Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana
Abstract
1. Introduction
1.1. Organic Waste
1.2. Enzymes
1.3. Hydorgen Production from Household Biowaste
1.4. Thermotoga Neapolitana
2. Materials and Methods
2.1. Examination of the Enzymes on Different Substrates
2.2. Pretreatment and Cultivation of Organic Waste
2.3. Cultivation and Strain Maintenance of T. neapolitana
2.4. Fermentation in the Anaerobic Bioreactor
2.5. Analysis of the Fluid Products
2.6. Analysis of the Gas
2.7. Calculation of the Yields
3. Results and Discussion
3.1. Hydrolysis Efficiency of the Enzymes
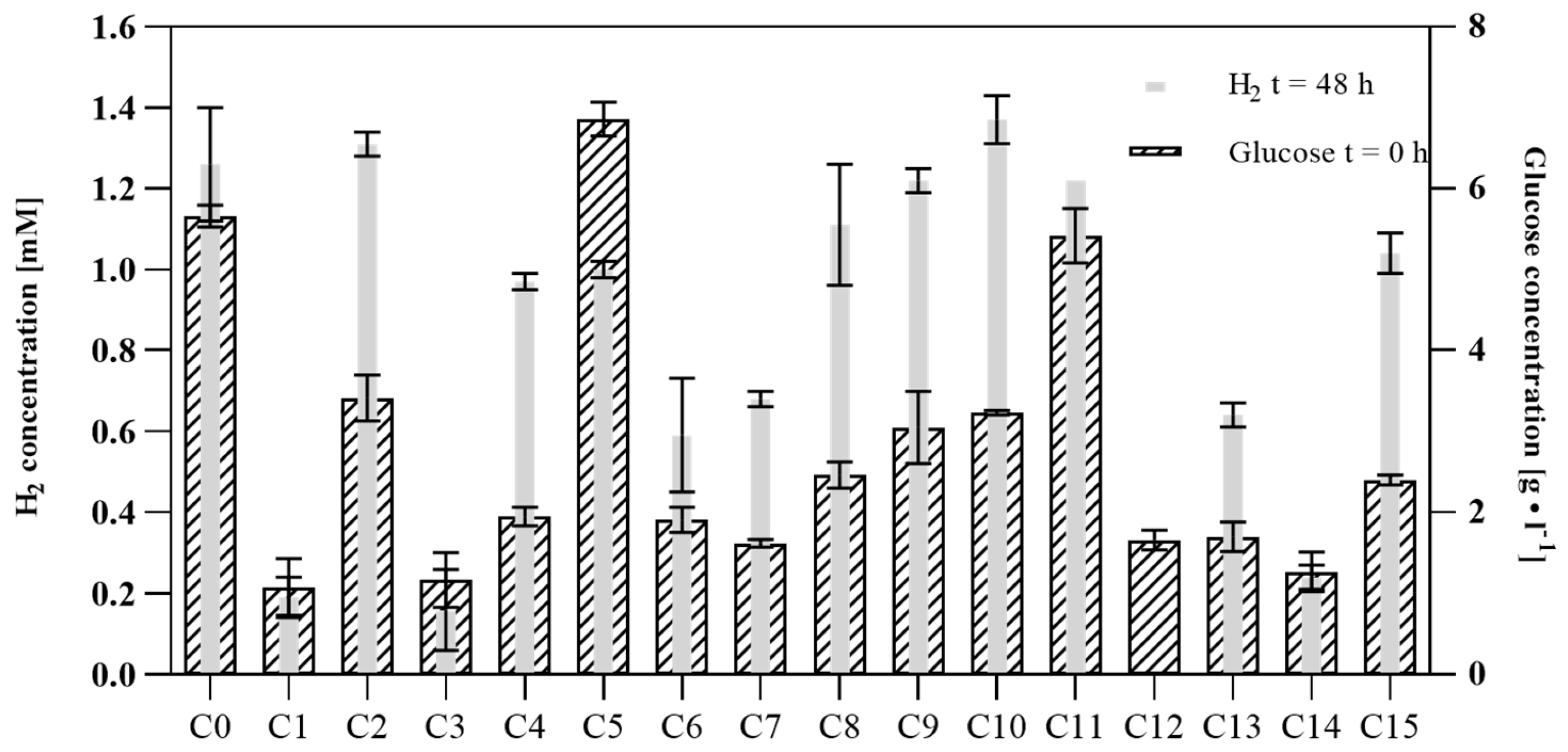
3.2. Set Fermentation with Hydrolyzate
3.3. Fermentation with Hydrolyzate in Anaerobic Bioreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Choi, H.S.; Park, C.; Kim, S.W. Current States and Prospects of Organic Waste Utilization for Biorefineries. Renew. Sustain. Energy Rev. 2015, 49, 335–349. [Google Scholar] [CrossRef]
- Esercizio, N.; Lanzilli, M.; Vastano, M.; Landi, S.; Xu, Z.; Gallo, C.; Nuzzo, G.; Manzo, E.; Fontana, A.; d’Ippolito, G. Fermentation of Biodegradable Organic Waste by the Family Thermotogaceae. Resources 2021, 10, 34. [Google Scholar] [CrossRef]
- Munro, S.A.; Zinder, S.H.; Walker, L.P. The Fermentation Stoichiometry of Thermotoga neapolitana and Influence of Temperature, Oxygen, and pH on Hydrogen Production. Biotechnol. Prog. 2009, 25, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.Y.; Calay, R.K.; Román, E. Biogas from Organic Waste—A Case Study. Procedia Eng. 2016, 146, 310–317. [Google Scholar] [CrossRef]
- Grim, J.; Malmros, P.; Schnürer, A.; Nordberg, Å. Comparison of Pasteurization and Integrated Thermophilic Sanitation at a Full-Scale Biogas Plant—Heat Demand and Biogas Production. Energy 2015, 79, 419–427. [Google Scholar] [CrossRef]
- Tippkötter, N. Grundlagen der biochemischen Umwandlung. In Energie aus Biomasse; Kaltschmitt, M., Hartmann, H., Hofbauer, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1447–1500. ISBN 978-3-662-47437-2. [Google Scholar]
- Gopinath, S.C.B.; Anbu, P.; Arshad, M.K.M.; Lakshmipriya, T.; Voon, C.H.; Hashim, U.; Chinni, S.V. Biotechnological Processes in Microbial Amylase Production. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and Industrial Applications of α-Amylase: A Review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W. Enzyme Research and Applications in Biotechnological Intensification of Biogas Production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef]
- Lipiński, K.; Umiejewska, K. Application of Multi-Enzymatic Hydrolysis for Improving the Efficiency of the Biogas Production in Solid Waste Fermentation Process in Ostróda WWTP. E3S Web Conf. 2017, 22, 00105. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Dark-Fermentative Biological Hydrogen Production from Mixed Biowastes Using Defined Mixed Cultures. Indian J. Microbiol. 2017, 57, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Nabgan, W.; Tuan Abdullah, T.A.; Nabgan, B.; Jalil, A.A.; Nordin, A.H.; Ul-Hamid, A.; Hassan, N.S.; Hussain, I.; Coelho, A.; Amin, A.; et al. Catalytic Biohydrogen Production from Organic Waste Materials: A Literature Review and Bibliometric Analysis. Int. J. Hydrogen Energy 2021, 46, 30903–30925. [Google Scholar] [CrossRef]
- 14,4 Millionen Tonnen Bioabfälle Im Jahr 2020. Available online: https://www.destatis.de/DE/Presse/Pressemitteilungen/2022/09/PD22_371_321.html (accessed on 29 November 2022).
- Mayer, F.; Bhandari, R.; Gäth, S.A.; Himanshu, H.; Stobernack, N. Economic and Environmental Life Cycle Assessment of Organic Waste Treatment by Means of Incineration and Biogasification. Is Source Segregation of Biowaste Justified in Germany? Sci. Total Environ. 2020, 721, 137731. [Google Scholar] [CrossRef] [PubMed]
- Verordnung (EU) Nr. 142/2011 der Kommission vom 25. Februar 2011 zur Durchführung der Verordnung (EG) Nr. 1069/2009 des Europäischen Parlaments und des Rates mit Hygienevorschriften für Nicht für den Menschlichen Verzehr Bestimmte Tierische Nebenprodukte Sowie zur Durchführung der Richtlinie 97/78/EG des Rates Hinsichtlich Bestimmter Gemäß der Genannten Richtlinie von Veterinärkontrollen an der Grenze befreiter Proben und WarenText von Bedeutung für den EWR. p. 254. Available online: https://eur-lex.europa.eu/legal-content/de/TXT/?uri=CELEX%3A32011R0142 (accessed on 30 November 2022).
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Overview of Hygienization Pretreatment for Pasteurization and Methane Potential Enhancement of Biowaste: Challenges, State of the Art and Alternative Technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Application of Hydrolytic Enzymes in Biorefinery and Its Future Prospects. In Microbial Strategies for Techno-Economic Biofuel Production; Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Eds.; Clean Energy Production Technologies; Springer: Singapore, 2020; pp. 59–83. ISBN 9789811571893. [Google Scholar]
- Bhati, N.; Shreya; Sharma, A.K. Cost-effective Cellulase Production, Improvement Strategies, and Future Challenges. J. Food Process Eng. 2021, 44, e13623. [Google Scholar] [CrossRef]
- Nidhi, C.; Sharma, B.; Singh, P.K. Energy Value in Biomass and Plastic Components of Municipal Solid Wast. MATTER Int. J. Sci. Technol. 2017, 13, 80–92. [Google Scholar] [CrossRef]
- Shahzadi, T.; Mehmood, S.; Irshad, M.; Anwar, Z.; Afroz, A.; Zeeshan, N.; Rashid, U.; Sughra, K. Advances in Lignocellulosic Biotechnology: A Brief Review on Lignocellulosic Biomass and Cellulases. Adv. Biosci. Biotechnol. 2014, 5, 246–251. [Google Scholar] [CrossRef]
- Rajnish, K.N.; Samuel, M.S.; John, J.A.; Datta, S.; Chandrasekar, N.; Balaji, R.; Jose, S.; Selvarajan, E. Immobilization of Cellulase Enzymes on Nano and Micro-Materials for Breakdown of Cellulose for Biofuel Production-a Narrative Review. Int. J. Biol. Macromol. 2021, 182, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- De Vrije, T.; Bakker, R.R.; Budde, M.A.; Lai, M.H.; Mars, A.E.; Claassen, P.A. Efficient Hydrogen Production from the Lignocellulosic Energy Crop Miscanthus by the Extreme Thermophilic Bacteria Caldicellulosiruptor Saccharolyticus and Thermotoga Neapolitana. Biotechnol. Biofuels 2009, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Sundarram, A.; Murthy, T.P.K. α-Amylase Production and Applications: A Review. Environ. Microbiol. 2014, 10, 166–175. [Google Scholar]
- Kamon, M.; Sumitani, J.; Tani, S.; Kawaguchi, T.; Kamon, M.; Sumitani, J.; Tani, S.; Kawaguchi, T. Characterization and Gene Cloning of a Maltotriose-Forming Exo-Amylase from Kitasatospora Sp. MK-1785. Appl. Microbiol. Biotechnol. 2015, 99, 4743–4753. [Google Scholar] [CrossRef]
- Chen, P.; Xu, R.; Wang, J.; Wu, Z.; Yan, L.; Zhao, W.; Liu, Y.; Ma, W.; Shi, X.; Li, H. Starch Biotransformation into Isomaltooligosaccharides Using Thermostable Alpha-Glucosidase from Geobacillus stearothermophilus. PeerJ 2018, 6, e5086. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Dai, Y.; Villani, T.; Giurleo, D.; Simon, J.E.; Wu, Q. Synthesis of Novel Flavonoid Alkaloids as α-Glucosidase Inhibitors. Bioorganic Med. Chem. 2017, 25, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Wei, X. Sugar Compositions, α-Glucosidase Inhibitory and Amylase Inhibitory Activities of Polysaccharides from Leaves and Flowers of Camellia Sinensis Obtained by Different Extraction Methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Safran, J.; Habrylo, O.; Cherkaoui, M.; Lecomte, S.; Voxeur, A.; Pilard, S.; Bassard, S.; Pau-Roblot, C.; Mercadante, D.; Pelloux, J.; et al. New Insights into the Specificity and Processivity of Two Novel Pectinases from Verticillium Dahliae. Int. J. Biol. Macromol. 2021, 176, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Rahman, M.S.; Qin, W. New Insights in Pectinase Production Development and Industrial Applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kognou, A.L.M.; Zhang, J.; Qin, W. Different Facets of Lignocellulosic Biomass Including Pectin and Its Perspectives. Waste Biomass Valor. 2021, 12, 4805–4823. [Google Scholar] [CrossRef]
- Rehman, H.U.; Baloch, A.H.; Nawaz, M.A. Pectinase: Immobilization and Applications (A Review). Trends Pept. Protein Sci. 2021, 6, 16. [Google Scholar]
- Yadav, S.; Yadav, P.K.; Yadav, D.; Yadav, K.D.S. Pectin Lyase: A Review. Process Biochem. 2009, 44, 1–10. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, Xylanase Families and Extremophilic Xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Sharma, D.; Chaudhary, R.; Kaur, J.; Arya, S.K. Greener Approach for Pulp and Paper Industry by Xylanase and Laccase. Biocatal. Agric. Biotechnol. 2020, 25, 101604. [Google Scholar] [CrossRef]
- Prade, R.A. Xylanases: From Biology to BioTechnology. Biotechnol. Genet. Eng. Rev. 1996, 13, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Maulana Hidayatullah, I.; Setiadi, T.; Tri Ari Penia Kresnowati, M.; Boopathy, R. Xylanase Inhibition by the Derivatives of Lignocellulosic Material. Bioresour. Technol. 2020, 300, 122740. [Google Scholar] [CrossRef] [PubMed]
- El Asri, O.; Fadlaoui, S.; Ramdani, M.; Errochdi, S. Microbial Degradation of Biowaste for Hydrogen Production. In Recent Advances in Microbial Degradation; Inamuddin, Ahamed, M.I., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 431–447. ISBN 9789811605178. [Google Scholar]
- Michalopoulos, I.; Lytras, G.M.; Mathioudakis, D.; Lytras, C.; Goumenos, A.; Zacharopoulos, I.; Papadopoulou, K.; Lyberatos, G. Hydrogen and Methane Production from Food Residue Biomass Product (FORBI). Waste Biomass Valor. 2020, 11, 1647–1655. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable Hydrogen Production by Dark-Fermentation: Current Status, Challenges and Perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the Potential of Bio-Hydrogen Production by Fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Talapko, D.; Talapko, J.; Erić, I.; Škrlec, I. Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation. Energies 2023, 16, 3321. [Google Scholar] [CrossRef]
- Moretti, P.; Morais De Araujo, J.; Borges De Castilhos, A.; Buffière, P.; Gourdon, R.; Bayard, R. Characterization of Municipal Biowaste Categories for Their Capacity to Be Converted into a Feedstock Aqueous Slurry to Produce Methane by Anaerobic Digestion. Sci. Total Environ. 2020, 716, 137084. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Gupta, R.S. Molecular Signatures for the Phylum (Class) Thermotogae and a Proposal for Its Division into Three Orders (Thermotogales, Kosmotogales ord. nov. and Petrotogales ord. nov.) Containing Four Families (Thermotogaceae, Fervidobacteriaceae fam. nov., Kosmotogaceae fam. nov. and Petrotogaceae fam. nov.) and a New Genus Pseudothermotoga gen. nov. with Five New Combinations. Antonie Van Leeuwenhoek 2014, 105, 143–168. [Google Scholar] [CrossRef]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.-H.; Stackebrandt, E. (Eds.) The Prokaryotes; Springer: New York, NY, USA, 2006; ISBN 978-0-387-25497-5. [Google Scholar]
- Dipasquale, L.; d’Ippolito, G.; Fontana, A. Capnophilic Lactic Fermentation and Hydrogen Synthesis by Thermotoga Neapolitana: An Unexpected Deviation from the Dark Fermentation Model. Int. J. Hydrogen Energy 2014, 39, 4857–4862. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Landi, S.; Esercizio, N.; Lanzilli, M.; Vastano, M.; Dipasquale, L.; Pradhan, N.; Fontana, A. CO2-Induced Transcriptional Reorganization: Molecular Basis of Capnophillic Lactic Fermentation in Thermotoga Neapolitana. Front. Microbiol. 2020, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T.; Nielsen, T.M.; Iversen, N. Hydrogen Production in Anaerobic and Microaerobic Thermotoga Neapolitana. Biotechnol. Lett. 2007, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Selig, M.; Schönheit, P. Glucose Fermentation to Acetate, CO2 and H2 in the Anaerobic Hyperthermophilic Eubacterium Thermotoga Maritima: Involvement of the Embden-Meyerhof Pathway. Arch. Microbiol. 1994, 161, 460–470. [Google Scholar] [CrossRef]
- Childers, S.E.; Vargas, M.; Noll, K.M. Improved Methods for Cultivation of the Extremely Thermophilic Bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 1992, 58, 3949–3953. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tewari, R.; Rana, S.S.; Soni, R.; Soni, S.K. Cellulases: Classification, Methods of Determination and Industrial Applications. Appl. Biochem. Biotechnol. 2016, 179, 1346–1380. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The Role of Pretreatment in Improving the Enzymatic Hydrolysis of Lignocellulosic Materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Nguyen, T.; Pyokim, J.; Sunkim, M.; Kwanoh, Y.; Sim, S. Optimization of Hydrogen Production by Hyperthermophilic Eubacteria, Thermotoga Maritima and Thermotoga Neapolitana in Batch Fermentation. Int. J. Hydrogen Energy 2008, 33, 1483–1488. [Google Scholar] [CrossRef]
- Khan, A.A.; Vincent, J.F.V. Mechanical Damage Induced by Controlled Freezing in Apple and Potato. J. Texture Stud. 1996, 27, 143–157. [Google Scholar] [CrossRef]
- Wasserstoff. Available online: https://gestis.dguv.de/data?name=007010 (accessed on 30 November 2022).
- Dreschke, G.; d’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Enhancement of Hydrogen Production Rate by High Biomass Concentrations of Thermotoga Neapolitana. Int. J. Hydrogen Energy 2018, 43, 13072–13080. [Google Scholar] [CrossRef]




| Enzyme | Concentration |
|---|---|
| Cellulase TXL | 10 µL·g−1 |
| Amylase FL | 0.2 µL·g−1 |
| Glucoamylase | 0.2 µL·g−1 |
| Pectinase | 0.33 µL·g−1 |
| Xylanase 2 | 50 µg·g−1 |
| Sample | Substrate |
|---|---|
| 1 | Compost from garden and household waste—collection summer/fall 2021 (2–6 months old) |
| 2 | Organic waste (1 week old) |
| 3 | Garden cuttings, spring cuttings—winter grasses and hedge cuttings (1 month old) |
| 4 | Garden cuttings, autumn—grass clippings and leaves (5 months old) |
| 5 | Mashed potatoes |
| 6 | Vegetable waste—carrots and asparagus |
| 7 | Vegetable waste—lettuce, peppers, carrots, cucumber |
| 8 | Organic waste from municipal waste management, sample 1.1—food residues mainly cabbage |
| 9 | Organic waste from municipal waste management, sample 1.2—food residues esp. spring onion, potato peel, cabbage |
| 10 | Organic waste from municipal waste management, sample 1.3—food residues, mainly spring onion, potato peel, cabbage |
| 11 | Organic waste from municipal waste management, sample 2.1—bread rolls, cucumber, potato peels, Thuja hedge trimmings |
| 12 | Organic waste from municipal waste management, sample 2.3—Thuja hedge trimmings |
| 13 | Organic waste from municipal waste management, sample 3.1—branch cuttings |
| 14 | Lawn cutting |
| 15 | Press cake lawn cut—Honroso (low) late cut |
| 16 | Defined organic waste 50% starch (potato + bread) + 25% lignocellulose (hedge trimmings + grass) + 25% vegetable/fruit peelings (apple + cucumber) |
| Sample | Cellulose | Lignin | Xylan | Pectin | Starch |
|---|---|---|---|---|---|
| BW1 | 50% | 25% | 25% | - | - |
| BW2 | 50% | - | 25% | - | 25% |
| BW3 | 25% | - | 25% | - | 50% |
| BW4 | 10% | 50% | 40% | - | - |
| BW5 | - | 50% | 40% | 10% | - |
| BW6 | 40% | - | 20% | 40% | - |
| Enzyme | Glucose Concentration g·L−1 | |||
|---|---|---|---|---|
| 0 h | 2 h | 24 h | 48 h | |
| Cellulase TXL | 0.86 ± 0.01 | 0.63 ± 0.01 | 0.77 ± 0.01 | 0.89 ± 0.01 |
| Amylase FL | 1.18 ± 0.01 | 1.49 ± 0.01 | 3.41 ± 0.03 | 4.19 ± 0.04 |
| Glucoamylase AN | 1.59 ± 0.02 | 3.74 ± 0.04 | 5.81 ± 0.06 | 6.51 ± 0.06 |
| Xylanase 2 | 0.84 ± 0.01 | 0.71 ± 0.01 | 0.80 ± 0.01 | 0.86 ± 0.01 |
| Pectinase L-40 | 0.82 ± 0.01 | 0.85 ± 0.01 | 1.80 ± 0.02 | 2.54 ± 0.02 |
| Total | 14.98 ± 0.15 | |||
| Enzyme mix | 1.29 ± 0.01 | 2.91 ± 0.03 | 5.27 ± 0.05 | 7.50 ± 0.07 |
| Hydrolysis | 0 h | 2 h | 24 h | 48 h |
|---|---|---|---|---|
| [g·L−1] | ||||
| UH11.1 | 1.29 ± 0.01 | 3.11 ± 0.03 | 5.10 ± 0.05 | 4.52 ± 0.04 |
| UH16.1 | 1.29 ± 0.01 | 2.91 ± 0.03 | 5.27 ± 0.05 | 7.50 ± 0.07 |
| UH16.2 | 1.50 ± 0.01 | 2.54 ± 0.02 | 5.47 ± 0.05 | 5.98 ± 0.06 |
| Fermentation | H2 [%] | H2 Volume [ml] | H2 Substance [mmol] | H2 [mol/kg DM Substrate] |
|---|---|---|---|---|
| UC11.1 | 60 ± 0.01 | 664.30 ± 16.30 | 27.07 ± 0.66 | 1.09 ± 0.03 |
| UC16.1 | 41 ± 0.01 | 211.95 ± 5.20 | 6.00 ± 0.21 | 0.35 ± 0.01 |
| UC16.2 | 45 ± 0.01 | 309.81 ± 7.60 | 12.63 ± 0.31 | 0.51 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tix, J.; Moll, F.; Krafft, S.; Betsch, M.; Tippkötter, N. Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana. Energies 2024, 17, 2938. https://doi.org/10.3390/en17122938
Tix J, Moll F, Krafft S, Betsch M, Tippkötter N. Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana. Energies. 2024; 17(12):2938. https://doi.org/10.3390/en17122938
Chicago/Turabian StyleTix, Julian, Fabian Moll, Simone Krafft, Matthias Betsch, and Nils Tippkötter. 2024. "Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana" Energies 17, no. 12: 2938. https://doi.org/10.3390/en17122938
APA StyleTix, J., Moll, F., Krafft, S., Betsch, M., & Tippkötter, N. (2024). Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana. Energies, 17(12), 2938. https://doi.org/10.3390/en17122938






