysis to estimate turbocharger speed fluctuation in diesel engines [46];
- (g)
- The design method of a typical engine fault identification model based on Simulink [47] and the prediction of toxic compound emissions in exhaust gases based on engine vibration and Bayesian optimized decision trees [48];
- (h)
- The fundamental diagnosis of diesel engines via displacement and velocity with Laser Doppler Vibrometry [49] and the acoustic emission characteristics of a single-cylinder diesel generator at various loads and with a failing injector [50];
- (i)
- An analytical approach to convert a vibration signal to combustion characteristics in diesel engines [51].
- Conclusion:
- The analysis of the set of scientific achievements in the field of diagnosing compression ignition engines clearly indicates the significance of the scientific issues discussed. There are various approaches to this type of problem. They are both in the context of theoretical, empirical, and model approaches, which make it possible to assess the operation of such objects, taking into account the area of their design and operation in specific conditions and for specific fuel. However, these achievements do not include the following aspects:
- (a)
- The empirical and mathematical assessment of the correctness of DME combustion with diesel fuel, taking into account estimates and characteristics of vibration processes;
- (b)
- Linking vibration characteristics with the assessment of emission parameters;
- (c)
- The assessment of the reliability of the impulsive and energy vibration parameters in terms of their vibration equivalents;
- (d)
- The assessment of the sensitivity and statistical reliability of each engine work cycle in relation to its reference representation for the particular fuel composition;
- (e)
- Mapping the kinetic representation of the combustion process in a mathematical model, taking into account engine work variables, fuel properties, and statistical significance of the data set (which is useful for modeling and testing with artificial intelligence methods—mapping continuous monitoring and process and design diagnostics in variable conditions, taking into account learning models for the early identification of irregularities).
The authors of this scientific article undertook empirical studies and constructed mathematical models for this research area, taking into account the above assessments. This study tried to bring closer the issues of assessing the kinetics of the combustion process for this type of thermodynamic energy conversion system and a specific DME composition with diesel fuel. The above composition was performed to meet the current and future requirements of emission regulations in the field of modern and early diagnosis of systems with high energy-conversion kinetics.
3. Experimental Set-Up
The entire research experiment was carried out in stationary conditions, characterized by constant engine operating conditions for its specific measurement point. The tests were carried out on an engine dynamometer to determine the specific thermodynamic stability of the object in order to minimize the impact of external factors on the measurement reliability. The structure of measurement systems for this type of experiment was selected in such a way that it suited the purpose and scope of the research as well as its specificity. The above specificity depended largely on the properties of dimethyl ether and the method of its preparation to constitute a specific physico-chemical composition for research on the combustion process in its kinetic phase. Dimethyl ether is an organic compound from the group of ethers, a colorless gas with a characteristic odor, which is moderately soluble in water.
As an alternative fuel, it can be used for liquid propane butane (LPG), liquid natural gas, gasoline, and diesel fuel. The subject of this research is the impact of its application to diesel fuel on specific operating characteristics and vibration processes. No need to break the C–C bond during ether oxidation results in its favorable oxidation to CO2. DME is suitable for powering compression ignition engines due to its high cetane number (above 55). The above chemical compound is also characterized by a 34% higher energy density (8.2 kWh/kg) compared to methanol, and during the oxidation reaction of its molecule, twice as many electrons are generated than during the combustion of methanol. Due to the differences in the physical and chemical properties of DME and diesel fuel, for example, regarding their states of matter, a special system was designed and built for research as part of the study, enabling the storage and transport of the tested fuel to a high-pressure pump. Thanks to this, differences in the compressibility of both fuel components and their phases were taken into account (Figure 1).
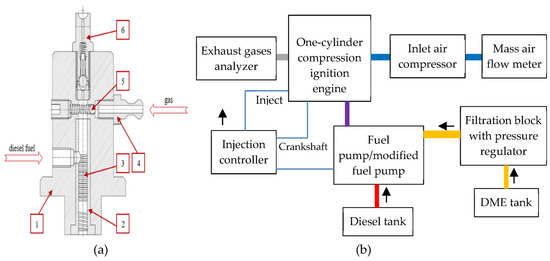
Figure 1.
The functional structure of a fuel pump (a) and a test stand (b) for DME combustion measurements: 1—body of the delivery section, 2—cylinder, 3—section labyrinth seal, 4—gas stub pipe, 5—one-way gas valve, 6—one-way outlet valve.
The fuel pump was driven by an electric motor and controlled by an inverter [1]. Bearing in mind that the value of a fuel temperature during its delivery to the engine may increase, appropriate corrections were made to ensure the stability of fuel injection parameters into the engine (in various operating conditions). The constant value of a diesel fuel pressure was maintained while supplying it to the pump (0.5 MPa) thanks to the use of a fuel pre-pump, while the gaseous fuel was fed directly to the system using conditioning (filtration and control of the dissolved gas share using a pressure reducer). During the entire test cycle, the concentrations of toxic exhaust gas components were also measured, constituting a secondary image of the quality of the fuel–air mixture formation and its combustion in the engine. The continuous concentration measurement was carried out for the following exhaust gas components: carbon monoxide (CO), hydrocarbons (HC), nitric oxide (NO), CO2, and O2 (obtained using the Axion R/S system). The measurement range and errors for each component were as follows: CO = 0–10% (±0.02%), HC = 0–4000 ppm (±8 ppm), NO = 0–4000 ppm (±15 ppm), CO2 = 0–16% (±0.3%), PM = 0–50 mg/m3 (±0.001 mg/m3), and O2 = 0–25% (±0.02%). The PM was measured using the process of a laser light scattering technique; the Non-Dispersive Infra Red method was used for CO, HC, and CO2; and the electrochemical method was utilized for NO and O2. Calibration was carried out before each test (using Axion R/S system software by LabVIEW, with reference gases).
The constant rotational speed and torque for a given engine operating point are important factors in determining the constant share of a dissolved gas concentration in diesel fuel, which, in this case, were maintained using an eddy current brake. The brake enabled torque measurement via a force transducer (vector moment of force). The fuel injection pressure was measured using a pressure sensor (the strain gauge inside sends an electrical signal to the controller and the electronic injection parameter measurement system). The fuel pump (Figure 1) was regulated in the ‘Filtration block with pressure regulator’ module.
The experiments were carried out on a single-cylinder compression ignition engine with direct fuel injection (into a toroidal combustion chamber in the piston) and a liquid cooling system. The geometric and operating parameters of the engine are shown in Table 1.

Table 1.
The tested engine specifications.
Measurements of vibration parameters (similarly as in [2]), as part of the author’s research, were carried out using a multichannel data acquisition system, Pulse, from Bruel&Kjær, which included the following aspects (Figure 2):
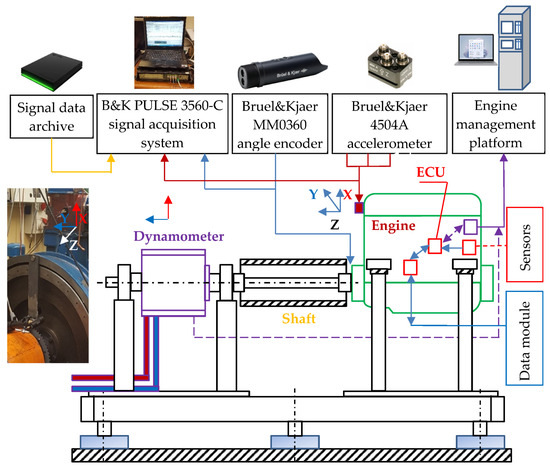
Figure 2.
Components of the test stand used for the vibration evaluation of DME combustion in different operating conditions (ECU—Electronic Control Unit).
- −
- The multichannel high-speed signal acquisition system: B&K Pulse type 3560C.
- −
- The advanced B&K Pulse software platform (version no. 17) for signal data acquisition and analysis (calibration was carried out before each test programmatically).
- −
- The three-axis vibration acceleration transducer: B&K type 4504A.
- −
- The laser encoder: B&K type MM0360 (synchronization of measurement signals with the specific position of an engine crankshaft).
The Bruel&Kjær Pulse 3560-C system for the dynamic and parallel acquisition of fast-changing and slow-changing signals is characterized by the following parameters [52]:
- −
- Number of parallel input/output channels: 5/1.
- −
- Number of auxiliary channels: 16.
- −
- Type of input channels: Direct, CCLD.
- −
- Frequency range [Hz]: 0–25,600.
- −
- Absolute amplitude precision (1 kHz, 1 V input): ±0.05 dB, typical ±0.01 dB.
- −
- Absolute maximum input [Vpeak]: ±35.
- −
- A/D conversion: 2 × 24 bits.
- −
- Voltage [V (DC)]: 10−32.
- −
- Nominal/max power consumption [W]: 30/42.
- −
- Dimensions: height/width/depth [m]: 0.105/0.376/0.300.
The Bruel&Kjær type 4504A vibration acceleration transducer is characterized by the properties described in Table 2 [53].

Table 2.
Properties of the Bruel&Kjær type 4504A vibration acceleration transducer [53].
The Bruel&Kjær type MM0360 laser angle encoder has the following features [54]:
- −
- Speed range [rpm]: 0–300,000.
- −
- Operating range: 1.5 (0.6) to > 70 cm (27″) and > 30° from the center line.
- −
- Laser spot: <Ø5 mm at a 70 cm distance.
- −
- Maximum continuous input voltage [V]: −5 to +30.
- −
- Laser class 3R. Visible 660–690 nm, CW, P [optical] < 2mW. Complies with EN/IEC 60825–1: 2007.
- −
- Operating temperature range [°C]: −10 to +50.
- −
- Input type CCLD (DeltaTron or ICP® inputs from 3 to 20 mA), U ≥ 20V.
4. Materials and Methods
4.1. Alternative Fuel Specifications and Their Attainment
The efficiency of thermodynamic processes carried out during kinetic combustion is strictly dependent on the type of fuel used and its preparation, as well as the shaping of the fuel injection process. The search for methods to increase the overall efficiency of the engine is reflected in studies on the area of composition of such fuel. They enable meeting current and future requirements in terms of the level of engine brake power (in a wide range of operating conditions) and the lowest possible emission values of harmful and toxic components, which are also competitive with the environmental burden of electric drives (energy consumption, emissions, toxicity problems of manufacturing and recycling battery cells, and costs of building and maintenance and modernization of the charging infrastructure). Therefore, the authors of the work focused on dimethyl ether as alternative fuel subjected to the combustion process in a single-cylinder compression ignition engine based on its physicochemical similarity to diesel fuel. The above properties of dimethyl ether are illustrated in Table 3.

Table 3.
Physicochemical properties of DME [55,56].
The above compound is obtained via dehydration of methanol or from a mixture of hydrogen and carbon monoxide [55,56]. The effect of dehydration of two methanol molecules is the formation of one DME molecule, which can be written as follows:
2CH3OH ⇄ CH3OCH3 + H2O,
DME synthesis is more efficient than the method described above because this compound can be obtained from fuels or biomass without the production of methanol. The production of dimethyl ether [55,56] from coal or biomass requires more unit processes than in the case of gases. Gasification is then necessary, thanks to which the synthesis gas is obtained. The efficiency of such a reaction is increased by, for example, pre-drying the biomass (necessary gas purification). If dimethyl ether is produced from methane, then synthesis gas is obtained as follows:
2CH4 + O2 + CO2 + heat → 3H2 + 3CO + H2O,
Acceleration of the DME oxidation can be achieved by using platinum (it facilitates breakage of the H–O bond in the ether molecule, which is active during dehydrogenation), but its disadvantage is the poisoning of the catalyst surface with products such as –CO [57]. This process can be avoided by using PtSn and PtRu alloys. Ru is mostly metallic, and Sn does not retain this state, being transformed into tin oxides and hydroxides. Platinum, on the other hand, acts as a catalyst while maintaining its metallic character [58,59]. The Pt poisoning effect is reduced by activating the dissociative adsorption of water (a larger amount of chemisorbed hydroxyl groups) [60]. The dynamics of DME oxidation are slower [61]; hence, Pd is added to PtRu and Pt [62,63,64].
DME limitations as fuel for engines:
- −
- It cannot be used on its own as a fuel for spark-ignition engines (due to its low self-ignition temperature, which increases the risk of knocking combustion);
- −
- Lower viscosity and lubricity than diesel fuel (possible mixture leaks, as well as faster fuel pump wear);
- −
- Chemical stability during storage, diffusion coefficient, and the risk of tank explosion during heating of DME pose a threat (hence the need to store it in liquid form, under moderate pressure, above 0.6 MPa);
- −
- Application requires modification of vehicle power supply systems and creation of a distribution system;
- −
- The calorific value of DME is lower than the calorific value of diesel fuel (larger tanks for DME).
The above barriers have been eliminated today through appropriate design changes and inclusion of appropriate additives refining the above fuel. Thanks to this, taking into account the numerous advantages of this chemical compound, the benefits of its use as an energy source in engines contribute to further research on this scientific problem.
4.2. Methods and Conditions of Empirical Studies
The active-passive experiment was chosen to evaluate rapidly changing thermodynamic processes in a mechanical system for converting energy obtained from various fuel compositions. The tested technical object operated in stationary conditions, in which a constant value of the engine speed was maintained equal to 900 rpm. For the above engine speed, the torque was changed every 10 N∙m in the scope of 0–50 N∙m (Table 4). The fuel injection pressure was kept constant at 40 MPa. The fuel injection time to the engine was 0.1 ms, and the fuel injection angle was 2.8125 degrees, which is related to dividing the engine operating cycle length of 720 degrees with the resolution value of 256 bits (depending on the encoder placement at the camshaft).

Table 4.
Engine operating field defined by the research program [3].
The experiment included three research series. The first one concerned tests using a conventional fuel injection system with a Bosch CP3 pump. The next two series included the use of a high-pressure injection system equipped with an original injection pump design ensuring proper gas dilution. The external controller was responsible for precise fuel pressure regulation, thanks to information from the pressure sensor in the common rail tank. The effect of this action was to make it possible to influence the electronic fuel valve dosing and the operation of the pressure regulator in the tank. The constant value of a fuel injection pressure was established for each of the research series, amounting to 40 MPa, thanks to which the stability of the system was achieved. The above equilibrium state was one of the basic conditions necessary for a reliable assessment of the effect of the gas dissolution process in a specific fuel. As a consequence, the appropriate conclusions regarding the functional relations between the correctness of this composition and the effects it brings in the form of obtaining specific changes in engine performance indicators, emission parameters, and point estimates of impulse and energy vibration processes (generated in the engine during combustion and the propagation of these interactions on the structure of the tested object) were obtained. The fuel injection time was similar for both conventional fuel (diesel fuel) and DME obtained for different supply pressures (Table 5).

Table 5.
Obtained values and differences of a fuel injection time for reference fuel (diesel fuel) and DME for different engine operating points and supply pressure [3].
Approximation errors were minimized by accurately reproducing the research conditions and accurate formulas. The permissible total error of measurement of the real value (including stochastics) was set at ±2.5%. The authors also isolated the external factors’ influence (engine dynamometer) on the creation of omission errors. The design of the measuring station allowed for the isolation of external sources of vibration process generation; hence, the measurements concerned only the observed source processes. The measurement process was repeated three times, preceded by calibration of the measurement paths, for both the emission and vibration measurement systems.
5. Results and Discussion
5.1. Assessment of Fuel Type Impact on Exhaust Compound Concentrations
As part of the research experiment, DME was used as an additive to diesel fuel, which is related to its advantages. The value of tangential forces increases when precision elements cooperate because the above chemical compound has low lubricity and viscosity, which increase friction losses and wear of the surface layer within the friction surfaces (injection system, piston–crank system). DME dissolves well in diesel fuel, so it is appropriate to use it as a solution that will be released at the right time and burned together with diesel fuel. This type of solution was used by the authors of this article, who observed the effects of the use of fuel compositions with DME with a specific share on operational parameters, emission indicators, and generated vibration processes (the assessment of the thermodynamic quality of combustion reflected in the multidimensional characteristics of the vibration process). The coexistence of a close relationship between the main process and its vibration representation may be the basis for inferring the course of tribological processes with the search for sources of the greatest importance in the generation of tangential forces affecting friction losses. This will make it possible to forecast tribological and diagnostic changes in real time during the period of facility operation, as well as the early detection of symptoms of process and design failures.
In the first stage of the research, considered in detail by the authors in earlier work [62], the identification and quantitative assessment of the effect of the given engine operating parameters (e.g., torque, fuel pressure in the fuel supply system) and fuel type on the obtained concentrations of exhaust toxic components—HC (hydrocarbon), PM (particulate matter), CO (carbon oxide), NOx (nitrogen oxides)—were of great importance (Table 6, Table 7, Table 8 and Table 9). Thanks to these studies, the existence of a mathematical relationship between the change of operating and fuel supply parameters, the composition of fuels (selected by the authors), and the concentration of the above toxic components was confirmed. The observed improvement in the quality of creating a combustible mixture and its combustion for these fuels verified the validity of dimethyl ether (DME) as an additive to diesel fuel, which can meet restrictive emission standards at present and in the future.

Table 6.
The relative concentration of hydrocarbons (HCs) in exhaust gases at different torques and supply pressures for DME fuel application [3].

Table 7.
The relative concentration of particulate matter (PM) in exhaust gases at different torques and supply pressures for DME fuel application (x1, x2—value for DME at 0.2 MPa and 0.4 MPa) [3].

Table 8.
The relative concentration of carbon oxide (CO) in exhaust gases at different torques and supply pressures for DME fuel application (x1, x2—value for DME at 0.2 MPa and 0.4 MPa) [3].

Table 9.
The relative concentration of nitrogen oxides (NOx) in exhaust gases at different torques and supply pressures for DME fuel application (x1, x2—value for DME at 0.2 MPa and 0.4 MPa) [3].
According to the data presented in Table 6, Table 7, Table 8 and Table 9, it was observed that the use of DME as an alternative fuel (in an appropriate composition with diesel fuel) achieved the following results:
- −
- It drastically reduced the HC concentration in exhaust gases, with a positive effect on the quality of the combustion and the efficiency of the entire fuel dose utilization in individual engine operation cycles (Table 6). This is related to the DME properties: a favorable O2/C/H2 in %mass (DME = 34.8/52.2/13; diesel = 0/86/14); a lower Molar mass/liquid density in [g/mol]/[kg/m3] (DME = 46.07/667; diesel = 170/831), a better C/H ratio (DME/diesel = 0.337/0.516). The HC concentration was only 1.7–4.1% of the value obtained for diesel fuel in relation to the pressure of 0.2 MPa and 1.0–3.9% of the concentration for the pressure of 0.4 MPa. The increase in load had a clear effect on the HC concentration reduction (regardless of the supply pressure value). A slight HC concentration reduction in the range of 0.2–0.9% (depending on the operating point) was observed when the pressure increased twice. However, this benefit may also constitute an added value to the total emission in the case of taking into account the entire set of vehicles in a given area and their traffic intensity (global effects expected currently and in the future from power sources, taking into account the criteria of maximizing the overall efficiency and minimizing environmental impact).
- −
- It significantly reduced the PM concentration (Table 7), which is more effective the higher the torque and supply pressure are. The amount of PM is 40.5–63.5% of the value obtained for diesel fuel (for a pressure of 0.2 MPa) and 33.8–78.4% of the reference value (for a pressure of 0.4 MPa). The above benefit results from the improvement in combustion conditions and reduction in the average droplet diameter during the work cycle, which affects their better evaporation and increases the share of fuel burned completely (fulfillment of the international postulate in PM emission reduction). The favorable chemical structure of DME, related to the oxygen share (34.8 %mass), lower carbon share by 17.2%, and improvement in the C/H ratio by 34.6%, results in a lower share of both the insoluble fraction (derived from C) and the soluble PM fraction (from the liquid form of HC). A twofold increase in the supply pressure reduces concentrations in the range of 2.6–52.6%. There was no beneficial effect only for the torque of 0 N∙m for the pressure of 0.2 MPa (too low of a supply pressure; lower efficiency of the diesel fuel oxidation process), which was not confirmed in the remaining operating conditions.
- −
- It reduced the CO concentration (Table 8), and the increase in torque resulted in a globally mild CO reduction for both supply pressures (an improvement in combustion stoichiometry conditions and efficiency of fuel conversion into mechanical work). A doubling of the supply pressure had a clear effect on reducing this concentration for higher torques (from 40 N∙m), and for lower values, it was ambiguous or small (0.7% for 0 N∙m).
- −
- It increased the NOx concentration (Table 9), and the more intense it was, the higher the torque value (which is consistent with the combustion theory). The improvement in combustion efficiency is accompanied by an unfavorable emission effect, which intensifies with the supply pressure increase (0.9–7.9%). It can be limited by appropriate corrections in the scope of fuel injection and its combustion, as well as by using DeNOx exhaust gas cleaning systems. The source of higher NOx emission during DME combustion is the higher O2 content, which improves the formation of a homogeneous mixture structure and faster fuel evaporation. This results in improved combustion conditions, visible in the form of higher pressure and temperature in the combustion chamber. The low autoignition temperature and better evaporation of DME favor this phenomenon. A higher cetane number is an additional measure in this area.
- −
- Precise mathematical equations were developed for individual HC, PM, CO, and NOx concentrations in relation to the engine torque, fuel type, and various supply pressures described in more detail in [64], for which the R2 indices were in the range of 0.88–0.96 (for HC), 0.80–0.98 (for PM), 0.95–0.99 (for CO) and 0.97–0.99 (for NOx).
The fundamental problem of assessing cause–effect relationships between a dynamic source process (of a given nature) and the nature of its generation and propagation is to assess not only whether the above relation exists, but also its determinism and strength and correlation uniqueness for object diagnostics. Following positive expectations in this area, the next stage concerns searching for quantitative changes and parametric representations, thanks to which detailed analyses can be performed and the obtained results and relationships can be applied in practice. The authors of the above work demonstrated in [3] that there is a close relationship between the changes that occur in the process of kinetic combustion of diesel fuel and DME and their corresponding representations in vibration acceleration signals. However, in that scientific work, the assessment was limited only to the assessment of the peak value and root mean square—RMS—omitting other vibration parameters. Therefore, in the above work, the analyses were extended to mean and peak-to-peak estimates to assess the rapidly changing thermodynamic process in a mechanical system for converting energy obtained from various fuel compositions. The diagnostic reliability study of these two point measures of the vibration process was carried out here, taking into account the time domain and the process values. The expected effect was to obtain mathematical dependencies of a deterministic nature in relation to the critical variables of the engine operation parameter vector (the object operation space), fuel structure vector (the fuel type and composition, the conversion efficiency, and emission effects), and injection system supply parameters vector (mixture formation conditions, the injection kinetics efficiency, and combustion quality). The authors of the paper chose the X axis for further evaluation in this article (from three directions of vibration signal recording) because it represented the direction of piston movement in the cylinder. The above choice was related to the greatest sensitivity of this recording plane to the kinetic combustion and the effects it had on the object structure (the unidirectionality, the signal strength, and the repeatability of energy mappings).
5.2. Evaluation of Fuel Type Impact on Vibration Acceleration Characteristics
The time histories of the vibration acceleration signals for each recording direction were subjected to a time selection process, choosing fragments that represented specific engine operating cycles separately for conventional fuel and for the diesel fuel+DME composition with specific supply pressure. In the next stage, fragments that represented the kinetic combustion in a vibration form were defined and further specified with respect to the beginning and end of the thermodynamic source process. Example results of time selection and their partial representation for the combustion are presented in Figure 3 and Figure 4.
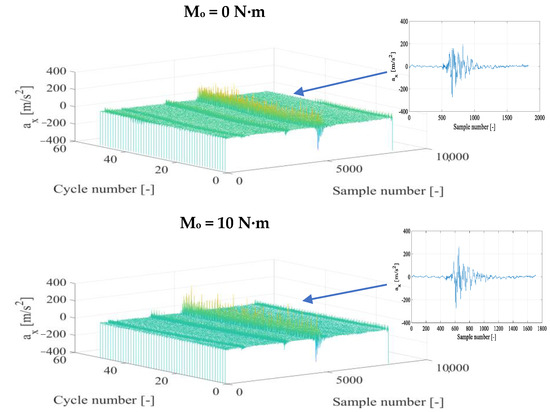
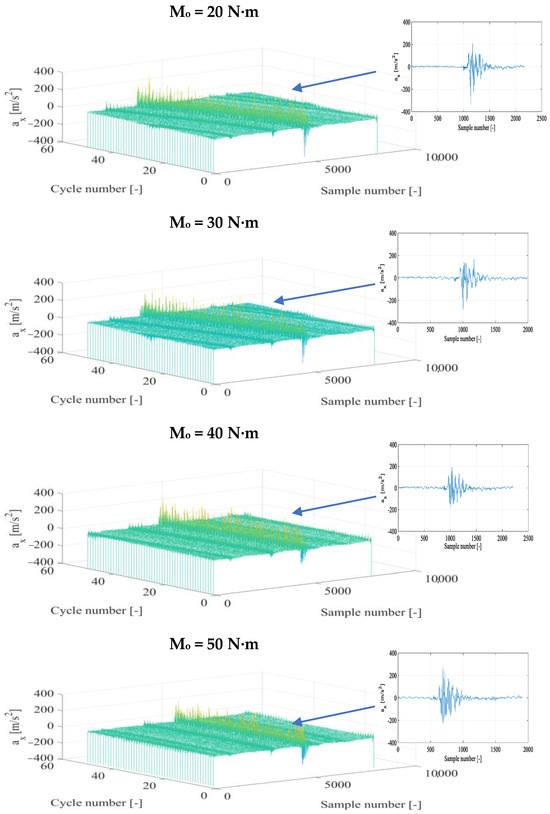
Figure 3.
Fragments of vibration accelerations in the X direction (ax) after time selection and the corresponding representation of the diesel fuel combustion for n = 950 rpm and different torques.
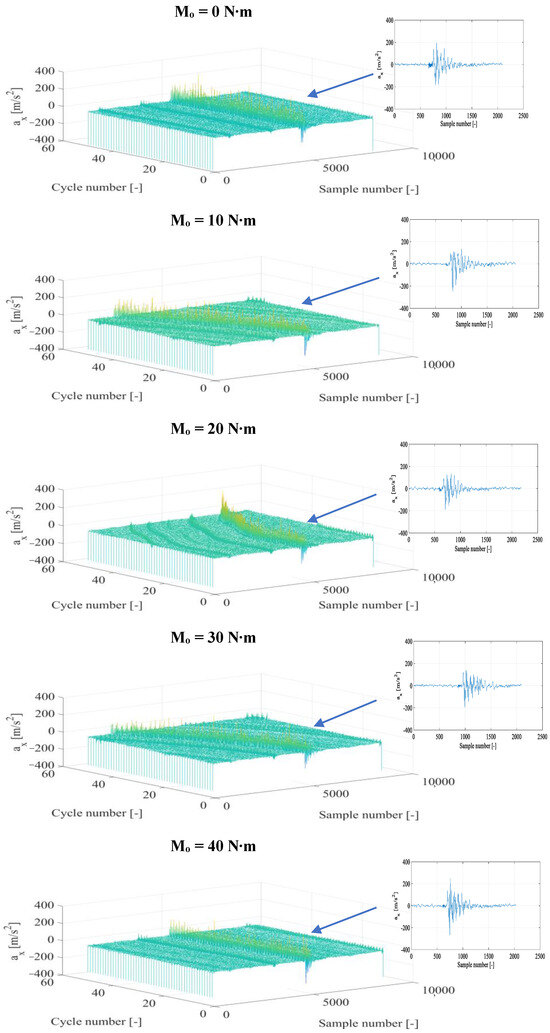
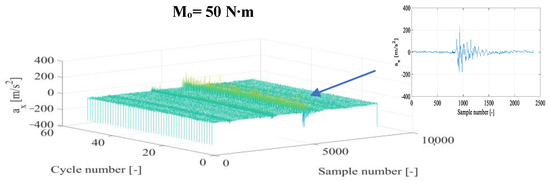
Figure 4.
Fragments of vibration accelerations in the X direction (ax) after time selection and the corresponding representation of the diesel+DME fuel combustion for n = 950 rpm and different torques.
The sample number (Figure 3 and Figure 4) denotes the number of the i-th discrete value obtained in the measurement process, representing a physical quantity that is in a direct (or indirect) functional relationship with changes occurring in the evaluated source process. The cycle number denotes the number of a given fragment of the engine’s operating cycle recorded in a specified measurement period (in a specific time course of subsequent engine operating cycles).
According to Figure 3 (a parallel graphic record of fragments representing single engine cycles for diesel fuel combustion), fragments of the combustion can be identified clearly. The nature of the above representations is similar both in particular cycles and for different torques. However, the higher its value, the higher the amplitude of vibration accelerations and the amount of energy subject to conversion from fuel into brake power. A single combustion in the vibration representation confirms the above relationship. The time selection indicates a certain amplitude variability in individual combustion cycles, which is related to the nature of this process in a high-speed piston engine. Therefore, an averaging operation and an analysis of the standard deviation are required in the final phase of the analysis of each individual fragment from the combustion. According to the three-dimensional graphics, a constant moment of combustion onset is observed in most engine load conditions, which confirms the duration stability of the initial diffusion phase of the combustion, in which the hydrocarbon bonds of diesel fuel—necessary to facilitate the kinetic combustion—are weakened.
The application of DME as an additive to diesel fuel (Figure 4) affects the preservation of the nature of the combustion in a specific vibration signal representation. However, changes in the amplitude of vibration accelerations and the energy amount of the vibration process can already be seen at this stage, which suggests that it is possible to obtain other levels of vibration amplitude (in relation to those obtained for diesel fuel). The increase in torque influenced the rise in the vibration amplitude and the degree of energy conversion from fuel burned in the cylinder working space into its useful value obtained in the appropriate vibration representation. The consistency of the moment of the kinetic combustion onset was preserved for Mo = 0, 40, and 50 N∙m and its angular parallel shift for Mo = 10 and 30 N∙m and for Mo = 20 N∙m. Parallelism was noted for half of the cycles with a subsequent delay in the onset moment of the combustion. Each of the combustion cycles in these latter two cases requires detailed time selection to obtain a representative combustion fragment in each of the engine cycles.
5.3. Assessment of Fuel Type Impact on Vibration Diagnostic Parameters
The assessment in the amplitude domain was carried out using point measures of the vibration process. In the presented paper, the average value of vibration accelerations and peak-to-peak vibration accelerations in the X direction (the direction of piston movement in the cylinder as the most sensitive to the combustion component) were used. Both measures were determined for each individual time fragment of vibration accelerations (representing the combustion). By obtaining a matrix of measures for a specific engine operating point and the direction of signal recording and fuel type, averaging was applied within a given measure, operating point, and fuel characteristics. The previously described amplitude variability was determined by calculating the standard deviation. The above STD value is also helpful in analyzing the signal stability (impulse and energy) of the thermodynamic process itself for the particular fuel composition. Figure 5 presents the effect of fuel type on the efficiency of the combustion expressed in the averaged mean value and peak-to-peak of the vibration process (depending on the engine torque).
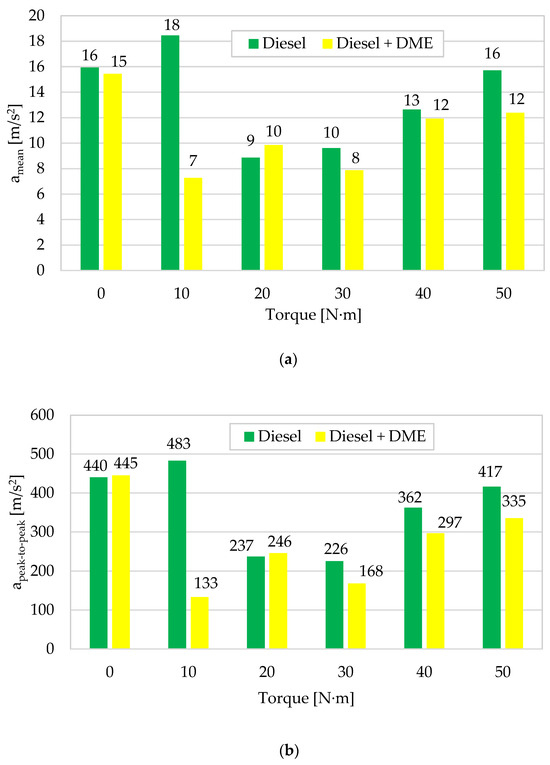
Figure 5.
The influence of the fuel type on the efficiency of the combustion (for a supply pressure of 0.2 MPa, n = 950 rpm) reflected in the point estimates of the vibration process recorded in the X direction (for a time domain) for different engine torques given in the averaged: (a) mean (amean); (b) peak-to-peak value (apeak-to-peak).
According to the data in Figure 5, the coexistence of the functional relationship between the emission data of harmful and toxic exhaust components (Table 6, Table 7, Table 8 and Table 9) and the mean and peak-to-peak values of the vibration process representing the combustion in its individual cycles is visible. This allows for the joint design of equations in diagnostic algorithms that take into account emission and vibration monitoring. These procedures are related to the correctness of thermodynamic processes and their impact on the energy efficiency of their kinetics and amplitude impact on the structure of the object (tribological monitoring of particular components and structural elements to predict the first symptoms of a failure). The use of DME as a fuel component causes a favorable reduction in the mean amplitude of the vibration process (compared to that obtained for diesel fuel), which is visible in the entire range of engine loads (except for Mo = 20 N∙m). The above data confirm a more stable combustion with a milder angular increase in the indicated pressure for the DME composition, which consequently also means a lower energy load on individual kinematic pairs and structural elements. Beneficial effects for the wide range of object operating conditions indicate their applicability in traction operation and the possibility of using them for engines with different applications.
The analysis of the peak-to-peak value for the conditions in Figure 5 also confirms the belief that these favorable energetic changes can also coexist with a favorable course of the maximum vibration amplitudes. The noticeable changes in this vibration estimate appeared when the engine torque increased. This indicates that the uniqueness of the emission-vibration functional relationships is also present in their maximum amplitude exposures to processes with high dynamics of changes. DME clearly reduces the peak-to-peak amplitude at each of the engine operating points with a specific load (no effect was noted only for Mo = 0 N∙m and 20 N∙m by ∆ = 1.1–3.5%). Lower amplitudes for each of the combustion cycles and their averaged values reduce the dynamic loads generated on particular elements of the object’s structure and increase its operational durability.
5.4. Estimation of Vibration Diagnostic Parameter Reliability
The quantitative dimensions of the differences in amplitude measures for both fuels are presented in Figure 6, which confirms the statistical significance and determinism of the data obtained from the tests and its lasting beneficial effect on the engine and its operation when using the tested alternative fuel.
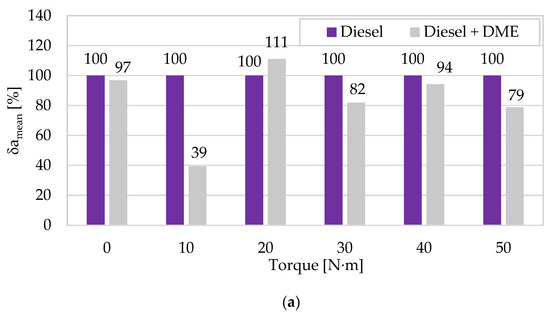
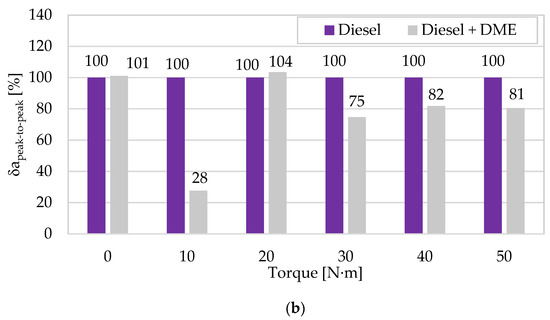
Figure 6.
The relative change of the averaged mean (a) and peak-to-peak (b) vibration accelerations in the X direction due to the use of DME as an alternative fuel (reference fuel: diesel fuel) for different engine torques (n = 950 rpm).
In the next phase of analyses, the authors of a paper tried to show how diagnostically good the presented amplitudes of vibration accelerations (average and peak-to-peak) for both fuels and the change in engine torque are related to those previously described in [62] for the RMS and peak amplitudes of vibration accelerations. As a result, appropriate characteristics describing them were built (categorizing energy and impulse estimates), referring each of them to the values obtained for diesel fuel (Figure 7). In the case of the mean and RMS values, a beneficial effect of using DME as a fuel to reduce vibration accelerations is observed for most engine operating points (in relation to the amplitudes obtained for diesel fuel), and lower values were noted for RMS (10–40 N∙m). In the case of the peak and peak-to-peak values, the differences between both measures are insignificant, and their beneficial effect was obtained for most measurement points (except for 0 N∙m and 20 N∙m), which means that they are equivalent and relevant for application in the diagnostic analyses of impulse processes. The increase in the engine torque resulted in higher values of both vibration amplitude point measures, which is related to the increase in the intensity of the combustion and the degree of conversion of the fuel’s chemical energy into work, and, consequently, the intensity of the vibration processes that are their sources.
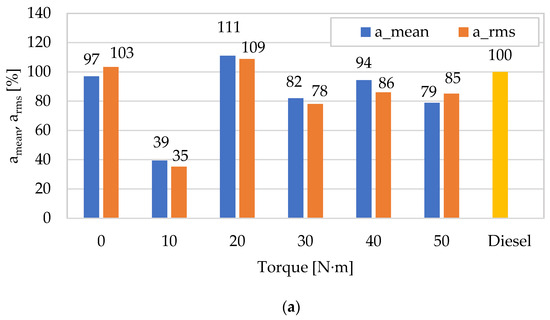
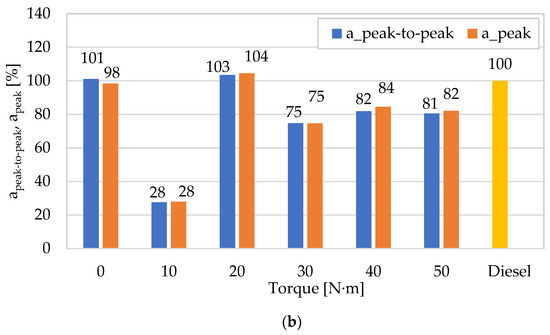
Figure 7.
Comparison of the dynamics of changes in the averaged mean value (amean) and peak-to-peak (apeak-to-peak) of vibration accelerations in the X direction (for n = 950 rpm) relative to the RMS (arms) and peak (apeak) values as a result of using DME as an alternative fuel (reference fuel: diesel fuel): (a) mean value versus RMS value; (b) peak-to-peak value versus peak value.
The next step in verifying the correctness of the DME application as an alternative fuel (made by the authors of this paper), which can be used in modern thermodynamic and mechanical systems based on fuel energy conversion into brake power (in their current and future applications), was to indicate the extent to which DME combustion affects the parametric stability of individual engine work cycles. In order to meet these requirements, it was necessary to determine the standard deviation. As a result, the dispersion of the obtained real values in individual combustion processes was determined in relation to their average value. The above data stability determines the degree of repeatability of the assessed thermodynamic process (from the point of view of its moment of generation and energy propagation) and, consequently, the stability of the efficiency of energy transformations and obtaining an approximately constant value of the mean effective pressure in individual cycles of the engine operation. For this purpose, the authors determined the relative change in the standard deviation δSTD, expressed as the difference in the STD values obtained for diesel fuel and DME fuel (relative to the values for diesel fuel), obtained by a specific point measure at a given engine operating point (Figure 8). Positive values in the figure indicate a decrease in the standard deviation value for DME fuel, which was, respectively (depending on the torque value, in the range of 20–50 N∙m): 8.8–49.1% (for the mean value), 17.7–41.2% (for the RMS value), 27.8–39.5% (for the peak value), and 28.8–39.5% (for the peak-to-peak value). In the case of no load and its low value, greater irregularity was noted for DME fuel, which was related to the less favorable thermodynamic conditions inside the combustion chamber (different physicochemical structures of both fuels and conditions of dissolution in the reference fuel).
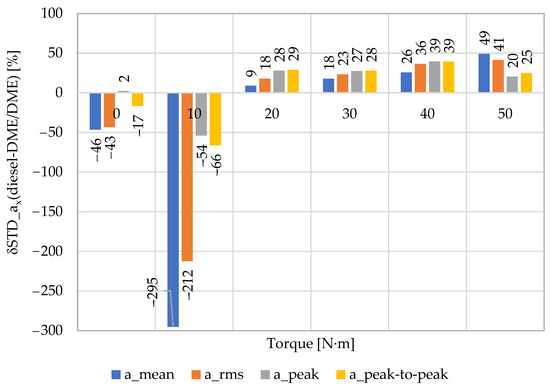
Figure 8.
Comparison of the STD changes in the averaged mean value (amean) and peak-to-peak (apeak-to-peak) of vibration accelerations in the X direction (for n = 950 rpm) relative to the RMS (arms) and peak (apeak) values as a result of using DME as an alternative fuel (reference fuel: diesel fuel).
The above STD reduction for the fuel composition with DME indicates the beneficial effect of this component on the mild run of the combustion, the impact of which is even greater for the increase in the engine load. Therefore, the short-term increase in dp/dt is smaller (also beneficial due to the intensity of NOx generation), thanks to which the levels of mechanical loads on the object structure and its individual kinematic pairs are reduced. As a consequence, this beneficial phenomenon increases the operational durability of the technical object in terms of mechanical and thermal loads. The above process improves the total ecological balance: demand for a specific energy source, exhaust emissions, and noise (lower acoustic signal amplitudes due to mild time characteristics of indicated pressure in individual engine cycles) in terms of the demand for specific design materials. Improvement in the active object safety in the conditions of the real operation, resulting from a smaller number of specific component failures (process and design), is an additional advantage of the DME as a fuel component. It constitutes, at the same time, a real alternative to meet future ecological, durability, and safety requirements for the engine in a vehicle, especially in conditions of high variability of traffic flows in urban agglomerations.
In order to meet the contemporary requirements for monitoring the operation of vehicles and machines in real time, it is advisable to supplement the above graphical and parametric characteristics with an accurate mathematical representation of the relationships between relevant variables. Their inclusion and indication of the accuracy of these relationships clearly indicate the reliability of empirical data and their possibility of application in specific real conditions. Thanks to them, it is also possible to implement the process of further improvement in the object model or its fragment in the scope of the adopted goals, with additional consideration of its further empirical verification. This is justified by the specific algorithmization of the observation of dynamic processes occurring in the engine and its optimization, especially in those cases when models based on artificial intelligence methods are used, for which the value of input data and the accuracy of the AI model have a special impact.
The sources of the above reductions of vibration acceleration amplitudes are both thermodynamic and physical causes. For the first group of phenomena that have a positive effect on the reduction in vibration energy, they share the following facts:
- −
- Faster fuel evaporation from the cylinders, as well as a shorter combustion time of the DME mixture with air.
- −
- High oxygen content in the molecule, which affects the lowering of the self-ignition temperature of the compound (faster combustion of the DME mixture with air).
- −
- A low boiling point supports faster mixing of air with fuel, which causes a significant acceleration of ignition (due to the lack of direct carbon bonds).
- −
- Even the course of combustion development, resulting from a slower increase in dp/dt in the kinetic phase of the process, is indirectly related to the high cetane number.
- −
- More intensive oxidation is related to the reduction in combustion enthalpy to a level lower than in the case of diesel fuel.
- −
- Bonds in DME disintegrate much faster, which results in a shorter ignition delay.
The above thermodynamic reasons generate less vibration energy (as a result of less expansive transfer of energy from combustion to its vibrational form), reflected in the form of forces acting on the object’s structure (resulting from the angular increase in combustion pressure in the entire volume of the combustion chamber). A milder dp/dt reduces impulse and energy increases in vibration amplitudes, and its even course in individual cycles affects the smaller amplitude fluctuation in subsequent cycles (amplitude stability and loads on the engine’s structure).
5.5. Vibration Diagnostic Parameters in Mathematical Relations
The presented graphical representations of the dependence between selected amplitude point estimates of the vibration process and the engine operating conditions, defined by the change of torque, are reflected in the mathematical equations describing them:
- (a)
- For the mean value of the vibration acceleration and diesel fuel:
y = 0.579x2 − 2.8546x + 12.109 (R2 = 0.99),
RMS value was [64]: y = 0.0607x2 − 0.042x + 0.9148 (R2 = 0.925),
- (b)
- For the mean value of the vibration acceleration and diesel fuel+DME:
y = 0.1275x2 + 0.208x + 6.733 (R2 = 0.722),
RMS value was [64]: y = 0.1222x2 − 0.4492x + 1.3273 (R2 = 0.753)
- (c)
- For the peak-to-peak value of the vibration acceleration and diesel fuel:
y = 16.495x2 − 81.039x + 320.5 (R2 = 0.899),
Peak value was [64]: y = 9.4642x2 − 12.405x + 128.75 (R2 = 0.908)
- (d)
- The peak-to-peak value of the vibration acceleration and diesel fuel+DME:
y = 4.1873x2 + 11.975x + 112.66 (R2 = 0.728),
Peak value was [64]: y = 16.315x2 − 60.381x + 173.24 (R2 = 0.709)
The above equations constitute the basis for creating predictive diagnostic models. They aim at continuous monitoring of the correctness of the process of creating and burning a combustible mixture using different fuels (including their different compositions) in engines that operate under variable load conditions. While the emission equations of a specific exhaust component are particularly important from the point of view of the real determination of the environmental impact by a given energy conversion system implemented in vehicles moving in urban agglomerations in real traffic conditions (with the additional function of eliminating sources of excessive exhaust emissions), the assessment of vibration processes is all the more important. It is based on the early detection of potential negative process and design changes that may affect the overall efficiency of the system and the generation and development of malfunctions. The latter affects the active and passive safety of system users and their environment. The usefulness of both categories of mathematical equations (obtained by the authors of this paper) allows for their further development, taking into account further variables, and the application of results to specific engineering solutions within classical diagnostics and those using artificial intelligence (AI) methods. Particularly in this respect, the potential of this issue is noticed, the application of which can be extended to the entire drive system, including systems based on the generation and accumulation of electrical energy (with an appropriate modification of models to their requirements).
6. Conclusions
This paper presents the issue of assessing the variability of kinetic combustion carried out in a mechanical system for converting fuel energy with a specific composition. The subject of the analyses was the efficiency of the combustion of diesel fuel and an alternative fuel with a specific share of dimethyl ether (in stationary conditions), taking into account changes in the engine load. The measures of the above variability assessment were empirical signals obtained from parallel data acquisition of the research object, while their basis was the expected functional relationship between the rapidly changing thermodynamic process (the formation and combustion of the mixture) and the vibration phenomenon reflecting the above observed process. The above relationship was confirmed based on the properties of the vibration acceleration signal (from a specific point on the engine) and amplitude point measures of the vibration process, which were the average value and peak-to-peak (qualification as a diagnostic parameter). The assessment of the reliability of their information value on process changes was obtained by presenting the results in the time and amplitude domains, also taking into account the variability in engine cycles.
In the first stage, the beneficial effect and purposefulness of using DME as an alternative fuel (added to the reference fuel) were proven thanks to the author’s measurement results of harmful and toxic exhaust components. According to Table 6, Table 7, Table 8 and Table 9, the use of DME caused a significant reduction in the specific negative exhaust components, and their concentrations were only as follows (relative to the value for diesel fuel):
- (a)
- For HC: 1.7–4.1% (pinj = 0.2 MPa) and 1.0–3.9% (for pinj = 0.4 MPa)—Table 6.
- (b)
- For PM: 40.5–63.5% (pinj = 0.2 MPa) and 33.8–78.4% (for pinj = 0.4 MPa)—Table 7.
- (c)
- For CO: 80.5–97.5% (pinj = 0.2 MPa) and 82.8–98.4% (pinj = 0.4 MPa)—Table 8.
- (d)
- For NOx: 101.2–108.1% (pinj = 0.2 MPa) and 103.9–110.6% (pinj = 0.4 MPa)—Table 9.
According to Figure 3 and Figure 4, in the first stage of vibration analyses (in the time domain), it was proven that there is a close relationship between the combustion and its vibration representation mapped in the vibration acceleration signal. The above effect was a close correlation of the vibration amplitude and energy variability in the set of analyzed combustion cycles. In these runs, the possibility of identifying the combustion of a given fuel type was also noted. As a result, it is also possible to assign parametric characteristics dependent on this variable. Their quantitative values were obtained in analyses in the amplitude domain, where the reliabilities of using both vibration parameters in assessing the combustion and their cyclic amplitude and energy fluctuations were determined (Figure 5 and Figure 6). In the case of the mean value of vibration accelerations, the DME application resulted in a reduction in the vibration amplitude in the range of 5.7–60.6%, and for the peak-to-peak estimate, this range was 18.2–72.4%, respectively (depending on the operating point). In both cases, the benefits resulting from the reduction in exhaust emissions and from the vibration amplitude indicate the possibility of their further application and use for the purpose of diagnosing the engine, improving the energy and emission balance of the thermodynamic system.
The effectiveness of both vibration parameters was realized by referring them to parameters equivalent to them in terms of their nature. The mean value was referred to RMS, and the peak-to-peak value was compared to the peak value (Figure 7) while supplementing the data with the value of the measures obtained for the reference fuel. Both measures representing the combustion of fuel with the DME component were lower than for the reference fuel, and the differences between both measures were dependent on the engine operating conditions, amounting to 3.8–8.2% (the mean value in relation to RMS) and 0.1–2.8% (the peak-to-peak value in relation to the peak value). This indicates the reliability of both measures and their equivalence in relation to the RMS and peak value. In the last stage of the analyses, it was proven that the DME has a beneficial effect on reducing the standard deviation for the tested vibration measures. This indicates that the amplitude and energy stability of the combustion process in individual engine cycles is obtained.
As a result, the repeatability of each work cycle for both vibration measures is proved, which intensifies when the engine load increases. Thanks to this, appropriate equations were determined for the dependence of vibration parameters on the engine load, both for diesel fuel and the composition with dimethyl ether, obtaining the parameter R2, respectively:
- (a)
- For the mean value: 0.99 (diesel fuel) and 0.722 (diesel+DME).
- (b)
- For the peak-to-peak value: 0.899 (diesel fuel) and 0.728 (diesel+DME).
- (c)
- For the RMS: 0.925 (diesel fuel) and 0.753 (diesel+DME).
- (d)
- For the peak value: 0.908 (diesel fuel) and 0.709 (diesel+DME).
The obtained mathematical relationships in the developed emission and vibration models allow for reliable analyses of the creation of an fuel–air mixture and kinetic combustion carried out in engines with high variability of thermodynamic processes. The reliability of the obtained analyses was confirmed in various engine operating conditions and for various fuels, determining variable combustion characteristics. Comparative analyses of data from the categories of vibration measures verified their suitability for a specific research task, and their relative differences indicated their real diagnostic value.
Thanks to this, it is possible to further extend the analyses with dimensionless parameters, as well as analyses in the frequency and time-frequency domains. Additionally, the obtained data constitute graphical representations, discrete data, and mathematical equations that can be used to optimize classical models and build AI models, as well as to test them (in the context of monitoring and diagnosing processes and detecting structural failures in their early generation phase).
Author Contributions
Conceptualization, M.W., M.B. and W.K.; methodology, M.W., M.B. and W.K.; validation, M.W., M.B. and R.S.; formal analysis, M.W.; investigation, M.W., M.B., W.K. and R.S.; data curation, M.W.; writing—original draft preparation, M.W.; writing—review and editing, W.K.; visualization, M.W. and J.P.; supervision, M.W.; project administration, M.W. and M.B.; funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Polish Ministry of Education and Science fund for Statutory Activities of the Institute of Powertrains and Aviation, PUT (PL) 0415/SBAD/0342.
Data Availability Statement
The original contributions presented in the study are included in the scientific paper; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bajerlein, M.; Karpiuk, W.; Smolec, R. Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design. Energies 2022, 15, 9163. [Google Scholar] [CrossRef]
- Waligórski, M.; Batura, K.; Kucal, K.; Merkisz, J. Empirical assessment of thermodynamic processes of a turbojet engine in the process values field using vibration parameters. Measurement 2020, 158, 107702. [Google Scholar] [CrossRef]
- Waligórski, M.; Bajerlein, M.; Karpiuk, W.; Smolec, R. Influence of dimethyl ether combustion in the compression-ignition engine on the peak and effective measures of the vibroacoustic process and toxic compounds emission. Adv. Sci. Technol. Res. J. 2024, 18, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Lijewski, P.; Waligórski, M. Exhaust Emissions from a Hybrid City Bus Fuelled by Conventional and Oxygenated Fuel. Energies 2022, 15, 1123. [Google Scholar] [CrossRef]
- Kamińska, M.; Urbański, P. Assessment of the environmental costs of Rail Buses in Poland based on research in real operating conditions. Adv. Sci. Technol. Res. J. 2024, 18, 215–228. [Google Scholar] [CrossRef]
- Yu, C.; Guo, L.; Sun, W.; Zhang, H.; Cheng, P.; Yan, Y.; Zhu, G.; Jiang, M.; Guo, Y.; Yue, F. Experimental and chemical kinetic study on effects of H2-DME fusion addition on laminar premixed flame speed and flame instability for ammonia composite combustion. Energy 2024, 310, 133175. [Google Scholar] [CrossRef]
- Diao, S.; Li, H.; Yu, M. Atomic insights into the combustion mechanism of DME/NH3 mixtures: A combined ReaxFF-MD and DFT study. Int. J. Hydrogen Energy 2024, 80, 743–753. [Google Scholar] [CrossRef]
- Hou, J.; Xi, S.; Wang, Z.; Li, S. Effects of biodiesel ratio and nozzle diameter on combustion and emissions of a biodiesel–DME-fueled engine. Trans. Can. Soc. Mech. Eng. 2023, 47, 308–316. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Zhu, W.; Long, W.; Bi, M. Experimental and simulated study of ammonia blending with DME steam reformed and cracked gases under lean-burn condition. Int. J. Hydrogen Energy 2024, 94, 464–473. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H. An experimental study on the flame instability of NH3/DME blends with H2 addition. Int. J. HydrogenEnergy 2024, 68, 813–822. [Google Scholar] [CrossRef]
- Lu, M.; Wan, K.; Zhu, X.; He, Y.; Zhu, Y.; Yuan, Y.; Cai, Q.; Gao, Z.; Jiang, C. Investigation of swirl premixed dimethyl ether/methane flame stability and combustion characteristics in an industrial gas turbine combustor. Energy 2024, 310, 133255. [Google Scholar] [CrossRef]
- Yu, M.; Luo, G.; Sun, R.; Wang, L.; Li, X.; Yao, H. Experimental and numerical study on NOx reduction from NH3/DME/air flame using fuel staging method. Fuel 2024, 372, 132100. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhao, P.; Jia, M.; Chang, Y.; Du, L. Exploring energy utilization of the methanol/dimethyl ether dual-fuel engine under the methanol reforming strategy: A comparison of different low-temperature combustion modes. Energy 2024, 312, 133659. [Google Scholar] [CrossRef]
- Shere, A.; Subramanian, K.A. Effects of hydrogen and EGR on energy efficiency improvement with ultra low emissions in a common rail direct injection compression ignition engine fueled with dimethyl ether (DME) under HCCI mode. Int. J. Hydrogen Energy 2024, 52, 1447–1474. [Google Scholar] [CrossRef]
- Chai, M.; Chen, Z.; Nourozieh, H.; Yang, M.; Xu, J.; Sun, Z.; Li, Z. Perspectives of dimethyl ether (DME) as a transitional solvent for enhanced oil recovery (EOR). Energy 2024, 310, 133191. [Google Scholar] [CrossRef]
- Shi, X.; Li, W.; Zhang, J.; Fang, Q.; Zhang, Y.; Xi, Z.; Li, Y. Exploration of NH3 and NH3/DME laminar flame propagation in O2/CO2 atmosphere: Insights into NH3/CO2 interactions. Combust. Flame 2024, 260, 113245. [Google Scholar] [CrossRef]
- Velamati, R.K.; Mohammad, A.; Eckart, S.; Veetil, J.E. Ignition and cool flame interactions of DME/H2/air blends in a micro-channel with a wall temperature gradient. Int. J. Thermofluids 2024, 24, 100891. [Google Scholar] [CrossRef]
- Xinyu Dong, X.; Fang, Z.; Tang, X.; Qiao, X.; Li, X.; Zhou, D.; Yang, K.; Wang, L. Thermodynamic and exergoeconomic analysis of a dual expansion and triple recuperation supercritical CO2 power cycle driven by DME-oxygen combustor. Energy Convers. Manag. 2024, 303, 118173. [Google Scholar] [CrossRef]
- Zhou, G.; Kong, Y.; Yu, J.; Zhang, Q.; Li, R.; Wang, D.; Fan, T.; Cui, Y.; Li, Z. Experimental study on the flame-dual field overpressure coupling evolution characteristics of LPG/DME blended gas explosion venting. J. Clean. Prod. 2024, 444, 141220. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, F.; Cao, S.; Ma, Y.; Niu, Y.; Zhao, D. Experimental study of emission characteristics for fully premixed combustion of dimethyl ether and hydrogen based on flame ions. J. Energy Inst. 2024, 114, 101654. [Google Scholar] [CrossRef]
- Li, N.; Kong, W.; Chen, Y.; Zhang, B.; Sui, C. Chemical kinetics analysis of ammonia/dimethyl ether combustion under water addition conditions. J. Energy Inst. 2024, 113, 101549. [Google Scholar] [CrossRef]
- Peinado, C.; Liuzzi, D.; Sluijter, S.N.; Skorikova, G.; Boon, J.; Guffanti, S.; Groppi, G.; Rojas, S. Review and perspective: Next generation DME synthesis technologies for the energy transition. Chem. Eng. J. 2024, 479, 147494. [Google Scholar] [CrossRef]
- Zhou, G.; Kong, Y.; Zhang, Q.; Li, R.; Qian, X.; Zhao, H.; Ding, J.; Li, Y.; Yang, S.; Liu, Y. Effect of dimensionless vent ratio on the flame-shock wave evolution dynamics of blended LPG/DME gas explosion venting. Fuel 2024, 358, 130205. [Google Scholar] [CrossRef]
- Kim, D.J.; Oh, S.Y.; Yoo, C.S.; Park, J.; Chung, S.H. Non-monotonic liftoff height behaviors in laminar nonpremixed coflow jet flames of DME with ambient temperature variation. Proc. Combust. Inst. 2024, 40, 105210. [Google Scholar] [CrossRef]
- Su, X.; Su, R.; Gao, N.; Chen, H.; Ji, Z.; Xu, H.; Wang, B. Investigation on combustion and emission characteristics of diesel polyoxymethylene dimethyl ethers blend fuels with exhaust gas recirculation and double injection strategy. J. Traffic Transp. Eng. (Engl. Ed.) 2024, 11, 614–630. [Google Scholar] [CrossRef]
- Li, H.; Zhao, M.; Xiao, H. Optimization of theoretical pressure prediction model for confined explosion of low-carbon fuels. Int. J. Hydrogen Energy 2024, 64, 381–388. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, Z.; Liu, Z.; Zhang, Q.; Zhang, Y. Study on the effect of pre-blended section ratio and hydrogen blended ratio on explosion dynamics of dimethyl ether-hydrogen blended gas. Combust. Flame 2024, 267, 113600. [Google Scholar] [CrossRef]
- Ergen, G. Comprehensive analysis of the effects of alternative fuels on diesel engine performance combustion and exhaust emissions: Role of biodiesel, diethyl ether, and EGR. Therm. Sci. Eng. Prog. 2024, 47, 102307. [Google Scholar] [CrossRef]
- Zander, L.; Kather, Y.; Vinkeloe, J.; Djordjevic, N. Influence of steam dilution on ignition delay time of dimethyl ether/air mixtures at high pressure measured behind reflected shock waves. Combust. Flame 2024, 259, 113191. [Google Scholar] [CrossRef]
- Huang, W.; Wu, H.; Sun, W.; Hong, C.; Tian, Z.; Yan, Y.; Huang, Z.; Zhang, Y. Probing the Pre-Ignition Behavior of Negative Temperature Coefficient Fuels at Low to High Temperatures: A Case Study of Dimethyl Ether. Energies 2023, 16, 2118. [Google Scholar] [CrossRef]
- Fabiś, P.; Flekiewicz, M. The Influence of LPG and DME Mixtures on Passenger Car Performance. Energies 2022, 15, 7144. [Google Scholar] [CrossRef]
- Youn, I.; Jeon, J. Combustion Performance and Low NOx Emissions of a Dimethyl Ether Compression-Ignition Engine at High Injection Pressure and High Exhaust Gas Recirculation Rate. Energies 2022, 15, 1912. [Google Scholar] [CrossRef]
- Yang, S.; Lee, C. Experimental Research on the Injection Rate of DME and Diesel Fuel in Common Rail Injection System by Using Bosch and Zeuch Methods. Energies 2018, 11, 273. [Google Scholar] [CrossRef]
- Yoon, S.H.; Han, S.C.; Lee, C.S. Effects of High EGR Rate on Dimethyl Ether (DME) Combustion and Pollutant Emission Characteristics in a Direct Injection Diesel Engine. Energies 2013, 6, 5157–5167. [Google Scholar] [CrossRef]
- Wen, M.; Liu, H.; Cui, Y.; Ming, Z.; Feng, L.; Yao, M. Optical diagnostics of methanol active-thermal atmosphere combustion in compression ignition engine. Fuel 2023, 332, 126036. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, G.; Guo, L.; Zhang, H.; Yan, Y.; Lin, S.; Zeng, W.; Zhang, X.; Jiang, M.; Yu, C. Optical diagnostic study of internal and external EGR combined with oxygenated fuels of n-butanol, PODE3 and DMC to optimize the combustion process of FT synthetic diesel. Fuel 2024, 355, 129390. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Xue, J.; Chen, M.; Dai, L.; Yin, Z.; Kang, Y. Optical test devices and methods for internal combustion engines and optical studies on spray combustion characteristics for three different alternative fuels: A review. J. Energy Inst. 2024, 117, 101845. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K.; Ferris, A.M.; Zetterberg, J.; Lacoste, D.A.; Fjodorow, P.; Wagner, S.; Cai, L.; Rudolph, C.; Zádor, J.; Li, Y.; et al. Chapter 14—Chemistry diagnostics for monitoring. In Developments in Physical & Theoretical Chemistry, Combustion Chemistry and the Carbon Neutral Future; Brezinsky, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 417–501. ISBN 9780323992138. [Google Scholar] [CrossRef]
- Vrublevskyi, O.; Wierzbicki, S. Measurement and theoretical analysis of the displacement characteristics of moving components in a solenoid injector in view of wave phenomena. Measurement 2022, 187, 110323. [Google Scholar] [CrossRef]
- Wen, M.; Liu, H.; Cui, Y.; Ming, Z.; Feng, L.; Wang, G.; Yao, M. Study on combustion stability and flame development of ammonia/n-heptane dual fuel using multiple optical diagnostics and chemical kinetic analyses. J. Clean. Prod. 2023, 428, 139412. [Google Scholar] [CrossRef]
- Zentgraf, F.; Johe, P.; Nicolas, A.; Barlow, R.S.; Böhm, B.; Peterson, B.; Dreizler, A. On the evolution of turbulent boundary layers during flame–wall interaction investigated by highly resolved laser diagnostics. Combust. Flame 2024, 261, 113276. [Google Scholar] [CrossRef]
- Ming, Z.; Liu, H.; Cui, Y.; Wen, M.; Zhang, X.; Yao, M. Optical diagnosis study of fuel volatility on combustion characteristics of spray flame and wall-impinging flame. Fuel Process. Technol. 2023, 250, 107880. [Google Scholar] [CrossRef]
- Gritsenko, A.V.; Glemba, K.V.; Petelin, A.A. A study of the environmental qualities of diesel engines and their efficiency when a portion of their cylinders are deactivated in small-load modes. J. King Saud Univ.—Eng. Sci. 2021, 33, 70–79. [Google Scholar] [CrossRef]
- Fragasso, J.; Moro, L. Structure-borne noise of marine diesel engines: Dynamic characterization of resilient mounts. Ocean Eng. 2022, 261, 112116. [Google Scholar] [CrossRef]
- Rao, X.; Sheng, C.; Guo, Z.; Yuan, C. A review of online condition monitoring and maintenance strategy for cylinder liner-piston rings of diesel engines. Mech. Syst. Signal Process. 2022, 165, 108385. [Google Scholar] [CrossRef]
- Chiavola, O.; Palmieri, F.; Recco, R. Vibration analysis to estimate turbocharger speed fluctuation in diesel engines. Energy Procedia 2018, 148, 876–883. [Google Scholar] [CrossRef]
- Bo, X.; Cheng, Z.; RuiJie, H.; Zhinong, J.; Zhilong, G. The construction method of typical engine fault identification model based on Simulink. IFAC-Pap. 2024, 58, 19–24. [Google Scholar] [CrossRef]
- Bortnowski, P.; Matla, J.; Sierzputowski, G.; Włostowski, R.; Wróbel, R. Prediction of toxic compounds emissions in exhaust gases based on engine vibration and Bayesian optimized decision trees. Measurement 2024, 235, 115018. [Google Scholar] [CrossRef]
- Sitnik, L.; Pentoś, K.; Magdziak-Tokłowicz, M.; Wrobel, R. The Laser Doppler Vibrometry in mechatronics diagnostics. Arch. Civ. Mech. Eng. 2015, 15, 962–970. [Google Scholar] [CrossRef]
- Dykas, B.; Harris, J. Acoustic emission characteristics of a single cylinder diesel generator at various loads and with a failing injector. Mech. Syst. Signal Process. 2017, 93, 397–414. [Google Scholar] [CrossRef]
- Hunicz, J.; Gęca, M.S.; Ratajczyk, E.; Andwari, A.M.; Yang, L.; Mikulski, M. An analytical approach to converting vibration signal to combustion characteristics of homogeneous charge compression ignition engines. Energy Convers. Manag. 2023, 294, 117564. [Google Scholar] [CrossRef]
- Available online: https://www.bksv.com/media/doc/bu0228.pdf (accessed on 5 November 2024).
- Available online: https://www.bksv.com/en/transducers/vibration/accelerometers/ccld-iepe/4504-a (accessed on 5 November 2024).
- Available online: https://www.bksv.com/media/doc/bp2288.pdf (accessed on 5 November 2024).
- Available online: https://www.acs.org/molecule-of-the-week/archive/d/dimethyl-ether.html (accessed on 15 December 2024).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dimethyl-Ether#section=GC-MS (accessed on 15 December 2024).
- Lasch, K.; Hayn, G.; Jörissen, L.; Garche, J.; Besenhardt, O. Mixed conducting catalyst support materials for the direct methanol fuel cell. J. Power Sources 2022, 105, 305–310. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Pieta, I.S.; Rutkowska, I.A.; Wadas, A.; Marks, D.; Klak, K.; Stobinski, L.; Cox, J.A. Electrocatalytic oxidation of small organic molecules in acid medium: Enhancement of activity of noble metal nanoparticles and their alloys by supporting or modifying them with metal oxides. Electrochim. Acta 2013, 110, 474–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, L.; Sun, G.; Sun, S.; Liu, J.; Tang, S.; Li, H.; Zhou, B.; Xin, Q. Structure and chemical composition of supported Pt–Sn electrocatalysts for ethanol oxidation. Electrochim. Acta 2005, 50, 5384–5389. [Google Scholar] [CrossRef]
- Herron, J.A.; Ferrin, P.; Mavrikakis, M. First-Principles Mechanistic Analysis of Dimethyl Ether Electro-Oxidation on Monometallic Single-Crystal Surfaces. J. Phys. Chem. C 2014, 118, 24199–24211. [Google Scholar] [CrossRef]
- Rutkowska, I.A.; Rytelewska, B.; Kulesza, P.J. Enhancement of oxidation of dimethyl ether through formation of hybrid electrocatalysts composed of Vulcan-supported PtSn decorated with Ru-black or PtRu nanoparticles. Electrochim. Acta 2021, 400, 139437. [Google Scholar] [CrossRef]
- Grinberg, V.A.; Maiorova, N.A.; Pasynskii, A.A.; Emets, V.V.; Shiryaev, A.A.; Vysotskii, V.V.; Gerasimov, V.K.; Matveev, V.V.; Nizhnikovskii, E.A.; Andreev, V.N. Nanostructured catalysts for direct electrooxidation of dimethyl ether based on Bi- and trimetallic Pt–Ru and Pt–Ru–Pd alloys prepared from coordination compounds. Russ. J. Coord. Chem. 2017, 43, 206–212. [Google Scholar] [CrossRef]
- Yudanov, I.V.; Matveev, A.V.; Neyman, K.M.; Roösch, N. How the C–O Bond Breaks During Methanol Decomposition on Nanocrystallites of Palladium Catalysts. J. Am. Chem. Soc. 2008, 130, 9342–9352. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Tong, Y.; Osawa, M.; Ye, S. Electrochemical and infrared study of electro-oxidation of dimethyl ether (DME) on platinum polycrystalline electrode in acid solutions. Electrochim. Acta 2008, 53, 6093–6103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).