Abstract
Food waste (FW) is a significant global issue with a carbon footprint of 3.3 billion tonnes (Bt), primarily generated due to improper food supply chain management, storage issues, and transportation problems. Acidogenic processes like dark fermentation, anaerobic digestion, and a combination of DF-AD can produce renewable biofuels (Bio-CH4, Bio-H2) by valorising FW, aligning with the UN SDGs. FW is an ideal substrate for acidogenic processes due to its high moisture content, organic matter, and biodegradability. However, the choice of FW valorisation pathways depends on energy yield, conversion efficiency, and cost effectiveness. Acidogenic processes are not economically viable for industrial scale FW treatment due to reduced energy recovery from stand-alone processes. So, this study reviews comparative studies on biogas, biohydrogen, and biohythane production from FW via acidogenic processes, focusing on energy yield, energy recovery, and environmental and economic impact to provide a clear understanding of energy recovery and yield from all acidogenic processes. Additionally, this review also explores the recent advancements in digestate slurry management and the synergistic effects of AD and HTC processes. Lastly, a futuristic integrated bio-thermo-chemical process is proposed for maximum energy recovery, valuing food waste to energy vectors (Bio-H2, Bio-CH4, and hydro-char) along with digestate management and biofertilizer production.
1. Introduction
Food waste (FW) is biodegradable waste generated from a variety of sources, such as households, food processing industries, restaurants, and other hospitality domains. Global food waste production is estimated to be 1.6 billion tonnes (Bt) annually [1], and the carbon footprint of food waste is estimated to contribute to greenhouse gas (GHG) emissions by approximately 3.3 Bt of CO2 annually. The generation of FW by consumers and the average of carbon dioxide emissions per capita from different regions across the globe are represented in Figure 1. FW generation is highest in North America and Oceania and Europe, with amounts of about 310 and 270 kg per capita per annum, respectively. Latin America and North Africa and West and Central Asia show consumer waste production of about 210 and 225 kg per capita per annum, respectively. South and Southeast Asia, Sub-Saharan Africa, and industrialised Asia generate consumer waste of about 125, 160, and 245 kg per capita per annum, respectively [2,3]. On the other hand, there is an enormous accumulation of FW due to improper food supply chain management, storage issues, and transportation problems [4].
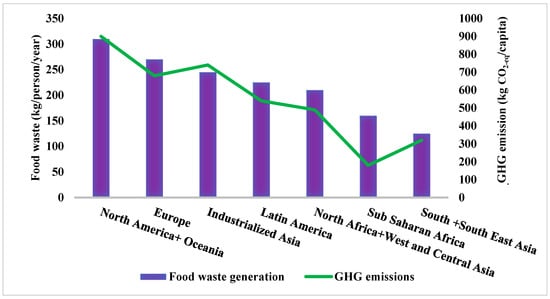
Figure 1.
Representation status of food waste generated per person per year with kgCO2-eq per capita around the globe. Authors’ elaboration based on data from [2,3].
According to studies [5], FW in some countries accounts for nearly half of total solid waste. Currently, more than 95% of FW management globally is performed via landfilling and incineration [6,7], often because they are less expensive than other treatments [8,9,10,11]. However, it is well known that FW landfilling is producing CH4-rich landfill gas, contributing considerably to climate change and accounting for 58% of fugitive methane emissions, and so it has to be avoided [12]. Indeed, it is internationally acknowledged that the following hierarchy should be applied to FW: prevent, donate or up-cycle, feed animals or leave unharvested, composting or anaerobic digestion, incineration or landfilling [13]. Thus, when prevention, donation, or animal feeding are not feasible, resource and energy are to be addressed. In particular, energy recovery has a role as a renewable and sustainable energy source, which is required in view of urban population increase (the United Nations predicts a 2.5 billion increase in urban population by 2050, with 90% of this growth occurring in Asia and the Africa UN [14], as well as energy demand increase by nearly 50% over the next 20 years IEA [15], depletion of fossil fuels, and the consequent climate change impact on the planet [16]).
Thermochemical and biochemical routes are available for FW energy valorisation. However, biochemical routes are preferred over thermochemical routes due to their simplicity, as well as less energy consumption with minimal generation of harmful emissions [17,18]. Therefore, acidogenic processes like dark fermentation (DF), anaerobic digestion (AD), and integrative processes, i.e., combination of both DF and AD, come into the picture as attractive options for producing renewable biofuel (H2 and CH4) by utilising FW, addressing waste management and the recovery of nutrients as a win-win approach. Indeed, the reduction in FW, its correct management, and the development of renewable energy systems all fit within the United Nations Sustainable Development Goals (SDGs) [19].
DF produces a mixture of H2 and CO2, often addressed as biohydrogen (bio-H2), while AD produces a mixture of CH4 and CO2, generally named biogas. Both processes are considered the most efficient and eco-friendly routes of FW valorisation. Production of bio-H2 via DF is a promising option, as it requires simpler mechanisms and less energy input compared to photo fermentation. However, while DF is still at low TRL (Technological Readiness Level) development, AD has a high TRL and is fully available industrially worldwide. Biogas produced from AD is commonly used as fuel for combined heat and power (CHP) application in reciprocating internal combustion engines (ICEs) and, less frequently, in gas turbines (GT), the former being more efficient for the typical sizes found in these applications (i.e., less than 1 MW). In the last decade, the upgrading of biogas to bio-methane by removing the CO2 and other contaminants attracted great interest as a viable vehicular fuel option [20,21]. Bio-H2, intended as the H2-CO2 mixture produced through DF, can be used as is to feed several types of fuel cells [22] or purified to obtain pure H2 [23]. However, the utilisation of vehicular-grade purified bio-H2 derived from biological processes, such as DF, is impeded by the issues of elevated flammability and storage difficulties [24].
Hythane® is a trademark introduced by Hydrogen Component Inc. in the early 90s, which studied blending Compressed Natural Gas (CNG) with hydrogen as a fuel for internal combustion performances. Hythane combustion is environmentally friendly, emitting nearly 45% less NOx gas than CNG [25]. It was observed by various researchers that combination of H2 and CH4, with approximately 10–25% H2 composition and 75–90% CH4, results in enhancing the overall economy and the effectiveness of the processes involved with increased energy recovery (10–40%) [24,26]. Hence, hythane may be an appropriate vehicular fuel, along with the dual benefits of H2 and CH4, like decreased NOX, SOX emissions, increased flammability, decreased combustion time, and improved heat efficacy. Biohythane, a mixture containing bio-H2, bio-CH4, and CO2, can be produced by integrating the DF and AD process, offering lower production costs, a lower carbon footprint, and environmental sustainability [26,27,28]. To promote the use of FW through DF, AD, and DF-AD, it is essential to compare these three processes in order to select the preferable valorisation pathway. Indeed, FW is an appropriate substrate for all acidogenic processes (i.e., AD, DF, and two-stage DF-AD processes) due to its high moisture content and high content of organic matters with excellent biodegradability, as well as its organic and nutrient-rich composition. However, due to the variable composition of food waste, it is important to select the best possible valorisation pathway (i.e., AD or DF or DF-AD) with respect to energy yield and conversion efficiency, as well as cost effectiveness. Consequently, it is crucial to investigate the advantages of valorising the FW process through individual processes (AD, DF) and integrated processes (two-stage DF-AD).
AD, DF, and DF-AD processes are sustainable methods for treating organic waste, but they also produce byproducts like digestate and effluents. Digestate, the residue left after anaerobic processes, poses challenges in large-scale plant operations due to high variability in effluent treatment. Treatment methods include fertilizer production and thermochemical processing, like hydrothermal carbonisation [29]. HTC converts organic matter into high-carbon content, producing solid fuel and soil ameliorant. Thermochemical conversion of digestate into energy vectors is a promising solution for waste effluent management in biogas plants. Integrated technologies combining anaerobic processes with thermochemical methods are being developed, with hydrothermal carbonisation being the most suitable due to its high moisture content [30]. This process generates renewable energy and hydrochar from the digestate, improves soil quality and fertility, and contributes to a circular economy [31,32]. Biorefinery concepts promote integrated technologies, with biomass waste being a valuable resource for bioenergy and biofertilizer production due to its abundant availability [29,30,33].
Several reviews dealt with the production of either H2 or CH4 from FW [34,35,36,37,38,39]. On the other side, the two-stage integrated DF-AD process is reported to be significantly superior to single-stage processes in terms of efficacy, efficiency, and stability; multiple studies demonstrate this [25,27,40]. Despite the fact that several researchers have achieved high production rates and improved energy recovery with emission of fewer pollutant from biomass waste streams, it is not yet economically viable on an industrial scale.
After an exhaustive literature survey, it has been observed that there is not a single past review available on comparative evaluation of the three acidogenic processes for the valorisation of FW, primarily from the viewpoint of energy yield, operational parameters, and economic and environmental evaluations. Comparative analysis of these three acidogenic processes for FW has yet to be explored, and, to the best of the author’s knowledge, there are no such comparative review studies available. So, there is need of a review of comparative analyses for all three biofuels produced from FW in terms of yield, economic and environmental viability, reactor configuration, etc. To fulfil the existing research gaps, this study took the opportunity to provide a systematic review on comparative studies on the production of biogas, biohydrogen, and biohythane from FW via anaerobic fermentation processes, with special emphasis on comparative analysis of these processes with regards to energy recovery/energy yield, environment impact, and economic impact for FW valorisation.
The review paper also explores the potential of integrated biochemical processes in food waste valorisation within circular biorefineries. It focuses on anaerobic digestion and dark fermentation, which enhance bio-CH4 and bio-H2. Further, it provides additional information regarding the future of digestate utilisation and value-added products, with a vision towards emerging integrative technologies within a circular acidogenic biorefinery framework. The review aims to highlight technological advancements and optimisation strategies for energy recovery from food waste. Lastly, a futuristic integrated bio-thermo-chemical process is proposed, with the production of Bio-CH4, Bio-H2, and hydrothermal carbonisation of digestate for hydrochar production. It provides a roadmap for researchers, industry professionals, and policymakers to develop and implement circular biorefineries, transforming food waste into valuable energy resources while adhering to sustainability and circularity principles. The study could provide a useful support for the selection of particular acidogenic processes based on the ultimate use of bioenergy, as well as insights into the technology efficiency and the investment, operational, and maintenance costs.
2. Trends in Publications Associated with Dark Fermentation, Anaerobic Digestion, and Two-Stage Integrated DF-AD of Food Waste
The literature items reviewed in this paper were selected from the bibliometric source of the Web of Science database. The keywords, such as food waste biogas, food waste biomethane, food waste biohydrogen, food waste biohythane, etc., were used individually and in different combinations. Currently, research interests within the “acidogenic biorefinery” are growing rapidly, as shown by the recent trends in publications associated with FW with regards to biogas, bio-H2, and biohythane production, as shown in Figure 2a–c, for the last ten years. FW to biogas has been the most explored research topic, as there are almost 3663 research and review articles in the past ten years, out of which research and review articles number 2645 and 1018, respectively. FW to bio-H2 is also gaining interest in the research community, as there are almost 2573 research and review articles published in the last 10 years. In comparison to biogas and bio-H2, biohythane production from FW has been a less explored research topic in the last 10 years, as there are only 227 research and review articles, out of which there are only 78 review articles till this date. The trend of research and review articles (published from 2014 to May 2023) in the field of biogas, bio-H2, and biohythane production from FW is shown in Figure 2a–c. As per the literature available, there is no review article which explores the comparative analysis of biogas, bio-H2, and biohythane production from FW.
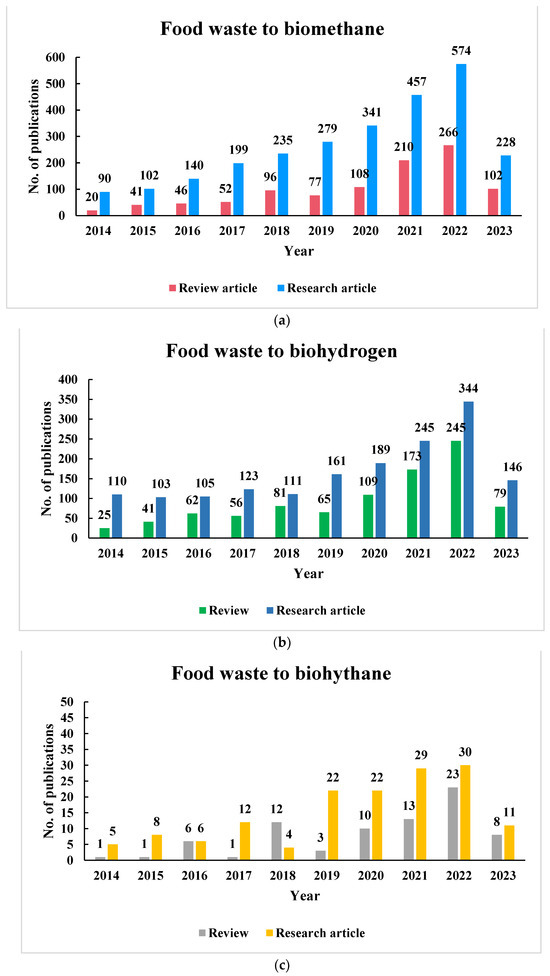
Figure 2.
(a) Representation of the number of scientific papers on the investigation of biomethane production from food waste from 2014 to May 2023. (b) Representation of the number of scientific papers on the investigation of biohydrogen production from food waste from 2014 to May 2023. (c) Representation of the number of scientific papers on the investigation of biohythane production from food waste from 2014 to May 2023.
3. Current Status of Food Waste
The food supply chain is broadly categorised in the primary production, processing, distribution, and consumption stages. FW in the primary production stage of the food supply chain comprises the biowaste generated during harvesting of crops and procurement of edible items such as meat and dairy products from animals. The FW in the processing stage includes the biowaste that remains after processing of useful items. In the distribution stage, biowaste generated from both the fresh and manufactured products are considered as FW. FW in the consumption stage is generally counted by including the biowaste generated from household and food services. According to a recent study, FW accounts for almost 20% of the total food produced in EU. Out of 129 Mt of FW, the maximum fraction of FW (approximately 46%) was generated in the consumption stage, followed by 25% in primary production, 23.6% in the processing stage, and 5.5% in distribution and retail (Figure 3) [41,42]. In addition, variation in the proportion of FW items, such as fruits, vegetables, cereals, fish, meat, etc., in different stages of the food supply chain is shown in Figure 4.
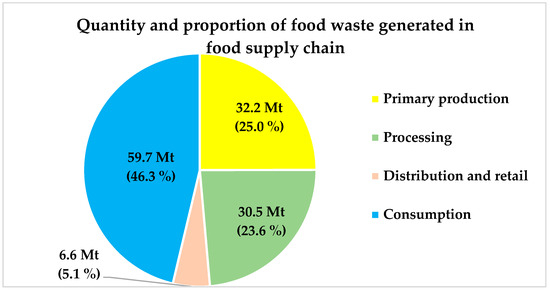
Figure 3.
FW generation in different stages of food supply chain [42].
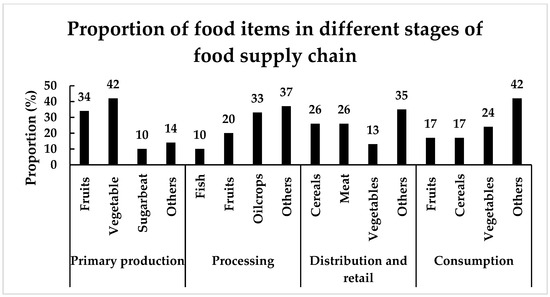
Figure 4.
Proportions of different food items in various food supply chains [42].
Characteristics of Food Waste
Characterisation of FW is an essential step before selecting any valorising technology for producing value-added products or biofuels. FW is generally categorised using its proximate, ultimate, and compositional properties [43,44]. Proximate properties, such as total solid (TS), moisture content (MC), volatile solid (VS), and ash content, are important parameters to decide the appropriate technology needed for conversion of any biomass to biofuel and value-added products [45]. Ultimate properties, such as carbon (C), nitrogen (N), hydrogen (H), oxygen (O), and total sulphur (S), are also essential properties to optimise the required parameters C/N, C/O, and C/H during any bioenergy conversion process and determine the theoretical biofuel potential [46]. Compositional properties like fat, proteins, and carbohydrates decide the biofuel potential of the biomass. Protein is an essential fraction of FW for biomethanation via the AD process [47], and carbohydrate fraction accounts in FW are helpful for deriving biohydrogen via DF. Liquid biofuel (biodiesel) yield can be enhanced through lipid content [48]. Carbohydrates, protein, and fat (lipids) are the major components of FW. Generally, FW containing carbohydrates, lipids, and protein were studied for biogas, bio-H2, and biohythane production. However, FW with high carbohydrate content and acidic pH is considered the most favourable feedstock for bio-H2 production. In contrast, lipids and protein are not considered suitable for bio-H2 production [35]. For FW-AD, FW comprising a balanced carbon to nitrogen ratio (C/N) and neutral to alkaline pH is considered a suitable feedstock for efficient biogas production [49]. For biohythane production, suitable substrates for the first stage and second stage are similar to the DF and AD, respectively [18]. Other than all the proximate, ultimate, and compositional properties, micronutrients such as total phosphorus, total potassium, and total nitrogen are essential parameters to evaluate the quality of organic fertilizer generated from valorisation of FW [50]. Depending on food habits, source, stage of food supply chain, etc., huge variation can be noted in various properties. Table 1 shows the properties of various FW with systematic categorisation into generic FW, kitchen waste, restaurant waste, vegetable waste, and synthetic FW. It has been noticed (Table 1) that the pH of FW varies from 4.1 to 6.5. There is also a huge variation in TS (6.80–96.70%) and vs. content (60.0–94.60%) in various FW. Carbohydrate, fat, and protein content also varies from a minimum value of 36.70%, 9.70%, and 10% to a maximum value of 73.70%, 40.00%, and 30%, respectively. Similar variations have also been observed in carbon (39.0–72.5%), hydrogen (5.10–9.20), nitrogen (1.0–5.70), sulphur (0.05–0.92), oxygen (25.0–51.04), and C/N ratio (7.90–55.80).

Table 1.
Proximate, ultimate, and compositional analysis of various food waste.
4. Acidogenic Process for Food Waste Valorisation
4.1. Anaerobic Digestion
AD is a chain of biochemical reactions in which microorganisms convert complex organic polymers into CH4 (50–70%), CO2 (30–50%) with traces of hydrogen sulphide (0–4000 ppm), siloxanes, and other impurities in the absence of oxygen [43]. The AD process for biogas production is well known and comprised of four steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Figure 5). In the hydrolysis step, complex organic molecules are converted into soluble monomers and catalysed by hydrolytic and fermentative bacteria-excreted enzymes (cellulase, protease, and lipase). This reaction produces soluble carbohydrates, amino acids, glycerol, and long-chain carboxylic acids. During the second stage, which is acidogenic fermentation, the soluble organic products of hydrolysis are converted into simple organic substances—primarily volatile fatty acids like propionic, formic, butyric, valeric, etc.—ketones, and alcohols. Further, in the subsequent phase of acetogenesis, they are transformed into acetate, carbon dioxide, and hydrogen. The acetogenesis of VFA compounds such as propionic and butyric acid can only occur in the presence of a very low H2 concentration. During this process, the protons function as electron acceptors and are therefore reduced to H2 gas [67]. In the final phase of reduction (methanogenesis), methanogenic archaea convert acetate and H2 into CH4 and CO2. The acetoclastic methanogens transform acetate into CH4 and CO2, while the hydrogenotrophs change H2 and CO2 into CH4 [68]. A number of factors affect the AD process, including pH, temperature, hydraulic retention time (HRT), organic loading rate (OLR), C/N ratio, and pretreatment technique. Optimising various operational conditions maximises the CH4 yield of the process and makes it more technically and economically feasible. The AD process has been recognised as an effective waste management method with the simultaneous production of considerable amounts of biogas as a source of energy, considering that the energy content of the mixture is directly linked to the CH4 content (LHV 50 MJ/kg). AD for biogas production will have a global installed capacity for power generation of 29.5 GW by 2022 [69,70].
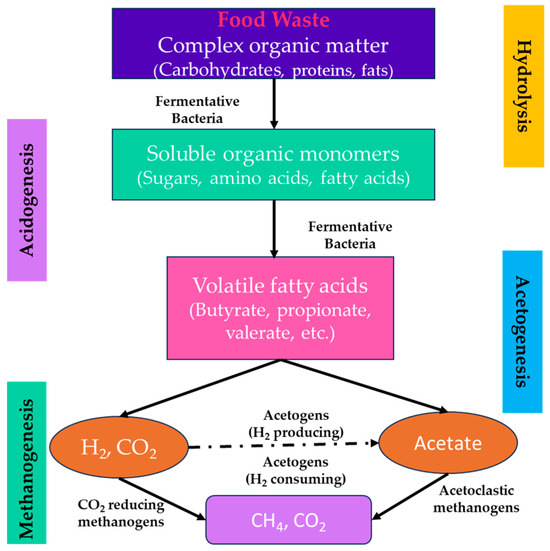
Figure 5.
Process flow diagram of anaerobic digestion process.
Due to its high organic and nutrient content, FW is a valuable biomass resource for biogas recovery via AD. The nutrient content of food residue, specifically its carbohydrate, protein, and lipid content, can have an effect on biogas production. According to research, lipids produce the greatest amount of biogas [71]. Among all categories of FW, the fat, oil, and grease (FOG) had the highest CH4 yield, reaching up to 1.1 m3 CH4/kg vs. added. The CH4 potential of lipids (1.014 m3/kg VS) is significantly higher than that of carbohydrates (e.g., glucose, 0.37 m3/kg VS) and proteins (0.74 m3/kg VS). Due to their slow biodegradability, however, a prolonged retention period is necessary to complete the process [71]. On the other hand, the rate of conversion for carbohydrates and proteins is much faster. Although AD is a well established and commonly employed technology for the treatment of organic-rich wastewaters, sewage sludge, and animal manure, adopting AD for FW management still faces a number of technical, economic, and social challenges, such as volatile fatty acids (VFAs) accumulation and process instability, foaming, low buffer capacity, and high transportation and operation costs [72].
4.2. Dark Fermentation
At present, the predominant method for producing H2 is the steam reforming of hydrocarbons and the coal gasification process, accounting for over 90% of total production. This reliance on fossil fuels has resulted in a major increase in dependence on these non-renewable resources. In order to address this issue, it is necessary to transition towards H2 production processes based on renewable sources, among which biological methods of H2 synthesis may play a role, producing a mixture of H2 and CO2, where the energy content is directly linked to the H2 content (LHV 120 MJ/kg) [73]. Microbial H2 production encompasses various mechanisms, such as direct and indirect biophotolysis of water, photo fermentation (PF), and dark fermentation (DF) [74]. DF has gained recognition as a highly feasible method due to its low cost, ease of operation, and the vast availability of renewable substrates [75]. The production of H2 gas via DF has a number of advantages over conventional techniques, such as steam reforming of natural gas or electrolysis of water. The use of renewable organic waste as a feedstock, low energy consumption, and low greenhouse gas emissions are some of these benefits [76].
The process of DF of FW is a biological phenomenon that involves the conversion of organic compounds into bio-H2 (intended here as the mixture of H2 and CO2) and other useful byproducts in the absence of light. The aforementioned procedure includes (as shown in Figure 6) the decomposition of complex organic compounds into simpler components, such as H2 gas, acetate, and CO2, through the activity of anaerobic microbes. Additional byproducts that can be derived from this process include VFAs, alcohols, and organic acids. These byproducts possess potential for industrial applications, particularly in the fields of bioenergy generation and chemical residues. In the process of DF, carbohydrates first undergo fermentation, resulting in the production of pyruvate. This metabolic pathway generates ATP and nicotinamide adenine dinucleotide (NADH) as energy sources [77]. The conversion of pyruvate into acetyl-CoA occurs via the process of ferredoxin reduction. In the event that acetyl-CoA is converted to acetate, both NADH and reduced ferredoxin are used to convert H+ to H2 via a metalloenzyme called hydrogenase or nitrogenase, yielding a maximal theoretical yield of 4 mol H2/mol hexose sugar [78]. The aforementioned method can also be applied to C-5 sugars; however, it is worth noting that the acetate pathway results in a production of 3.3 mol H2/mol pentose sugar. The enzyme hydrogenase plays a crucial role in facilitating the production of H2 through the process of anaerobic fermentation, wherein it effectively combines protons and electrons. DF is commonly conducted under controlled environments, wherein parameters such as temperature, pH, and substrate concentration are carefully regulated. The technology exhibits promising prospects for use in the fields of renewable energy production and waste treatment. Nevertheless, there are certain obstacles that need to be addressed in order to obtain optimal H2 yields, including poor H2 yields and the presence of competition among microorganisms. The low yields of H2 can be ascribed to the existence of creatures that consume H2, such as homoacetogens, hydrogenotrophic methanogens, sulphate-reducing bacteria, nitrate-reducing bacteria, and propionate makers. These organisms contribute to a drop in the overall production of bio-H2 [79]. Notwithstanding these obstacles, the process of DF has the potential to make a significant contribution towards waste diversion from landfills and the mitigation of GHGs. A number of studies on the utilisation of FW for H2 production via DF have been published. Kim et al. (2009) reported a 148.7 mL H2/g vs. added yield of H2 produced via batch mode DF of cafeteria FW pretreated at 90 °C in conjunction with a low lactate production [80]. Low substrate conversion efficiency, which is affected by factors such as the type and composition of FW, the presence of inhibitors and contaminants, and the operating conditions, can impede DF of FW [81]. Researchers are working on identifying optimal feedstocks and pretreatment methods to enhance H2 yields. Scale-up challenges include process control, substrate availability, and waste byproduct management [82]. Even though H2 would be produced from waste materials in an eco-friendly manner, it is still uncertain whether DF of FW is economically viable. Energy efficiency is a key research goal, aiming to minimise energy input for optimal conditions. DF faces efficiency and scalability challenges, making it less sustainable for hydrogen production and waste management, often requiring integration with other biochemical or thermochemical processes for economic viability [83,84].
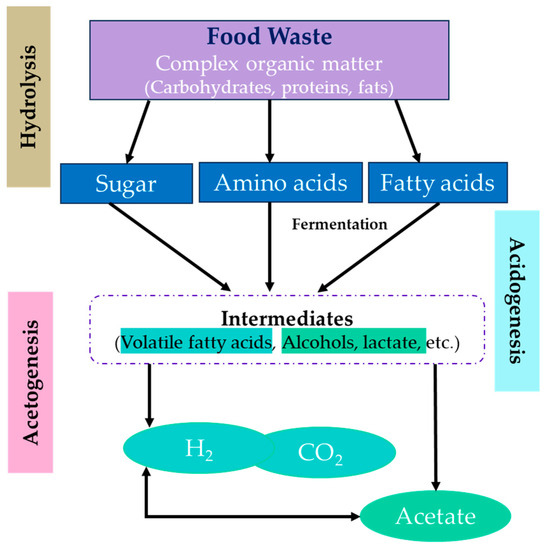
Figure 6.
Process flow diagram of dark fermentation process.
4.3. Integrated Fermentative Biohydrogen and Biogas Production in Two-Stage DF-AD Process
Both bio-H2 and biogas are promising alternative energy carriers that have significant and sustainable importance. The two-stage AD process is a sustainable and efficient method for harnessing energy from FW, depicted in Figure 7. The term “two-stage AD process” typically refers to either stage-1 AD and stage-2 AD or stage-1 DF and stage-2 AD. Both processes share similarities; however, in the AD-AD configuration, the products generated in both stages are CH4 and CO2. On the other hand, in the DF-AD configuration, the products in the first stage are H2 and CO2, while in second stage, the products are CH4 and CO2. It should be noted that in our situation, two-stage AD refers to the DF-AD setup, which is the default setting for the entire manuscript. So here, this integrated process consists of two unique stages, each accompanied by specific microorganisms and metabolic pathways, in order to generate bio-H2 gas (H2 & CO2) and biogas (CH4 & CO2) from the same substrate material [27]. In Stage 1, organic waste materials are subjected to biological reactions under strictly anaerobic conditions, focusing on the production of H2 gas. This stage includes hydrolysis, acidogenesis, fermentation, and a mixture of H2 and CO2. During Stage 2, the residual volatile fatty acids (VFAs) obtained from Stage 1, including acetic acid, propionic acid, and butyric acid, are consumed by methanogenic bacteria such as Methanosarcina and Methanobacterium. Through the process of methanogenesis, these bacteria convert the VFAs into a mixture of biogas consisting of CH4 and CO2.
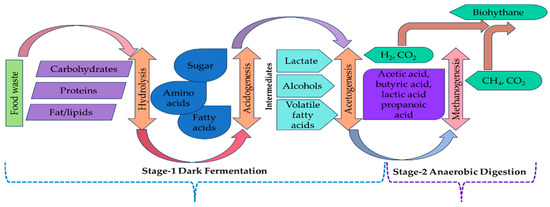
Figure 7.
Integrated two-stage dark fermentation and anaerobic digestion process.
The gases generated from the first and second stages can form a mixture comprising bio-H2 and biogas, generally known as biohythane, with different proportions [85]. The ratio of H2 to CH4 can vary significantly depending on the process parameters and content of the feedstock [86]. The biohythane mixture obtained from these two stages offers several advantages compared to individual fuel biogas or bio-H2. These advantages include a wider range of flammability, lower ignition temperature and time, absence of nitrous oxide (NOx) emissions, and improved engine performance without the need for specific modifications [82]. The two-stage integrated process optimises energy recovery from FW by optimising metabolic pathways and microorganisms, improving efficiency compared to single-stage anaerobic digestion. It separates bio-H2 and biogas production, optimising temperature conditions and producing higher gas yields. This process reduces energy requirements, allowing more energy to be generated from the same feedstock. The success of this process depends on factors like microorganism choice, feedstock composition, temperature control, and system design [87,88].
Han and Shin (2009) found that the coproduction of H2 and CH4 from FW markedly increased the bioenergy conversion efficiency from 8% in only H2 production to 78% [89]. Qin et al. (2019) studied the co-production of H2 and CH4 and the co-digestion of FW and paper waste for recirculated two-stage anaerobic digestion. The results show that bio-H2 and biogas were simultaneously and stably produced, 79 NL-H2/kg-VS and 329 NL-CH4/kg-VS in the long-term operation (50 h) [90]. Cavinato et al. (2012) used a mixture of minced organic waste as a substrate for two-stage AD under thermophilic conditions with an HRT of H2–3.3 days and CH4–12.6 days, leading to the production of 66.7 and 720 L/kg-VS of H2 and CH4, respectively. Both stages in two-stage AD are regulated by specific microorganisms that thrive under different optimal process conditions. Hence, achieving biohythane production necessitates a delicate equilibrium among process variables, including pretreatment, substrate/feedstock type, temperature, pH, reducing equivalents, etc. [85].
4.4. Factors Affecting Acidogenic Processes (DF, AD, and DF-AD)
Organic loading rate (OLR), hydraulic retention time (HRT), pH of fermentation medium, and temperature are crucial parameters that have strong influence on the production rate, composition and quality, and yield of biogas, bio-H2, and biohythane. The detailed analysis of effects of all these factors on all these processes is discussed below.
4.4.1. Temperature
Temperature is a key factor in AD, DF, and integrated AD-DF of FW. It affects the yield of biogas, bio-H2, and biohythane by influencing the metabolism of microbiome, kinetics of biochemical reactions, dissociation of intermediate products, and gas–liquid transfer. According to the process temperature (Table 2), this process can be categorised as psychrophilic (<25 °C), mesophilic (30–40 °C), and thermophilic (50–60 °C). AD, DF, and AD-DF of FW at psychrophilic conditions are rarely explored, and most of these studies are in the mesophilic and thermophilic temperature range. AD of FW under the mesophilic temperature range was considered as an energy-efficient and more favourable strategy than the thermophilic range due to the lesser energy demand and lesser chance of rapid acidification [49]. However, in the case of DF of FW, thermophilic temperature (50–60 °C) conditions were suggested as the effective strategy to extract the maximum bio-H2 from FW [31]. In the case of integrated AD-DF, the thermophilic temperature for the first stage and mesophilic conditions for the second stage are recommended for efficient biohythane production [18].
4.4.2. pH
The pH of the intermediate process is the major factor that is responsible for stable microbial activity in different stages of AD, DF, and the AD-DF process. Acidic pH (due to accumulation of VFAs) or alkaline pH (due to accumulation of ammoniacal nitrogen compounds) both cause decline in biomethane production due to reduced activities of methanogens. Generally, for the AD of FW, pHs of 4–5, 5–6.5, and 6.8–7.5 are considered as ideal conditions for hydrolysis, acidogenesis, and the methanogenesis process, respectively [49]. Controlling the pH in the whole reactor volume during different stages of AD is not technically feasible; hence, the pH of the feed is adjusted to an almost neutral pH ranging from 6.8 to 7.2. In the case of DF of FW, acidic pH (4.5–6) conditions of the media are recommended for efficient bio-H2 production [31]. In the case of biohythane production, acidic pH (4.5–6) for H2 and neutral pH (6.5–7) for CH4 production (Table 2) can enhance the overall productivity of biohythane [26].
4.4.3. Oganic Loading Rate
OLR is the measure of the rate of addition of substrate, and it is a crucial parameter which confirms the harmony of microorganisms and the food waste at equilibrium conditions. OLR above the optimum range causes accumulation of VFAs due to inefficient conversion of VFAs by microbes into biogas and consequently reduces the biogas yield. Similarly, OLR below the optimum range also causes reactor failure due to insufficient supply of nutrients to the microbial community. In the case of FW-AD, OLR is also influenced by the process temperature; as for mesophilic and thermophilic conditions, the optimum OLR was recommended to be 2–3 kg VS/m3/d and 4–5 kg VS/m3/d (Table 2). DF of FW in continuous operation is generally conducted at high OLR of 17–106 kg VS/m3/d [49]. For the AD-DF process of FW, the OLR ranges from 16 to 18 kg VS/m3/d in the first stage and from 4 to 6 kg VS/m3/d in the second stage [18].
4.4.4. Hydraulic Retention Time
Hydraulic retention time (HRT) is the residence time of biomass in the bioreactor. It generally represents the linkage between the reactor volume and the feeding rate of biomass in the reactor. Optimum HRT is a crucial parameter for efficient functioning of AD, DF, and AD-DF reactors. Short HRT causes the insufficient use of FW due to incomplete degradation by the microorganism [49]. In contrast, extremely high HRTs may also result in inhibition of the process due to accumulation of VFAs and ammoniacal nitrogen inside the reactor. In the single-stage AD of FW, optimum HRT ranges from 25 to 40 days for both thermophilic and mesophilic temperature conditions [18]. In the case of bio-H2 using different types of continuous reactors, the HRT is generally reported (Table 2) in the range of 1–12 h [91]. For the integrated AD-DF process, usually short HRTs (1–3 d) for DF of FW and longer HRTs (15–30 d) for AD were recommended for enhanced rate and yield of biohythane production [26].
4.4.5. Reactor Configuration
The reactor type and its configuration is critical in any type of biofuel production, as both of them play an important role in growth and activity of microbial community. Generally, in the case of FW-AD, a continuous stirred tank reactor (CSTR) (either in batch or continuous mode) is recommended due to its ability to handle high solid content. As per the literature summarised in Table 2, other reactors, such as the upflow anaerobic sludge reactor (UASB), internal circulation reactor (IC), expanded granular sludge bed reactor (EGSB), etc., were also explored for FW-AD due to their enhanced buffering capacity and ability to handle high OLR, but the requirement of high upflow velocity limits their usage in FW-AD [92]. On the other hand, apart from CSTR and UASB, other reactors such as the packed bed reactor (PBR), anaerobic sequencing batch reactor (ASBR), anaerobic fluidised bed reactor (AFBR), and membrane bioreactor (MBR) were reported to be utilised for FW-DF [91]. In the case of biohythane, two reactors with either identical or different configurations were noted to be utilised. CSTR, UASB, and ASBR reactors are generally used for bio-H2 production in the first stage, while CSTR and UASB are utilised for biogas production in the second stage [26].

Table 2.
Comparative analysis of AD, DF, and DF-AD processes on operating parameters [18,26,91,93].
Table 2.
Comparative analysis of AD, DF, and DF-AD processes on operating parameters [18,26,91,93].
| Acidogenic Process | pH | HRT | Temperature | Products | OLR (g VS/L/d) | Reactor Configuration | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Biogas (single-stage AD process) | 6.5–7 | 25–40 d | Psychrophilic (10–30 °C), mesophilic (30–40 °C), and thermophilic (50–60 °C) conditions | Methane 50–70% | 2–3 (mesophilic) 4–5 (thermophilic) | CSTR AFBR UASB AMFR | Less expensive Simple operation | Longer HRT Low OLR |
| Bio-H2 (dark fermentation) | 4.5–6 | 4 h–5 d | mesophilic (30–40 °C) and thermophilic (50–60 °C) conditions | Hydrogen 30–60% | 17–106 | AFBR, UASB, MBR, CSTR, | Intermediate products from fermentation could be utilised as valuable products Utilisation of feedstock with lower pH | Low H2 yield |
| Biohythane (two-stage AD process) | Stage-1: 4.5–6 Stage-2: 6.5–7 | Stage-1: 1–3 days Stage-2: 15–30 days 14–20 days (Thermophilic) (14–40 days) mesophilic | Stage-1: Mesophilic/Thermophilic Stage-2: Mesophilic | Methane 50–60% Hydrogen 5–30% | Stage-1 16–18 (kg VS/m3/d) Stage-2 4–6 | CSTR LBR AMFR | Shorter HRT in comparison of single stage AD | VFA accumulation |
5. Energy Yield from AD, DF, and Integrated DF-AD Processes
Energy yield is an essential parameter that can be utilised to assess the economic viability, efficiency, and environmental impact of bio-CH4, bio-H2, and biohythane production. Additionally, energy yield has a significant role in comparing energy produced from the standalone AD process, DF, and two-stage integrated AD-DF processes. Various types of FW, such as kitchen waste, synthetic FW, raw FW, liquid FW, and solid FW, have been evaluated in many studies for production of biofuels such as bio-CH4, bio-H2 and biohythane through single-stage AD, dark fermentation, and two-stage DF-AD process. All of these technologies were explored on a batch, semi-continuous scale using different reactors, such as batch, CSTR, ASBR, UASB, etc. Due to the various technological interventions, such as pretreatment, co-digestion, trace element addition, etc., biogas production from AD is comparatively the most explored and matured technology. As per the literature review mentioned in Table 3, biogas production from FW may vary from 116 to 684 LCH4/kg vs. and bioenergy yield may vary from 3.9 to 22.9 MJ/kg vs. (calculated as multiplying the CH4- yield by CH4 -LHV). They can be harnessed from the corresponding biogas yield. In a comparison of biomethane and bioenergy yield through the AD of FW, bio-H2 and bioenergy yield through the DF route is very low, as the bio-H2 ranges from 63 to 360 LH2/kg vs. while bioenergy yield varies from 0.7 to 3.9 MJ/kg VS. In contrast, biohythane production from FW through two-stage AD and integrated DF-AD is comparable in terms of energy yield, which is 3.8–19 MJ/kg VS. Comparing energy yield of food waste through different pathways of energy production, the highest energy yield of 22.9 MJ/kg vs. was observed for AD. Energy yield through the integrated DF-AD pathway is comparable to energy yield through AD, and there is a huge scope to enhance it through further technological interventions.

Table 3.
Comparative analysis of various food waste to biogas, biohydrogen, and biohythane production on energy yield and reactor type.
It is noted that comparing the volumetric or mass yields of CH4, H2, or their mixture is rather meaningless, since their LHVs are very different. Thus, we decided to convert all the collected results into energy yields, which makes it possible to compare the three processes. Further, gas yields are often reported in the reviewed articles in different ways: as biogas or CH4 in the case of AD or referred to as different substrate characterisation parameters (TS, VS, chemical oxygen demand (COD), total organic carbon (TOC), etc.). Thus, summarised results are obtained, converting the different ways for yield expression into a single comparable one, which is volume of CH4 or H2 produced per unit mass of VS.
6. Economic and Environmental Aspects/Feasibility (Environmental Analysis/Sustainability of CO2 Emissions)
Acidogenic processes-based bioenergy production aids us with moving towards a low carbon-based global energy economy. So, it is important to understand the economic and environmental aspect of these processes for technoeconomic feasibility of the entire system, since it is an essential tool for scaling up of process. So far, only limited literature studies have documented the technoeconomic aspect of biogas, bio-H2, and biohythane production from FW. The technoeconomic feasibility of biogas from AD, bio-H2 from DF, and biohythane production from a two-stage AD process can vary depending on several factors, including the feedstock cost (which in the case of FW is reduced to the eventual collection costs), the efficiency of the conversion process, the capital and operating costs of the plant, and the market demand for these energy sources [24,117]. Biogas has a well-established market for CHP production and in the transportation sector, where it can be used as a substitute for natural gas. Bio-H2 is still in the development phase, but it has the potential to replace fossil fuels in the transportation and industrial sectors [118].
The integration of dark fermentation and anaerobic digestion for the production of biohythane is a relatively new concept, with limited research on its technical and economic feasibility. However, extensive research has been conducted on individual steps, highlighting the potential of this innovative method [119,120]. Biohythane is a promising energy source, as it allows for the recovery of both CH4 and H2, which can be used in various applications. With proper planning and management, all three technologies have the potential to provide a sustainable solution to waste management and energy production [24,117,121].
In reference to technoeconomic feasibility of biogas production from FW, [122] reported a capital investment of USD 1,928,652 and operational expenses of USD 11/t VS/y to generate a profit of USD 19/t VS/d for FW-based biogas production. Similarly, Bastidas-Oyanedel and Schmidt (2018) reported comparatively higher economic benefits of USD 95/t VS/y for biogas production from FW on a capital investment of USD 2,183,379 and operational expenses USD 11/t VS/y [121]. Micolucci et al. (2018) conducted a cost study for a pilot-scale two-stage thermophilic anaerobic digester on FW, calculating an annual income of EUR 540,874/y [123]. Another study documented a capital expenditure of EUR 12,687.7 for every 13.4 tonnes of waste biomass in two-stage anaerobic digestion [122]. Theaker (2020) explored large-scale AD installations in developed nations and the social, technological, and environmental benefits of micro-scale AD. This study focused on a 2 m3 micro-scale plant in London, UK, to determine how flexible biogas production might boost AD profitability. Technoeconomic analysis showed a 5.4–11.9-year payback period for the micro-scale AD plant [124]. Al Naami, A. (2017) presented the potential of biogas production using FW collected from the region of Kartamantul in Yogyakarta, Indonesia, reporting the production of 13,087 m3 of biogas from 120 tonnes of FW and the economic benefits of utilising biogas for cooking purposes and electricity generation for a life span of 20 years. Biogas utilisation for cooking purposes gave higher economic benefits in the form of a Net Present Value (NPV) of USD 5.82 M, while electricity generation showed a NPV of USD 2 M. The breakeven for electricity generation from biogas was noted to be USD 13.8 cents/kWhe, while it was USD 25.5 cents/m3- biogas when utilised for cooking [125].
For economic viability of FW-based bio-H2 production, Han et al. (2016) evaluated a combined bioprocess for fermentative H2 production from FW using solid state fermentation. The plant was designed to convert 3 tonnes of FW into H2 daily. The total capital cost and annual production cost were USD 583,092 and USD 882,98.1, respectively. The plant’s return on investment, payback period, and internal rate of return were 26.75%, 5 years, and 24.07%, respectively [126]. In another study, Krishnan et al. (2019) recommended a H2-producing facility with a 50 m3 capacity for economic viability. The net present value, payback duration, and internal rate of return for a scale of 50 m3 were determined to be USD 526,551, 6.9 years, and 9.25%, respectively [127]. Similarly, Dinesh et al. (2018) examined the economics of hydrogen production on a 200 m3 capacity plant treating 100 tonnes of feedstock daily. With the capital expenditure of USD 1,600,000, the plant was noted to produce a yearly profit of USD 360,000 with yearly operational expenses of 548,568 USD. As per the literature, the H2 price and annual production cost exerted the most significant influence on the net present value [128]. Hence, more research is necessary to address the technological and economic challenges associated with large-scale DF bio-H2 production in order to establish it as a feasible and competitive technology.
Recently, Byun and Han (2023) used experimental kinetic data to simulate FW treatment for H2 production and analysed economic viability by considering process factors and economic parameters [129]. This project designed a high-capacity FW treatment system for H2 production using AD and steam methane reforming. The results confirmed the biohydrogen production of 0.2 tonnes from 50 tonnes of FW every day, with a MSP of H2 USD 26.3/kg calculated using discounted cash flow analysis, a simulation model, and current economic parameters. The study advocated for the influence of plant capacity on production cost and minimum selling price. On scaling up the plant size to treat FW of 2000 t/d, the minimum selling price was noted to drop to USD 6.2/kg (matching the price of fossil-based H2). The economics of H2 production systems rely directly on the technological advancement, cost, and accessibility of raw materials, as well as the capital and operational costs of the process. From a sustainability perspective, it is imperative for energy sectors and environmental regulations to prioritise the production of low-carbon H2 while adhering to the highest environmental standards. Additionally, it will bestow a competitive edge upon them and enhance customer receptiveness. Previous studies reveal that in order to achieve economic feasibility, bio-H2 must be generated from renewable feedstocks. However, the task of generating bio-H2 from renewable feedstock is proving to be more arduous than initially expected. In order to achieve a high bio-H2 yield, it is necessary to overcome certain challenges. Over the past two decades, there has been significant progress in the development of bio-H2, transitioning from laboratory experiments to pilot-scale applications and successfully addressing numerous obstacles along the way [130].
Finally, DF biohydrogen production still has a long way to becoming a competitive technology. A study by Jarunglumlert et al. (2018) explored the scaling up of biohydrogen production from food waste, revealing that annual revenue includes income from the sale of hydrogen and CO2, solid waste in fermentation sold as animal food, and FW disposal service charges. The study found that the minimum production scale of 40 m3 would provide positive benefits with a 0.63% internal rate of return and a payback period of 9.7 years. The study also found that the highest benefit analysed by Aspen was achieved at 100 m3 with 81% of the annual return rate [131]. The environment impact of converting any biomass into biofuel or value-added products is considered the crucial assessment for any conversion technology. In this regard, Takata et al. (2013) studied comparative methane yield from different experimental results for an LCA study on methane yield (m3 CH4/t-VS) and concluded that methane production from food waste treatment can reach 500 m3 CH4/t-VS in wet AD, suggesting the use of a value of 450 m3 CH4/t-VS for the inventory of LCA studies. In dry AD, methane production does not exceed 300 m3 CH4/t-VS, and m3 CH4/t-VS is suitable for assessing GHG reduction from biogas yield. This research group also summarised that the wet AD had high overall GHG emissions (62 kg CO2-eq/t waste, 100% operating rate) due to pretreatment, deodorisation, and wastewater treatment facilities. Simple wet AD with no additional equipment produced the lowest GHG emissions (20 kgCO2-eq/t waste). The integrated composting system had the second-highest total GHG emissions (35 kg-CO2eq/t waste) due to energy usage, while the simple system had 12 kgCO2-eq/t waste. [132]. Moult et al. (2018) conducted a study that revealed a comparison indicating that GHG emissions associated with conventional biogas production from FW amounted to 89 kg CO2-eq/kg FW [133].
Similarly, Byun and Han (2023) conducted a study on environmental impact and analysed the lifecycle inventory of 1 kg of H2 production from FW using simulation model data. It was found that 1 kg of H2 consumed 255 kg of FW and 2.70 kWh of electricity, with direct CO2 emissions from DF and heat production being 45.2 kg. This analysis considers the potential CO2 emissions from conventional FW treatment methods like landfill, incineration, dump, and composting as an alternative to the current treatment process [129]. Aydin and Dincer (2022) also reported a GWP of 0.5 kgCO2-eq/kg H2 from hydrogen production from FW via the DF method [134]. Sun et al. (2019) conducted a study that employed a life-cycle approach to thoroughly evaluate the energy conversion properties and environmental consequences of two-stage biohythane synthesis using microalgae and FW. The system’s overall GHG emissions amounted to 0.173 kgCO2-eq/MJ, mostly driven by power generation, CO2 release in pressurised water, and energy recovery [135].
7. Treatment of Byproducts Generated from Anaerobic Processes: Digested Slurry Management
It can be seen that AD is the most promising and sustainable process for the treatment of organic waste. However, attention should also be paid to the AD/DF process byproducts: the digestate (semi-solid residue) and the effluents (liquid digestate as well as the leachate from composting reactors and maturation field). Digestate, the residue left after anaerobic conversion (AD and DF) of organic waste, poses several challenges in large-scale plant operations. Furthermore, due to the high variability of the volume and composition of the effluent, treatments have been found to be difficult. These include nutrient management, volume and handling, moisture content, pathogens, odour control, regulatory compliance, long-term sustainability, energy consumption, market demand, and feedstock variability [31].
Nutrient management is crucial to prevent environmental pollution when digestate is used as fertilizer. Large-scale biogas/biohydrogen plants must implement efficient digestate management practices, invest in appropriate equipment and technology, and collaborate with regulators, researchers, and the agricultural community to find sustainable solutions for digestate utilisation. Also, it has to be noted that, compared to single-stage anaerobic digestion for the biogas production, the dark fermentation–methanation system increases the overall organic matter utilisation and provides a more stabilised digestate [32,136].
Several means of digestate treatment/management are available. Most often, it is used as a fertilizer [31]. However, seasonality and excess formation in biogas plants necessitate additional tanks for storage. Another process includes thermochemical processing such as gasification, co-pyrolysis, hydrothermal carbonisation (HTC), etc. [30,137]. The demand for alternative digestate markets and land recycling is increasing, with a focus on digestate enhancement technologies like pyrolysis and hydrothermal carbonisation. These technologies aim to enhance the digestion process by converting digestate into carbonaceous solid and liquid fractions [33]. Thermochemical conversion of digestate into energy vectors (solid or gaseous fuel) is a promising solution to waste effluent management issues in biogas plants [137]. Also, anaerobic processes often fail to recover energy and biofuels from biomass, making digestate a promising material for energy production. Therefore, slurry management in biogas plants is an urgent issue.
Among the thermochemical processes, HTC is considered a promising technology for high-moisture biomass due to its unique advantages, such as no pre-drying process, milder reaction conditions, fast reaction rates, and low energy input [138]. HTC is a specific type of hydrothermal treatment process of biomass in a liquid environment, employing elevated temperatures (180–350 °C) and pressures [137,138,139,140]. The procedure yields a solid substance known as hydrochar, which is assumed to mimic the natural coal formation. Hydrochar is frequently utilised as a solid fuel for combustion, but it also serves multiple other purposes, such as soil amendment, energy storage, and absorption [29,138]. Furthermore, the HTC process yields a byproduct known as process water. Thus, integrating HTC as a post-treatment method for AD digestate in order to fully utilise wet organic wastes in a biorefinery-oriented manner is a very promising and emerging technology. Also, HTC is regarded as a potential technique for enhancing digestate by producing a solid hydrochar and process water containing a high level of organic carbon [33].
AD processes have an energy conversion efficiency of 33–50%, with over half remaining in digestate slurry. Digestate slurry treatment aims to reduce volume and recover nutrients in concentrated form. Treatments like HTC improve efficiency and convert organic matter into high-carbon content, producing various products like solid fuel and soil ameliorant [139]. The hydrochar produced via the HTC of AD digestate slurry can enhance soil quality and fertility, but its properties depend on feedstock types and anaerobic digestion conditions due to their heterogenic nature. Though HTC process water can enhance soil and nutrient availability, it can also cause oversalting, affecting plant growth in long-term applications. So, monitoring of soil salinity levels is crucial for mitigation. Strategies include adjusting application rates, using other soil amendments, and using specific irrigation practices [140,141]. By utilising the HTC method, the pathogenic effects of the digestate on human health and the environment can be also mitigated due to the high temperature inactivation [141]. HTC uses high temperatures and pressures to reduce heavy metal bioavailability in biogas digestate slurry, generating hydrochar as renewable energy. This energy can be used in thermal power facilities, reducing fossil fuel use and GHG emissions. Lastly, HTC also efficiently utilises carbonaceous raw materials, saving energy for slurry drying [29,139]. In addition, the hydrochar produced through the HTC process typically has a favourable energy density, allowing it to serve as an additional energy source for the treatment of FW, leading to a circular economy. In recent years, there has been a growing interest in integrating HTC with AD to enhance the energy extraction from a feedstock. Various integration approaches are there, but there is limited information on the most energetically viable route for valorisation [142]. Integrating HTC with AD processes leads to high energy recovery from the biomass valorisation [9,143].
8. Envisaged Futuristic Integrated Bio-Thermo-Chemical Processes: An Innovative Anaerobic Biorefinery Concept
Integrated biorefineries combine multiple processes within a single facility, maximising resource utilisation, enhancing synergies, reducing environmental impact, and incorporating energy recovery processes [144,145]. Thus, integrating bio-chemical with thermo-chemical (i.e., bio-thermo-chemical) processes for waste may lead to higher energy yields and more efficient solutions for biomass valorisation. In particular, combined and integrated thermo-chemical and biological processes for waste can be included under the umbrella of waste biorefineries [146]. They produce a variety of valuable products, including biogas, biohydrogen, biofuels, bio-based chemicals, hydrochar, and nutrient-rich digestate. Waste generation is minimised by using waste materials as feedstock, contributing to a sustainable approach to waste management. Integrated biorefineries also offer economies of scale, flexibility, and adaptability, making the system more economically viable. They also serve as hubs for research and innovation, driving advancements in bioprocesses and technology. However, designing and operating these facilities can be complex and require expertise in multiple disciplines [147,148,149].
Thus, the research should focus on an integrated holistic model to improve resource management sustainability. So, the proposed envisions for integrated processes gives a connected biorefinery system that combines biofuels and co-products with environmentally friendly practices on greener footprints. Figure 8 represents the futuristic sustainable model for integrated bio-thermo-chemical processes for the FW valorisation process. An integration of HTC with two-stage DF-AD can overcome the drawbacks associated with thermal or biological processing alone. The combination of two-stage DF-AD with HTC post-treatment for digestate slurry management creates intricate and diverse methods to transform organic matter into different value-added products while tackling waste management difficulties. The challenge lies in learning to integrate technologies for biological systems with bio-thermo-chemical processes and extending the loops, such as operating parameters across waste management systems. Innovation can create sustainable industrial structures by combining technologies in partnerships and with favourable economics. Understanding the integration of different technologies and the flow of materials across waste management systems is crucial. This integrated knowledge in low-carbon technologies will underpin sustainability and closed-loop business objectives, driving modern bioeconomy aspirations [150,151]. However, this approach is still in its infancy, with most studies conducted at laboratory scale and few at pilot scale. The innovation and industrial sectors should consider this approach to drive modern bioeconomy aspirations.
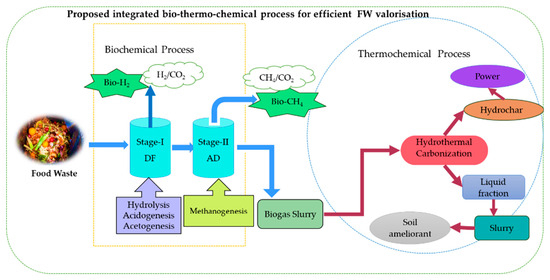
Figure 8.
Conceptualizing the integrated bio-thermo-chemical processes for food waste valorisation for recovery of value-added products.
9. Conclusions and Future Perspectives
Food waste (FW) significantly impacts the environment and society by increasing carbon footprints and depleting resources. Transitioning to circular biorefineries using FW and organic waste valorisation will promote environmental stewardship in bioenergy production and sustainable practices. The growing amount of FW presents opportunities for biofuels, but the type and diverse composition of FW still pose challenges. However, the adoption of integrated biorefineries to utilise FW for maximum energy and product recovery through the combination of biological, chemical, thermal, or electro-chemical processes could be a viable alternative for the future. Single-stage AD of FW is widely studied for its simplicity and economic viability, while two-stage AD/DF-AD processes are promising for producing biofuels like biogas, bio-H2, and biohythane and value-added products. The integration of FW into the bioeconomy requires efficient and cost-effective production methods. Effective FW valorisation pathways can manage and reduce carbon footprints, while government interventions and policies are crucial for a circular economy and sustainable development. Eventually, an integrated bioeconomy for FW management necessitates scientific, social, and political advancements for a cleaner, more sustainable future. Commercial digestate utilisation is challenging due to digestion conditions and limited commercialisation potential of specific technologies.
The integration of hydrothermal and anaerobic processes for simultaneous bioenergy production and digestate management could lead to higher economic outputs. Nevertheless, bottlenecks need to be addressed, including optimising factors, developing low-cost valorisation methods, and slurry post-treatment. This integration could result in production of biogas, bio-H2, biohythane, hydrochar, soil ameliorant, and other value-added products.
The circular bioeconomy helps us by conserving resources for a longer duration of time and also works towards the “waste-to-wealth” concept, which led to the emergence of new technologies, types of employment, and livelihoods, apart from other intrinsic environmental benefits, ultimately achieving zero waste and a significant reduction in emission of greenhouse gases. Hence, the closed-loop system of circular bioeconomy can be used as a tool to ease the reduction in FW and deal with associated remediation in the future. Further, to develop practical biomass valorisation technologies using integration of hydrothermal and biochemical techniques, multi-dimensional analysis of carbon footprints, environmental loads, energy sustainability via life cycle assessment studies, and economic benefits is needed.
Author Contributions
S.S.: conceptualisation; original draft preparation; writing—review and editing; methodology; formal analysis. S.K.: writing—review and editing; formal analysis. L.L.: supervision; conceptualisation; methodology; writing—review and editing; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Shivali Sahota acknowledges the BBCircle project for the grant during which this research was carried out (BBCircle-Biomateriali, Biocombustibili, Sequestro della CO2 e Circolarità. Studio sull’implementabilità di Bioraffinerie nella Regione Lazio-Avviso Pubblico “Gruppi di Ricerca 2020”-Determinazione n. G08487 del 19/07/2020 U and Determinazione n. G10624–POR FESR LAZIO 2014–2020–Progetto n. A0375-2020-36701).
Data Availability Statement
Data will be made available on request.
Acknowledgments
Sobodh Kumar would like to thank Late Ram Chandra, Indian Institute of Technology, Delhi, India, for his guidance and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maalouf, A.; Agamuthu, P. Waste management evolution in the last five decades in developing countries—A review. Waste Manag. Res. 2023, 41, 1420–1434. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/state-of-food-agriculture/2019/en/ (accessed on 5 December 2023).
- Cut Food Waste-China Water Risk. Available online: https://www.chinawaterrisk.org/wp-content/uploads/2022/03/CWR-Report-Together-We-Can-Action-6.pdf (accessed on 1 March 2023).
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Crutchik, D.; Barboza, J.; Vázquez-Padín, J.R.; Pedrouso, A.; Del Río, Á.V.; Mosquera-Corral, A.; Campos, J.L. Integrating food waste management into urban wastewater treatment: Economic and environmental impacts. J. Environ. Manag. 2023, 345, 118517. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing global food waste problem: Pinpointing the facts and estimating the energy content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Nordin; Kaida, N.; Othman, N.A.; Akhir, F.N.M.; Hara, H. Reducing Food Waste: Strategies for Household Waste Management to Minimize the Impact of Climate Change and Contribute to Malaysia’s Sustainable Development. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 7th AUN/SEED-Net Regional Conference on Natural Disaster, Kuala Lumpur, Malaysia, 25–26 November 2019; IOP Publishing: Bristol, UK, 2020; Volume 479, p. 012035. [Google Scholar]
- Edjabou, M.E.; Takou, V.; Boldrin, A.; Petersen, C.; Astrup, T.F. The influence of recycling schemes on the composition and generation of municipal solid waste. J. Clean. Prod. 2021, 295, 126439. [Google Scholar] [CrossRef]
- Ahmed, A.; Li, W.; Varjani, S.; You, S. Waste-to-energy technologies for sustainability: Life-cycle assessment and economic analysis. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 599–612. [Google Scholar]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A critical review on food waste management for the production of materials and biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Tang, R.; Liu, Y.; Ma, R.; Zhang, L.; Li, Y.; Li, G.; Wang, D.; Lin, J.; Li, Q.; Yuan, J. Effect of moisture content, aeration rate, and C/N on maturity and gaseous emissions during kitchen waste rapid composting. J. Environ. Manag. 2023, 326, 116662. [Google Scholar] [CrossRef]
- Harmsen, M.; van Vuuren, D.P.; Bodirsky, B.L.; Chateau, J.; Durand-Lasserve, O.; Drouet, L.; Fricko, O.; Fujimori, S.; Gernaat, D.E.; Hanaoka, T.; et al. The role of methane in future climate strategies: Mitigation potentials and climate impacts. Clim. Chang. 2020, 163, 1409–1425. [Google Scholar] [CrossRef]
- EPA. Wasted Food Scale. 2023. Available online: https://www.epa.gov/sustainable-management-food/wasted-food-scale (accessed on 1 November 2023).
- United Nations. The Sustainable Development Goals Report 2022; United Nations: San Francisco, CA, USA, 2022; pp. 1–68. Available online: https://unstats.un.org/sdgs/report/2022/The-Sustainable-Development-Goals-Report-2022.pdf (accessed on 1 August 2023).
- IEA. Tracking Clean Energy Progress 2023; IEA: Paris, France, 2023; License: CC BY 4.0; Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 30 August 2023).
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, D.; Mohanty, P.; Sarangi, P.K. Biochemical and thermochemical routes of H2 production from food waste: A comparative review. Chem. Eng. Technol. 2023, 46, 191–203. [Google Scholar] [CrossRef]
- Dangol, S.; Ghimire, A.; Tuladhar, S.; Khadka, A.; Thapa, B.; Sapkota, L. Biohythane and organic acid production from food waste by two-stage anaerobic digestion: A review within biorefinery framework. Int. J. Environ. Sci. Technol. 2022, 19, 12791–12824. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food waste biorefinery: Pathway towards circular bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2021, 19, 3377–3400. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Ferraren-De Cagalitan, D.D.T.; Abundo, M.L.S. A review of biohydrogen production technology for application towards hydrogen fuel cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- D’Silva, T.C.; Khan, S.A.; Kumar, S.; Kumar, D.; Isha, A.; Deb, S.; Yadav, S.; Illathukandy, B.; Chandra, R.; Vijay, V.K.; et al. Biohydrogen production through dark fermentation from waste biomass: Current status and future perspectives on biorefinery development. Fuel 2023, 350, 128842. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent developments in biohythane production from household food wastes: A review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrogen Energy 2019, 44, 17363–17380. [Google Scholar] [CrossRef]
- Meena, R.A.A.; Banu, J.R.; Kannah, R.Y.; Yogalakshmi, K.N.; Kumar, G. Biohythane production from food processing wastes–challenges and perspectives. Bioresour. Technol. 2020, 298, 122449. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Trably, E.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G.; Cazier, E.A.; Escudié, R. Effect of total solids content on biohydrogen production and lactic acid accumulation during dark fermentation of organic waste biomass. Bioresour. Technol. 2018, 248, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, T.; Subramanian, S. Biohythane as a high potential fuel from anaerobic digestion of organic waste: A review. Renew. Sustain. Energy Rev. 2021, 152, 111700. [Google Scholar] [CrossRef]
- Wilk, M.; Gajek, M.; Śliz, M.; Czerwińska, K.; Lombardi, L. Hydrothermal Carbonization Process of Digestate from Sewage Sludge: Chemical and Physical Properties of Hydrochar in Terms of Energy Application. Energies 2022, 15, 6499. [Google Scholar] [CrossRef]
- Timofeeva, S.S.; Karaeva, J.V.; Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V. Steam gasification of digestate after anaerobic digestion and dark fermentation of lignocellulosic biomass to produce syngas with high hydrogen content. Int. J. Hydrogen Energy 2023, 48, 7559–7568. [Google Scholar] [CrossRef]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; d’Imporzano, G.; Adani, F. Short-term experiments in using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Albini, E.; Pecorini, I.; Ferrara, G. Improvement of digestate stability using dark fermentation and anaerobic digestion processes. Energies 2019, 12, 3552. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion; Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic digestion of food waste—A short review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Kabir, S.B.; Khalekuzzaman, M.; Hossain, N.; Jamal, M.; Alam, M.A.; Abomohra, A.E.F. Progress in biohythane production from microalgae-wastewater sludge co-digestion: An integrated biorefinery approach. Biotechnol. Adv. 2022, 57, 107933. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Rouez, M.; Crest, M.; Steyer, J.P.; Delgenès, J.P.; Escudié, R. Food waste valorization via anaerobic processes: A review. Rev. Environ. Sci. Bio/Technol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- Sanchez Lopez, J.; Patinha Caldeira, C.; De Laurentiis, V.; Sala, S.; Avraamides, M. Brief on Food Waste in the European Union; Avraamides, M., Ed.; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Sahota, S.; Vijay, V.K.; Subbarao, P.M.V.; Chandra, R.; Ghosh, P.; Shah, G.; Kapoor, R.; Vijay, V.; Koutu, V.; Thakur, I.S. Characterization of leaf waste based biochar for cost effective hydrogen sulphide removal from biogas. Bioresour. Technol. 2018, 250, 635–641. [Google Scholar] [CrossRef]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: An overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Kumar, S.; Gandhi, P.; Yadav, M.; Paritosh, K.; Pareek, N.; Vivekanand, V. Weak alkaline treatment of wheat and pearl millet straw for enhanced biogas production and its economic analysis. Renew. Energy 2019, 139, 753–764. [Google Scholar] [CrossRef]
- Kumar, S.; D’Silva, T.C.; Chandra, R.; Malik, A.; Vijay, V.K.; Misra, A. Strategies for boosting biomethane production from rice straw: A systematic review. Bioresour. Technol. Rep. 2021, 15, 100813. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Velu, C.; Rajesh Banu, J.; Heimann, K.; Karthikeyan, O.P. Food waste valorization by microalgae. In Waste to Wealth; Springer: Singapore, 2018; pp. 319–342. [Google Scholar]
- Dahiya, S.; Kumar, A.N.; Sravan, J.S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Assis, T.I.; Gonçalves, R.F. Valorization of food waste by anaerobic digestion: A bibliometric and systematic review focusing on optimization. J. Environ. Manag. 2022, 320, 115763. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Sun, L.; Douieb, Y.; Sun, J.; Luo, W. Anaerobic digestion of municipal solid waste composed of food waste, wastepaper, and plastic in a single-stage system: Performance and microbial community structure characterization. Bioresour. Technol. 2013, 146, 619–627. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, Y.Y.; Liu, H.C.; Chen, T.C.; Hung, C.H.; Chen, C.H.; Ong, H.C. A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl. Energy 2019, 237, 283–291. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, Y.Y.; Liu, H.C.; Chen, T.C.; Hung, H.C.; Chen, C.H. Analysis of physicochemical properties of liquefaction bio-oil from food waste. Energy Procedia 2019, 158, 61–66. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S. Steam gasification with torrefaction as pretreatment to enhance syngas production from mixed food waste. J. Environ. Chem. Eng. 2021, 9, 104722. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, H.; Yao, D.; Zhang, J.; Zhu, Z.; Wang, Y.; Cui, P. Modeling and comprehensive analysis of food waste gasification process for hydrogen production. Energy Convers. Manag. 2022, 258, 115509. [Google Scholar] [CrossRef]
- Deheri, C.; Acharya, S.K. An experimental approach to produce hydrogen and methane from food waste using catalyst. Int. J. Hydrogen Energy 2020, 45, 17250–17259. [Google Scholar] [CrossRef]
- Park, C.; Lee, N.; Kim, J.; Lee, J. Co-pyrolysis of food waste and wood bark to produce hydrogen with minimizing pollutant emissions. Environ. Pollut. 2021, 270, 116045. [Google Scholar] [CrossRef]
- Chang, J.I.; Hsu, T.E. Effects of compositions on food waste composting. Bioresour. Technol. 2008, 99, 8068–8074. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Chang, J.I.; Chen, Y.J. Effects of bulking agents on food waste composting. Bioresour. Technol. 2010, 101, 5917–5924. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Han, W.; Ye, M.; Zhu, A.J.; Zhao, H.T.; Li, Y.F. Batch dark fermentation from enzymatic hydrolyzed food waste for hydrogen production. Bioresour. Technol. 2015, 191, 24–29. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Zhang, Z.; O’Hara, I.M.; Mundree, S.; Gao, B.; Ball, A.S.; Zhu, N.; Bai, Z.; Jin, B. Biofuels from food processing wastes. Curr. Opin. Biotechnol. 2016, 38, 97–105. [Google Scholar] [CrossRef]
- Lytras, G.; Lytras, C.; Mathioudakis, D.; Papadopoulou, K.; Lyberatos, G. Food waste valorization based on anaerobic digestion. Waste Biomass Valorization 2021, 12, 1677–1697. [Google Scholar] [CrossRef]
- Torsha, T. A Novel Approach for Anaerobic Treatment of Food Waste under Psychrophilic Temperature. Ph.D. Thesis, Concordia University, Concordia, CA, USA, 2023. [Google Scholar]
- He, Q.; Li, L.; Zhao, X.; Qu, L.; Wu, D.; Peng, X. Investigation of foaming causes in three mesophilic food waste digesters: Reactor performance and microbial analysis. Sci. Rep. 2017, 7, 13701. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Patel, S.K.; Das, D.; Kim, S.C.; Cho, B.K.; Kalia, V.C.; Lee, J.K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Factories 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, M.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Arun, J.; Sasipraba, T.; Gopinath, K.P.; Priyadharsini, P.; Nachiappan, S.; Nirmala, N.; Dawn, S.S.; Chi, N.T.L.; Pugazhendhi, A. Influence of biomass and nanoadditives in dark fermentation for enriched bio-hydrogen production: A detailed mechanistic review on pathway and commercialization challenges. Fuel 2022, 327, 125112. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Poggi-Varaldo, H.M. Hydrogen production by fermentative consortia. Renew. Sustain. Energy Rev. 2009, 13, 1000–1013. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Shin, H.S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Lens, P.N.; Pirozzi, F.; Esposito, G. Biohythane production from food waste in a two-stage process: Assessing the energy recovery potential. Environ. Technol. 2022, 43, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Modestra, J.A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Waste-Derived Renewable Hydrogen and Methane: Towards a Potential Energy Transition Solution. Fermentation 2023, 9, 368. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: A long-term pilot scale experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Parajuli, A.; Khadka, A.; Sapkota, L.; Ghimire, A. Effect of hydraulic retention time and organic-loading rate on two-staged, semi-continuous mesophilic anaerobic digestion of food waste during start-up. Fermentation 2022, 8, 620. [Google Scholar] [CrossRef]
- Sambusiti, C.; Monlau, F.; Ficara, E.; Musatti, A.; Rollini, M.; Barakat, A.; Malpei, F. Comparison of various post-treatments for recovering methane from agricultural digestate. Fuel Process. Technol. 2015, 137, 359–365. [Google Scholar] [CrossRef]
- Phanduang, O.; Lunprom, S.; Salakkam, A.; Liao, Q.; Reungsang, A. Improvement in energy recovery from Chlorella sp. biomass by integrated dark-photo biohydrogen production and dark fermentation-anaerobic digestion processes. Int. J. Hydrogen Energy 2019, 44, 23899–23911. [Google Scholar] [CrossRef]
- Han, S.K.; Shin, H.S. Performance of an innovative two-stage process converting food waste to hydrogen and methane. J. Air Waste Manag. Assoc. 2004, 54, 242–249. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef]
- Ayodele, D.T.; Ogunbiyi, O.D.; Akamo, D.O.; Otun, K.O.; Akinpelu, D.A.; Adegoke, J.A.; Fapojuwo, D.P.; Oladoye, P.O. Factors affecting biohydrogen production: Overview and perspectives. Int. J. Hydrogen Energy 2023, 48, 27513–27539. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste–A review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Yun, S.; Fang, W.; Du, T.; Hu, X.; Huang, X.; Li, X.; Zhang, C.; Lund, P.D. Use of bio-based carbon materials for improving biogas yield and digestate stability. Energy 2018, 164, 898–909. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef]
- Agyeman, F.O.; Tao, W. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Ultrasonication for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 2896–2903. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Shin, H.S.; Youn, J.H.; Kim, S.H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, H.S. Effects of base-pretreatment on continuous enriched culture for hydrogen production from food waste. Int. J. Hydrogen Energy 2008, 33, 5266–5274. [Google Scholar] [CrossRef]
- Kim, D.H.; Wu, J.; Jeong, K.W.; Kim, M.S.; Shin, H.S. Natural inducement of hydrogen from food waste by temperature control. Int. J. Hydrogen Energy 2011, 36, 10666–10673. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.C. A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int. J. Hydrogen Energy 2009, 34, 245–254. [Google Scholar] [CrossRef]
- Chu, C.F.; Li, Y.Y.; Xu, K.Q.; Ebie, Y.; Inamori, Y.; Kong, H.N. A pH-and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrogen Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Jung, K.W.; Moon, C.; Cho, S.K.; Kim, S.H.; Shin, H.S.; Kim, D.H. Conversion of organic solid waste to hydrogen and methane by two-stage fermentation system with reuse of methane fermenter effluent as diluting water in hydrogen fermentation. Bioresour. Technol. 2013, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yeshanew, M.M.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G. Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresour. Technol. 2016, 220, 312–322. [Google Scholar] [CrossRef]
- Kobayashi, T.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen–methane fermentation process treating food wastes. Int. J. Hydrogen Energy 2012, 37, 5602–5611. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen production from food waste in batch and semi-continuous conditions: Evaluation of a two-phase approach with digestate recirculation for pH control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Ricci, M.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew. Energy 2019, 130, 1108–1115. [Google Scholar] [CrossRef]
- Chu, C.F.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Hydrogen and methane potential based on the nature of food waste materials in a two-stage thermophilic fermentation process. Int. J. Hydrogen Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Lee, D.Y.; Ebie, Y.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Continuous H2 and CH4 production from high-solid food waste in the two-stage thermophilic fermentation process with the recirculation of digester sludge. Bioresour. Technol. 2010, 101, S42–S47. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef]
- Pisutpaisal, N.; Nathao, C.; Sirisukpoka, U. Biological hydrogen and methane production in from food waste in two-stage CSTR. Energy Procedia 2014, 50, 719–722. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process: Substrate conversion and energy yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef]
- Nathao, C.; Sirisukpoka, U.; Pisutpaisal, N. Production of hydrogen and methane by one and two stage fermentation of food waste. Int. J. Hydrogen Energy 2013, 38, 15764–15769. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Biohythane Production in Hydrogen-Oriented Dark Fermentation of Aerobic Granular Sludge (AGS) Pretreated with Solidified Carbon Dioxide (SCO2). Int. J. Mol. Sci. 2023, 24, 4442. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ng, K.H.; Vo, D.V.N.; Lam, M.K.; Lim, J.W. Bio-hydrogen production from steam reforming of liquid biomass wastes and biomass-derived oxygenates: A review. Fuel 2022, 311, 122623. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; Van Hulle, S.W.; Rousseau, D.P.; Garfí, M. Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: Natural pigments, biofertilizer and biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef]
- Marangon, B.B.; Magalhães, I.B.; Pereira, A.S.A.P.; Silva, T.A.; Gama, R.C.N.; Ferreira, J.; Castro, J.S.; Assis, L.R.; Lorentz, J.F.; Calijuri, M.L. Emerging microalgae-based biofuels: Technology, life-cycle and scale-up. Chemosphere 2023, 326, 138447. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.R.; Schmidt, J.E. Increasing profits in food waste biorefinery—A techno-economic analysis. Energies 2018, 11, 1551. [Google Scholar] [CrossRef]
- Ljunggren, M.; Zacchi, G. Techno-economic analysis of a two-step biological process producing hydrogen and methane. Bioresour. Technol. 2010, 101, 7780–7788. [Google Scholar] [CrossRef] [PubMed]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Theaker, H. Can Micro-Scale Anaerobic Digestion Be Viable? An Investigation of the Techno-Economics and Flexibility of Micro-scale Anaerobic Digestion for Future Routes to the Market. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2020. [Google Scholar]
- Al Naami, A. Techno-economic Feasibility Study of a Biogas Plant for Treating Food Waste Collected from Households in Kartamantul Region, Yogyakarta. Master’s Thesis, Gadjah Mada University, Yogyakarta, Indonesia, 2017. [Google Scholar]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Din, M.F.M.; Taib, S.M.; Ling, Y.E.; Puteh, H.; Mishra, P.; Nasrullah, M.; Sakinah, M.; Wahid, Z.A.; Rana, S.; et al. Process constraints in sustainable bio-hythane production from wastewater. Bioresour. Technol. Rep. 2019, 5, 359–363. [Google Scholar] [CrossRef]
- Dinesh, G.K.; Chauhan, R.; Chakma, S. Influence and strategies for enhanced biohydrogen production from food waste. Renew. Sustain. Energy Rev. 2018, 92, 807–822. [Google Scholar] [CrossRef]
- Byun, J.; Han, J.H. Economic feasible hydrogen production system from carbohydrate-rich food waste. Appl. Energy 2023, 340, 121044. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Sneha, N.P.; Rafi, S.M.; Sarkar, O. Dark fermentative hydrogen production: Potential of food waste as future energy needs. Sci. Total Environ. 2023, 888, 163801. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Prommuak, C.; Putmai, N.; Pavasant, P. Scaling-up bio-hydrogen production from food waste: Feasibilities and challenges. Int. J. Hydrogen Energy 2018, 43, 634–648. [Google Scholar] [CrossRef]
- Takata, M.; Fukushima, K.; Kawai, M.; Nagao, N.; Niwa, C.; Yoshida, T.; Toda, T. The choice of biological waste treatment method for urban areas in Japan—An environmental perspective. Renew. Sustain. Energy Rev. 2013, 23, 557–567. [Google Scholar] [CrossRef]
- Moult, J.A.; Allan, S.R.; Hewitt, C.N.; Berners-Lee, M. Greenhouse gas emissions of food waste disposal options for UK retailers. Food Policy 2018, 77, 50–58. [Google Scholar] [CrossRef]
- Aydin, M.I.; Dincer, I. A life cycle impact analysis of various hydrogen production methods for public transportation sector. Int. J. Hydrogen Energy 2022, 47, 39666–39677. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X. Life-cycle assessment of biohythane production via two-stage anaerobic fermentation from microalgae and food waste. Renew. Sustain. Energy Rev. 2019, 112, 395–410. [Google Scholar] [CrossRef]
- Schievano, A.; Sciarria, T.P.; Gao, Y.C.; Scaglia, B.; Salati, S.; Zanardo, M.; Quiao, W.; Dong, R.; Adani, F. Dark fermentation, anaerobic digestion and microbial fuel cells: An integrated system to valorize swine manure and rice bran. Waste Manag. 2016, 56, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Mikusińska, J.; Kuźnia, M.; Czerwińska, K.; Wilk, M. Hydrothermal Carbonization of Digestate Produced in the Biogas Production Process. Energies 2023, 16, 5458. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate management and processing practices: A review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling hydrothermal carbonization and anaerobic digestion for sewage digestate management: Influence of hydrothermal treatment time on dewaterability and bio-methane production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, S.; Wang, R.; Li, H.; Song, G.; Li, H.; Yin, Q. Life cycle assessment of food waste energy and resource conversion scheme via the integrated process of anaerobic digestion and hydrothermal carbonization. Int. J. Hydrogen Energy 2023, 52, 122–132. [Google Scholar] [CrossRef]
- Alibardi, L.; Astrup, T.F.; Asunis, F.; Clarke, W.P.; De Gioannis, G.; Dessì, P.; Lens, P.N.; Lavagnolo, M.C.; Lombardi, L.; Muntoni, A.; et al. Organic waste biorefineries: Looking towards implementation. Waste Manag. 2020, 114, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sharma, G.D.; Cysneiros, D.; Nayak, S.C.; Thakur, V.K.; Naidu, R.; Pandey, A.; Gupta, V.K. Minimizing hazardous impact of food waste in a circular economy–Advances in resource recovery through green strategies. J. Hazard. Mater. 2021, 416, 126154. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food waste utilization for reducing carbon footprints towards sustainable and cleaner environment: A review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Badawi, M.; Bonilla-Petriciolet, A.; Aminabhavi, T.M. Valorization of biowastes for clean energy production, environmental depollution and soil fertility. J. Environ. Manag. 2023, 332, 117410. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.N.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S. Integrated microalgal biorefinery–Routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).