Abstract
Various waste streams including municipal solid waste (MSW), polymer waste from personal protective equipment (PPE) used in medical fields, and composite waste from wind turbine blades (WTBs) demand modern waste management and recycling approaches. Ultimate and proximate analysis of mentioned samples revealed a higher content of carbon—28.2 ± 8.0, 80.1 ± 2.3, and 50.3 ± 2.3, respectively—exhibiting sufficient potential to be converted into secondary carbon-based compounds. For this purpose, oxidative liquefaction of selected waste materials was carried out following a detailed experimental plan, a centred composite design for WTBs, and a central composite face-centred plan for MSW and PPEs. Temperature, pressure, oxidant concentration, reaction time, and waste-to-liquid ratio were the parameters of key interest, and their values were tested at a range of 200–350 °C, 20–40 bar, 15–60%, 30–90 min, and 3–25%, respectively, depending upon the type of waste. As a result, total polymer degradation (TPD) was recorded for three types of waste and the results were satisfactory, encouraging the decomposition of primary waste in liquid oxygenated chemical compounds (OCCs). Gas Chromatography with Flame Ionisation Detection (GC-FID) helped us quantify the number of OCCs for each waste sample. Energy consumption during the process was also recorded and optimisation of the experimental plan based on maximum TPD and OCCs yields against the minimum energy consumption was performed to make the process tech-economic.
1. Introduction
The issue of rapid increases in various waste streams is a significant challenge in both industrial and societal contexts. The countries within the European Union, comprising a population of around 400 million people, produce a significant amount of waste. Between the years 1995 and 2019 [1], there was a notable rise in the annual growth of municipal solid waste (MSW) generation per individual in the European Union (EU) from 198 million metric tonnes (equivalent to 467 kg per capita) to 224 million metric tonnes (equivalent to 502 kg per capita), representing an increase of over 30% [2]. The escalation in demand for goods across all sectors has resulted in an increase in waste generation. The available data indicate a positive relationship between waste generation and household income [3,4]. An elevated level of household income frequently results in heightened consumption patterns, thereby leading to a greater generation of waste. Furthermore, the progressions in technology and industrial procedures have also yielded an escalation in waste production, as novel items and materials swiftly become outmoded or old, hence engendering a greater turnover of waste.
According to an estimation in 2018, around 29.1 million metric tons of plastic waste was collected in Europe alone, out of which 32.5% underwent recycling, 42.6% underwent energy recovery, while the remaining 7.2 million metric tons was deposited in landfills (24.9%) [5]. Germany, France, Italy, Spain, and Poland were the top five nations in the EU in 2020 for plastic waste production, producing 11 million metric tonnes of plastic waste, of which 38% was recycled [4]. One of the main causes of the higher rates of accumulation of plastic waste in the environment over the past two years has been the COVID-19 waste produced by the usage of personal protective equipment (PPE). According to the data [6] presented, 8.4 ± 1.4 million tons of pandemic-associated plastic waste has been generated and unfortunately, 25.9 ± 3.8 thousand tons has been dumped into the world’s oceans [7]. Current renewable energy regulations require Europe to obtain 32% of its energy from renewable sources by 2030 [8]. Wind, solar, and biomass are all renewable energy sources, but wind energy is most rapidly increasing, with an estimated 170 GW of electricity generation in 2017 [9]. The European Commission (2016) expects wind turbines to generate 25% of Europe’s power by 2050.
The average lifespan of a wind turbine is 20 years [10], and various models age almost at the same rate. The first batch of European onshore wind turbines has been reaching its End of Life for 10 years. Also, four offshore wind projects were dismantled [11].
The typical wind turbine requires 10 kg of blade material for 1 kW of generating capacity [10]. Wind turbine blades are typically glass or carbon composites and global waste WTB production is expected to reach 225,000 metric tons by 2034, with 100,000 metric tons in Europe [12]. So, at this moment the three major waste streams, i.e., MSW, plastic waste from PPE, and composite waste, are a major threat to the environment and are generating demand for modern waste management strategies.
Despite the implementation of many alternative waste disposal methods and a significant reduction of 50% in landfilling practices over the past 25 years, it is noteworthy that a substantial proportion of 20% of the waste generated in the EU continues to be disposed of through conventional means. The management of solid waste is a significant concern. Although various methodologies may possess distinct potentials, certain methods exhibit equivalent environmental impacts to that of landfilling. One significant issue pertains to the variation in legal remedies inside the EU. Most of the EU countries regard landfilling as the most detrimental method of waste disposal, with many nations even prohibiting its practice. Nonetheless, a notable absence of uniformity persists within the EU in relation to the implementation of waste management methodologies. For example, many nations place a high priority on the implementation of recycling programs and the establishment of waste-to-energy facilities, whilst others primarily depend on the practice of landfilling. The divergence in methodologies poses a barrier to the advancement of a more environmentally sustainable resolution for the management of solid waste in the area. The implementation of a comprehensive strategy and enhanced adherence to regulatory measures pertaining to waste management are crucial in mitigating the ecological consequences associated with the disposal of solid waste within the EU.
The management of waste should adhere to a waste hierarchy, which establishes a prioritised order of waste treatment options aimed at minimising the environmental consequences of waste. This hierarchy emphasises waste avoidance, reuse, recycling, energy or chemical recovery, and, as a last resort, disposal through landfilling. The “3Rs” (reduce, reuse, recycle) are widely implemented on a global scale [13,14]. The optimal practices are waste reduction, waste preparation for reuse, waste recycling, and waste disposal. The EU has achieved notable strides in mitigating the environmental consequences associated with solid waste disposal by adherence to the waste hierarchy and implementation of the 3Rs (reduce, reuse, recycle). Efforts have been made to implement waste avoidance and reduction techniques with the aim of promoting a generation of less waste through enterprises and individuals. The practice of reusing and recycling waste products serves to both preserve resources and diminish the necessity for landfilling and incineration. Nevertheless, in situations where disposal becomes imperative, it is important to adhere to appropriate waste management protocols to mitigate the adverse consequences on the environment.
Many unrecyclable wastes, especially multilateral plastic composites, wind up in landfills and the ocean, posing environmental concerns. Therefore, plastic waste chemical recycling through oxidative liquefaction might be a challenge with the best results, both environmental and economic. The oxidative liquefaction method involves the hydrothermal treatment of composite waste in the presence of water, together with the introduction of an oxidising agent such as hydrogen peroxide (H2O2) at moderate concentrations. So far, the research has been aimed at experimentally investigating the Wind Turbine Blades (WTBs), PPE, and MSW polymer matrix degradation during the oxidative liquefaction process in a batch reactor at variable temperatures, processing times, waste-to-liquid ratios, and oxidiser concentrations to measure total solid reduction, resin and polymer degradation, glass fibre yield, and quality. This study employs a blend of statistical methodologies and empirical observations to ascertain the most favourable parameters for the oxidative liquefaction procedure. By studying the multidimensional data, the researchers can determine the main characteristics that significantly affect the yield of the intended product fractions. This insight can then be used to optimise the process and produce the greatest possible results in terms of product quality and quantity. This article explores the steps required to identify the necessary conditions for processing non-recyclable waste using an innovative oxidative liquefaction technique. The aim is to achieve optimal outcomes and obtain useful products that can be reintegrated into the original production processes, aligning with the principles of the circular economy.
2. Materials and Methods
2.1. Materials
The End-of-Life Wind Turbine Blade (WTB) parts were supplied by ANMET, a business that has specialised in WTB recycling since 2015. The initial stipulation for the samples used in this investigation was segments of 25 × 30 cm. The initial step involved dividing the samples into smaller segments measuring 0.5 cm in width using an angle grinder. Subsequently, these segments were further processed into fine chips measuring 0.5–1.5 cm in length using a shredder mill. The acquired WTB samples primarily comprised glass fibre and polymer matrix architectures, with no significant presence of metal, wooden, or foam-filling components.
To prevent the transmission of viruses among the individuals involved in material handling, surplus personal protective equipment (PPE) was acquired from a nearby medical establishment. The personal protective equipment (PPE) comprised disposable face masks and gloves, N-95 face masks, protective gowns, and bedsheets (in the equilibrium relationship) that were manually segmented into fragments of 1–2 cm using scissors.
The municipal solid waste (MSW) analysed in this study was a diverse and intricate mixture of waste components, usually found in urban and suburban areas of Poland. The selected MSW type, consisting of a wide variety of materials including metals, glass, polymers, organic matter, and other components, was subjected to the detailed sorting process before experimental investigation. It was crucial to carefully choose representative MSW samples to ensure that the study’s findings may be targeted to real-world waste streams for assessment of waste-to-resource conversion systems and their possible economic and environmental advantages. The test samples were provided by the proficiency testing providers, which prepared the samples according to the ISO standards for this type of material.
2.2. Elemental Analysis of the Waste Sample
The LECO TruSpec CHN and SC 632 analyser were employed for the purpose of conducting the final analysis, namely, to ascertain the levels of elemental carbon, oxygen, hydrogen, nitrogen, and sulphur. The sample’s total moisture, volatile matter, and ash levels were measured using a weighing method in accordance with the CEN/TS 15414-2:2010 [15], EN 15403:2011 [16], and EN 15402:2011 [17] standards, respectively. The experimental protocol adhered to established standards and protocols, which involved the use of high-temperature combustion coupled with infrared detection [18].
2.3. Design of Experiments
The investigation of the oxidative liquefaction of certain multicomponent wastes was carried out by an experimental study using a central composite face-centred plan (CCF) and Statistica Software (v. 13.3.0) [19]. The variable denoted as n in the context of the CCF pertains to the quantity of distinct permutations of factor levels that will be subjected to experimentation. The design incorporates a combination of factorial points, axial points, and centre points. The factorial points yield insights into the primary impacts and interactions of the factors, whilst the axial points offer insights into the curvature of the response surface. The utilisation of centre points serves the purpose of estimating the variance of error and assessing the presence of a lack of fit. A total of five independent variables were evaluated, including temperature, pressure, residence duration, waste-to-liquid ratio, and oxidant concentrations.
The experimental matrix for the chosen multicomponent waste was designed using two different approaches: central composition design for five variables, each with three levels, and central composition design for three variables, also with three levels, presented in Table 1. This pertained to the categorisation of multilateral waste and its corresponding physical and chemical characteristics. Certain materials, like as WTB, have notably superior physical and chemical robustness in comparison to PPE or MSW. This study encompassed the modification of process conditions for the treatment of PPE and MSW, along with the elimination of statistically insignificant variables, such as pressure, during the analysis of the test results from WTB processing. Additionally, variables like residence time were optimised, leading to a reduction in the number of required experiments for subsequent waste treatment.

Table 1.
Process parameter levels compared to coded values using a central composite face-centred plan.
In the context of WTB, a comprehensive analysis and optimisation of the process, including five variables, namely oxidative liquefaction process temperature, residence duration, pressure, oxidant amount, and waste-to-liquid ratio, necessitated the execution of 29 tests. The experimental investigation involved a total of 17 tests conducted on less durable waste materials, specifically PPE and MSW. The tests were conducted to examine the effects of three variables, namely the temperature of the oxidative liquefaction process, the amount of oxidant used, and the waste-to-liquid solution ratio.
2.4. Oxidative Liquefaction
In the present study on oxidative liquefaction processes, the Parr reactor series 4650 was applied. A detailed description of the Parr reactor in the [20] experimental configuration employed in the process of oxidative liquefaction of specific waste materials has been provided in the earlier study [20]. The Parr reactor series 4650, manufactured by Parr Instruments, located in Illinois, USA, was employed in the research they were conducting. The reactor has a total volume of 500 mL and is equipped with a high-temperature spiral for heating purposes. A Parr 4838 reactor controller, with an operational range of 0 to 800 °C, makes it easier to control reactor temperature. This controller possesses a readout and setpoint resolution of 1 °C, thereby enabling precise temperature adjustments. Furthermore, the system accuracy of this controller is ±2 °C, ensuring reliable and consistent temperature control within the reactor. The pressure gauge installed on the reactor head has the capability to accurately monitor pressure within a wide range of 350 bar. There are two straight valves that can be utilised, with one of them serving as a mechanism to link the reactor to a pressure source. This connection enables the reactor to be filled with an inert gas, such as nitrogen. The second valve is utilised for the purpose of either extracting flue gases or gathering valuable gases subsequent to the aforementioned operation. The reactor head consists of an aperture that allows for entry to the thermowell, which contains a J-type thermocouple for the purpose of monitoring the temperature of the reactor over the course of the operation. The regulation of temperature during the operation is accomplished through the utilisation of a Parr controller, which employs Proportional Integral Derivatives (PIDs), each assigned with a distinct temperature value.
2.5. Total Polymer Degradation
To assess the extent of degradation of organic matrices during the process, the calculation of total polymer degradation (TPD) was performed and can be expressed using Equation (1). In the given context, the variable mf denotes the mass of the solid product after the process of drying, whereas mi indicates the beginning mass of the waste material being tested [21].
To enable the utilisation of the aforementioned formula, it is vital to initially conduct filtration of the entire mixture subsequent to the completion of the reaction. This filtration process involves the employment of filter paper to effectively segregate the liquid and solid constituents. The solid sample is thereafter exposed to a drying process in a laboratory dryer for a duration of 8 h at a temperature of 105 °C. Following the drying process, the sample is then re-weighed. After the filtration procedure, the liquid products are enclosed within a hermetically sealed container and then sent for further analysis.
2.6. Oxygenated Chemical Compounds Concentration
The liquid products derived by oxidative liquefaction are subjected to analysis using Gas Chromatography with Flame Ionisation Detection (GC-FID). This research employed DB-FAT WAX UI capillary columns within a Clarus 500 gas chromatograph equipped with a flame ionisation detector. Helium was utilised as the carrier gas. The temperature protocol for the furnace commenced at an initial value of 40 °C, lasting for a period of 4 min. Subsequently, the temperature increased gradually at a rate of 5 °C per minute until reaching a final value of 240 °C. Subsequently, the temperature was consistently maintained for a duration of 15 min. Calibration curves were established to facilitate quantitative measurement of specific oxygenated chemical compounds (OCCs).
3. Results
3.1. Comparison of the Elemental Characterisation of the Studied Waste
The composition of the multicomponent waste materials that were investigated, including WTBs, PPEs, and MSW, is detailed in Table 2. Through a comparative analysis of the final and near-final analytical findings, it becomes evident that there are significant alterations in the elemental composition of WTBs, PPEs, and MSW. The PPEs exhibit a moisture content of 0.1%, an ash content of 8.7%, a significantly high volatile matter content of 97.0%, a carbon content of 80.1%, a hydrogen content of 12.8%, a nitrogen content of 0.17%, a chlorine level of 0.04%, and a sulphur content below 0.03%. The current composition exhibits predominantly organic and combustible properties, suggesting the potential for energy harnessing and the production of chemically enhanced molecules with added value. In contrast, the samples of WTBs exhibit a relatively elevated moisture content of 1.3%, a significantly augmented ash content of 57.9%, a volatile matter content of 40.7%, a carbon content of 28.2%, a hydrogen content of 2.7%, a nitrogen content of 1.2%, an oxygen content of 8.2%, a sulphur content below 0.5%, and a chlorine concentration below 0.01%. The increased amounts of oxygen seen in WTBs can be attributed to the presence of epoxy resins, whereas the greatest ash content among the studied samples can be attributed to the inclusion of the glass fibre filler. The samples of municipal solid waste (MSW) exhibit a moisture content of 2.5%, a significant ash content of 15.1%, a volatile matter content of 84.9%, a carbon content of 50.3%, a hydrogen content of 7.2%, a nitrogen content of 1.1%, an oxygen content of 23.3%, a sulphur content below 0.5%, and a chlorine concentration of 0.03%. The high quantities of oxygen detected in municipal solid waste (MSW) can be attributed to the presence of a diverse range of materials, including both polymeric substances and those of natural origin, such as paper and biomass.

Table 2.
Comparison of characteristic physical and chemical parameters of studied waste.
The potential impact of various elemental compositions present in WTBs, PPEs, and MSW on their capacity to generate oxygenated chemical compounds (OCCs) and degrade polymers under oxidative liquefaction warrants consideration. Understanding these differences is crucial for tailoring and improving oxidative liquefaction systems to achieve efficient resource recovery and waste management.
3.2. Comparison of the Total Polymer Degradation in the Studied Waste
The combined data from 29 experiments carried out for WTB and 17 trials, each conducted for PPE and MSW, revealed that the polymer degradation range under the studied conditions varied from 21.1% to 49% (Figure 1). WTB exhibited the lowest total polymer degradation (TPD) value compared to the other two examined waste materials, PPE and MSW, due to the chemical resistance of its resins. It is evident that the TPD exhibited a highly consistent direction of change, while the variations in the results are attributed to the specific kind and chemical makeup of the examined polymer waste. The TPD values for PPE and MSW were greater compared to those for WTB, reaching 50% and 52%, respectively. These values were reached under the same process parameters as WTB, which included a temperature of 250 °C, oxidant level of 15%, and waste-to-liquid ratio of 25%. The experiment, conducted under significantly different process conditions, specifically at a temperature of 350 °C for WTB and 300 °C for PPE and MSW, with oxidant levels of 45% for WTB and 60% for PPE and MSW, respectively, and with waste-to-liquid mass ratios of 5% for and 3% for PPE and MSW, respectively, shows the highest values of TPD polymer WTB matrix degradation. The polymer matrix TPD degradation values of 49.0, 96.0, and 97.5% were achieved for WTB, PPE, and MSW, respectively, at these process conditions.
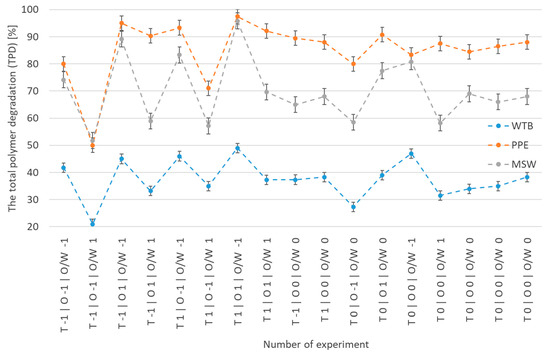
Figure 1.
Range of total polymer degradation for WTB, PPE, and MSW in the selected process conditions (T—temperature, O—oxidative level, O/W—waste–liquid ratio).
The standard deviations recorded at the centre point were 1.25, 1.43, and 1.85 for WTB, PPE, and MSW, respectively. The results confirm the uniformity of the tested materials. The homogeneity of PPE is significantly higher compared to WTB due to its predominantly single-polymer composition (polypropylene—PP). In contrast, WTB is characterised by a heterogeneous mixture of many materials, including polymers, resins, and fillers such as fibreglass.
3.3. Comparison of OCCs in the Studied Waste
Figure 2 displays the progression of OCC values resulting from the oxidative liquefaction of WTBs, PPE, and MSW. The experimental conditions that yielded the highest measured OCC values for the analysed materials exhibited several common features. Specifically, these tests frequently involved a lower or average operating temperature, as opposed to the previously mentioned TPD parameter. This implies that to achieve the highest yield of oxygen compounds, it is necessary to control the temperature and turbulence of the oxidation process. The procedure should be carried out at moderate temperatures to optimise the results. Furthermore, the findings suggest that in the context of OCC, the various variables examined have a reciprocal impact on the parameter under investigation. This phenomenon is observable in WTBs, PPE, and MSW. However, it is particularly prominent in the case of MSW. The OCC levels varied from 13 gOCC/kg waste for MSW and more than 200 gOCC/kg waste for WTBs and PPE. The most elevated values were recorded under relatively gentle process conditions, specifically a temperature of 200 °C, a low oxidant concentration of 30%, and a low waste-to-liquid ratio of 3%. Concentration values of OCCs ranging from 150–180 g/kg were also observed. When the process is conducted at a higher temperature, it is advisable to use a lower oxidant concentration, and vice versa. Conversely, when the temperature is lower, a higher oxidant concentration is necessary. In the case of WTB (T0; O0; O/W-1), a lower waste-to-oxidant solution ratio is required. High temperatures promote the intensification of oxidation, resulting in further oxidation of organic compounds to simpler ones like carbon dioxide. By raising the temperature and increasing the amount of oxidant, we lower the number of desirable aerobic compounds.

Figure 2.
Range of oxygenated chemical compounds for WTBs, PPE, and MSW in the selected process conditions (T—temperature, O—oxidative level, O/W—waste–liquid ratio).
The current conditions were advantageous for the substantial manufacturing of OCC. It is important to mention that varying waste-to-liquid ratios were recorded in these high-efficiency scenarios, suggesting a less immediate impact on the production or OCC.
3.4. The OCCs Yield Optimisation with Minimum Energy Consumption and the Maximisation of Total Polymer Degradation
Maximising the efficiency of the process was crucial to guarantee its economic viability, as decreasing energy usage would enhance the likelihood of broad commercial adoption. The optimisation technique depicted in Figure 3, Figure 4 and Figure 5 enhances the probability of attaining commercial feasibility of the process by efficiently controlling the OCC efficiency and maximum TPD in relation to the energy consumption associated with the process. During the precise optimisation procedure, the study examined five response parameters for WTB. However, the number of parameters for PPE and MSW was limited to three, while maintaining constant pressure and reaction time. The reduction in the number of variables from five to three was determined by the findings of the research carried out with WTB. When it comes to temperature, there are notable distinctions in the characteristics between WTB, PPE, and MSW. The total polymer degradation (TPD) in WTB exhibited an initial decline followed by an ascent with rising temperature. Conversely, when it comes to PPE and MSW, the breakdown of polymers exhibited an almost straight-line rise in respect to temperature. However, in both scenarios, there was an inverse relationship between the rise in temperature and the levels of OCC, which was not the desired outcome. These results lead to the conclusion that the minimal temperatures are optimal for attaining maximal TPD and OCC production, while minimising energy consumption.
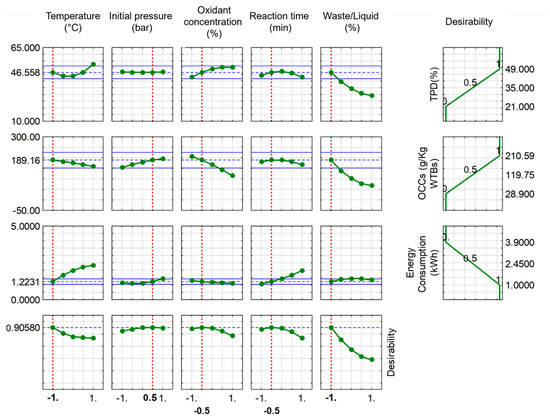
Figure 3.
Process optimisation through profiles of predicted values for WTB (the blue lines represent the desired optimal value of a parameter (TPD, OCC, EC) with +/− limits; the vertical broken red line represents the optimal conditions determined by the optimisation process performed; −0.5 and 0.5 represent the optimal calculated value between the minimum (−1)/maximum (1) and middle (0) values for a given variable).

Figure 4.
Process optimisation through profiles of predicted values for PPEs (the blue lines represent the desired optimal value of a parameter (TPD, OCC, EC) with +/− limits; the vertical broken red line represents the optimal conditions determined by the optimisation process performed; −0.5 and 0.5 represent the optimal calculated value between the minimum (−1)/maximum (1) and middle (0) values for a given variable.).
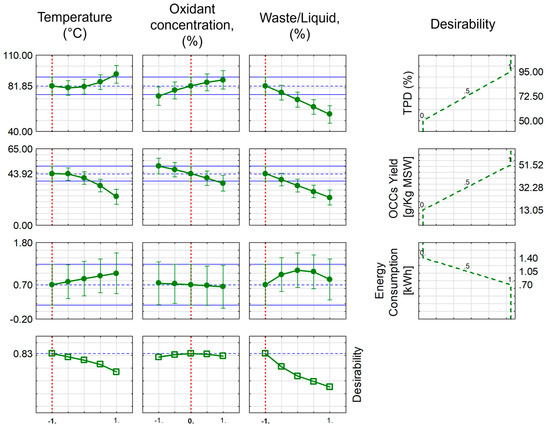
Figure 5.
Process optimisation through profiles of predicted values for MSW (the blue lines represent the desired optimal value of a parameter (TPD, OCC, EC) with +/− limits; the vertical broken red line represents the optimal conditions determined by the optimisation process performed; −0.5 and 0.5 represent the optimal calculated value between the minimum (−1)/maximum (1) and middle (0) values for a given variable).
In the experiments involving PPE and MSW, the pressure is maintained at a constant level. However, variations in pressure levels have a notable impact on the total breakdown of polymers in WTB.
The concentration of oxidants in the reactants has a significant impact on the extent of polymer degradation and the production of OCC. Elevated amounts of oxidants were observed to greatly expedite the overall breakdown of polymers in both materials. However, the waste materials that were evaluated showed a linear drop in OCC concentrations as the oxidant concentration increased. Consequently, it is preferable to have lower levels of oxidants for PPE and middling ones for MSW and WTB.
The energy usage during an experiment is exactly proportional to the reaction time. Prolonged response times have an adverse impact on both overall polymer degradation and OCC generation, leading to heightened energy usage.
The waste-to-liquid ratio has a comparable impact on the overall degradation of polymers and the formation of OCCs for PEEs and MSW. The decline in both ratios was substantial under conditions of elevated waste concentrations and reduced liquid volumes. Hence, it is beneficial to minimise the waste-to-liquid ratio to achieve the desired oxidative liquefaction outcomes for the waste materials under examination. Table 3 summarises the optimised values of all parameters investigated for WTB, PPE, and MSW.

Table 3.
Optimal reaction conditions for maximum TPD and OCC production from oxidative liquefaction of WTBs, PPE, and MSW.
4. Discussion
A considerable quantity of organic matter, mostly polymeric polymers, which can decompose to yield useful secondary chemicals, made up the multi-material waste under investigation [22]. The concentration of carbon and hydrogen in the waste polymeric materials examined in this research is crucial for the production of OCCs through an oxidative liquefaction method. Thorough studies were conducted to analyse and optimise the process in relation to total polymer degradation (TPD) and the presence of oxygenated chemical compounds (OCCs). These studies successfully identified significant effects resulting from the variables under investigation. The process outcomes were influenced by various variables, namely temperature, oxidant concentration, waste-to-liquid ratio, pressure, and reaction time. Within this specified range of conditions, it was noted that changes in pressure and reaction time had a minimal impact on the overall performance of the WTB system. Therefore, these variables were kept constant for the PPE and MSW systems as well. The association between higher temperatures and enhanced thermal decomposition of wind turbine blades (WTBs), personal protective equipment (PPE), and municipal solid waste (MSW) can be ascribed to distinctive characteristics of the near-critical conditions prevailing in the reactor [18,21].
The specific attributes of the process environment, such as enhanced diffusivity, the diminished dielectric constant, decreased density, and heightened water properties, facilitated the more efficient decomposition of resin materials [23]. The decline in OCC performance found in PPE as the temperature rose can be ascribed to the heightened oxidation of intermediates generated during the liquefaction process. Raising the temperature may enhance the speed of oxidation reactions, leading to a reduction in the overall yield of desired OCCs and promoting the conversion of reactants into CO2. However, within the context of MSW, the reduction in OCC yield can be ascribed to the properties of the materials employed. Consequently, this can potentially result in the formation of various intermediate products or expedite their oxidation, thereby contributing to the observed decline in OCC yield as the temperature rose. The performance of the polymeric multi-material wastes in the oxidative liquefaction process was affected by the intricate interplay between the waste-to-liquid ratio and oxidant concentration. A direct relationship existed between a decrease in waste-to-liquid ratio and an increase in TPD and OCC yields. The observable impact has several facets. Due to a higher waste-to-liquid ratio, meaning a larger amount of water in the reactants, the increased water content in this framework serves two purposes: it acts as both a solvent and a catalytic agent. A higher water concentration leads to an increase in the number of hydroxonium ions H3O+. These ions play a crucial role in initiating and developing several chemical reactions related to oxidative liquefaction [24]. The efficiency and desired outcomes of the process, such as high yields of OCC from WTB, PPE, and MSW liquefaction, are heavily influenced by the concentration of oxidants present in the reactants. Raising the oxidant concentration has the ability to elevate total polymer degradation levels due to the promotion of reduced carbon production resulting from greater oxygen content. However, excessively high concentrations can lead to negative repercussions. A high concentration of oxygen may result in excessive oxidation, which can be deleterious to the formation of liquid OCCs. This is because it breaks down intermediates into smaller and less stable molecules [25]. The prevailing conditions lead to the production of undesired by-products, namely carbon dioxide and water, which hinder the desired objective of acquiring advantageous OCCs. Furthermore, the excessive presence of oxidants results in an unanticipated elevation in temperature, therefore augmenting the probability of thermal degradation of the liquid products generated. Hence, it is imperative to meticulously regulate and enhance the levels of oxidant in order to attain the desired outcome while preventing any detrimental reactions or degradation of the product.
5. Conclusions and Prospects
Oxidative liquefaction holds promise for efficiently eliminating polymer waste from composite polymer matrices, such as WTBs, PPE, and MSW, among others. The waste plastic and composite materials examined in the conducted studies exhibited notable disparities in their composition, hence influencing the investigated process. The waste generated by WTB exhibited variation in physicochemical qualities and had a high proportion of inorganic residues. In contrast, the PPE and MSW were composed of polymers with similar characteristics (mainly thermoplastic polymers), which reacted more easily under the process conditions studied, and contain relatively moderate (MSW) and low (PPE) levels of inorganic residues. The optimisation attempts examined the intricate impact of multiple aspects on the materials under investigation. Through manipulation of the process variables, it was shown that the temperature of the liquefaction process had a non-linear impact on the extent of polymer degradation in the case of WTB. In contrast, PPE and MSW exhibited a more linear rise in degradation. Pressure fluctuations had a notable impact on WTB, resulting in substantial alterations in the production of oxidised chemicals. By determining the optimal value of WTB, it was possible to exclude it as a variable in the analysis of other waste materials. The optimal parameters for obtaining the maximum amount of OCC with the minimum amount of energy required for the process are as follows: For WTB, temperature 250 °C, initial pressure 35 bar, H2O2 concentration 22.5%, residence time 45 min, waste-to-liquid ratio 5%. For PPE and MSW, the conditions were the same for parameters such as temperature (200 °C), initial pressure (30 bar), residence time (45 min), and waste-to-liquid ratio (3%). Only the concentrations of H2O2 are different, being 30% for PPE and 45% for MSW. Furthermore, the research revealed that the alteration in oxidant concentration is a crucial determinant in the liquefaction process, namely the OCC content of the resulting products. Elevated oxidant concentration led to enhanced degradation of the total polymer content. Nevertheless, this had an adverse impact on the synthesis of oxygenated chemicals from the initial substances used in the experiment. The investigation demonstrated that both reaction time and waste-to-liquid ratio played crucial roles in the experiment. Elevated response time led to augmented energy expenditure without commensurate advantages. Conversely, a reduced ratio of waste to liquid was essential in order to obtain favourable outcomes for both substances. These findings establish a vital foundation for the development of eco-friendly industrial techniques, aligning with initiatives to minimise waste and enhance resource utilisation in the processing of polymer waste.
Author Contributions
R.M., M.S., S.S., H.M. and S.W., conceptualisation; R.M., M.S. and H.M., methodology, formal analysis, and investigation; R.M. and M.S., writing—original draft preparation; R.M., M.S., S.S., H.M. and S.W., writing—review and editing; R.M. and M.S., visualisation. All authors have read and agreed to the published version of the manuscript.
Funding
This work is prepared within the frame of the project Opus 21 “Oxidative liquefaction of plastic waste. Experimental research with multidimensional data analysis using chemometric methods” financed by the National Science Center, Poland (reg. number 2021/41/B/ST8/01770).
Data Availability Statement
The data presented in this study are openly available in Mendeley Data at DOI:10.17632/btbff8krrm.1, and DOI:10.17632/jwtzwhtbkv.1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Wei, T.; Park, D. Macroeconomic Impacts of Energy Productivity: A General Equilibrium Perspective. Energy Effic. 2019, 12, 1857–1872. [Google Scholar] [CrossRef]
- Amantayeva, A.; Shehab, E.; Meiirbekov, A.; Suleimen, A.; Tokbolat, S.; Sarfraz, S. Challenges and Opportunities of Implementing Industry 4.0 in Recycling Carbon Fiber Reinforced Composites. Adv. Sci. Technol. 2022, 116, 67–73. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, G.; Zheng, L.; Zhang, Y.; Qi, X.; Ke, S.; Gao, L.; Bai, R.; Liu, G. Modeling Waste Generation and End-of-Life Management of Wind Power Development in Guangdong, China until 2050. Resour. Conserv. Recycl. 2021, 169, 105533. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 4 December 2022).
- COVID-Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 7 February 2023).
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- Musiał, W.; Zioło, M.; Luty, L.; Musiał, K.; Bianco, V.; Klepacki, B.; Stolarski, M.J. Energy Policy of European Union Member States in the Context of Renewable Energy Sources Development. Energies 2021, 14, 2864. [Google Scholar] [CrossRef]
- Sayigh, A. Up-Date: Renewable Energy and Climate Change. Renew. Energy Environ. Sustain. 2021, 6, 13. [Google Scholar] [CrossRef]
- Liu, P.; Barlow, C.Y. Wind Turbine Blade Waste in 2050. Waste Manag. 2017, 62, 229–240. [Google Scholar] [CrossRef]
- Topham, E.; McMillan, D. Sustainable Decommissioning of an Offshore Wind Farm. Renew. Energy 2017, 102, 470–480. [Google Scholar] [CrossRef]
- Albers, H.; Greiner, S.; Seifert, H.; Kuehne, U. Recycling of Wind Turbine Rotor Blades. Fact or Fiction?; Recycling von Rotorblaettern Aus Windenergieanlagen. Fakt Oder Fiktion? DEWI-Magazin. 2009. Available online: https://www.osti.gov/etdeweb/biblio/21214142 (accessed on 20 December 2023).
- Kazerooni Sadi, M.A.; Abdullah, A.; Sajoudi, M.N.; Bin Mustaffa Kamal, M.F.; Torshizi, F.; Taherkhani, R. Reduce, Reuse, Recycle and Recovery in Sustainable Construction Waste Management. Adv. Mater. Res. 2012, 446–449, 937–944. [Google Scholar] [CrossRef]
- Marques, C.T.; Fritzen Gomes, B.M. Reuse, Reduce, Recycle. In Responsible Consumption and Production; Springer: Cham, Switzerland, 2019; pp. 1–9. [Google Scholar] [CrossRef]
- CEN/TS 15414-2:2010—Solid Recovered Fuels—Determination of Moisture Content Using the Oven Dry. Available online: https://standards.iteh.ai/catalog/standards/cen/1f025ad5-653e-4847-8293-2c181d215582/cen-ts-15414-2-2010 (accessed on 23 December 2023).
- EN 15403:2011—Solid Recovered Fuels—Determination of Ash Content. Available online: https://standards.iteh.ai/catalog/standards/cen/0c9908dd-e915-4470-b9b9-b7c2011b71fa/en-15403-2011 (accessed on 23 December 2023).
- EN 15402:2011—Solid Recovered Fuels—Determination of the Content of Volatile Matter. Available online: https://standards.iteh.ai/catalog/standards/cen/32c296e3-e4fa-443b-a9ae-fafcae85ef14/en-15402-2011 (accessed on 23 December 2023).
- Mumtaz, H.; Sobek, S.; Sajdak, M.; Muzyka, R.; Drewniak, S.; Werle, S. Oxidative Liquefaction as an Alternative Method of Recycling and the Pyrolysis Kinetics of Wind Turbine Blades. Energy 2023, 278, 127950. [Google Scholar] [CrossRef]
- TIBCO Software TIBCO Statistica™, StatSoft, Software Release 13.3.0, June 2017. Available online: https://docs.tibco.com/products/tibco-statistica-13-3-0. (accessed on 20 December 2023).
- Mumtaz, H.; Sobek, S.; Werle, S.; Sajdak, M.; Muzyka, R. Hydrothermal Treatment of Plastic Waste within a Circular Economy Perspective. Sustain. Chem. Pharm. 2023, 32, 100991. [Google Scholar] [CrossRef]
- Mumtaz, H.; Sobek, S.; Sajdak, M.; Muzyka, R.; Werle, S. An Experimental Investigation and Process Optimization of the Oxidative Liquefaction Process as the Recycling Method of the End-of-Life Wind Turbine Blades. Renew. Energy 2023, 211, 269–278. [Google Scholar] [CrossRef]
- Skrzyniarz, M.; Sajdak, M.; Zajemska, M.; Biniek-Poskart, A.; Iwaszko, J.; Skibiński, A. Possibilities of RDF Pyrolysis Products Utilization in the Face of the Energy Crisis. Energies 2023, 16, 6695. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Kolaczkowski, S.T.; Plucinski, P.; Beltran, F.J.; Rivas, F.J.; McLurgh, D.B. Wet Air Oxidation: A Review of Process Technologies and Aspects in Reactor Design. Chem. Eng. J. 1999, 73, 143–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).