Abstract
The purpose of this research was to evaluate the process of enzymatic biodiesel synthesis by directly using rapeseed as a raw material, extracting the oil contained within and interesterifying with a mixture of methyl formate and mineral diesel, choosing the amount of mineral diesel so that the ratio between it and the rapeseed oil in the seeds was 9:1. As the final product of the interesterification process, a mixture of mineral diesel and biodiesel was obtained directly, which is conventionally produced by mixing the mineral diesel and biodiesel. The tests were performed using enzymatic catalysis using the lipase Lipozyme TL TIM. Process optimization was performed using the response surface methodology. A model describing the interaction of three independent variables and their influence on the yield of rapeseed oil methyl esters was developed. The physical and chemical indicators of the product obtained under optimal interesterification conditions were evaluated.
1. Introduction
The aim to reduce net greenhouse gas emissions by at least 55% by 2030 compared to 1990 levels is indicated in the legal acts adopted by the European Commission. This goal can be achieved by replacing a part of mineral fuel with biofuel. The advantage of biofuel production and use is reductions in mineral diesel consumption, increased energy independence from countries that export mineral energy resources, and reductions in negative impacts on the environment [1].
The most popular method of biodiesel production is transesterification of oil with alcohols using homogeneous [2,3] or heterogeneous catalysts [4]. The advantage of heterogeneous catalysts is the possibilities of multiple use of a catalyst [5]. As much as 70% of the energy costs of the entire biodiesel production cycle are oil extraction and refining processes [6]. In order to reduce the cost of biodiesel production, use less energy, and, at the same time, have the least negative impact on the environment, a simultaneous process of oil extraction from oily raw materials and its transesterification into biodiesel (in situ) can be applied. In this process, biodiesel is produced directly from crushed oilseeds using various alcohols and catalysts [7]. Many researchers who have studied the in situ process have found that it occurs faster and more efficiently using solvents [8]. The most commonly used solvent is hexane [9] or, less often, petroleum ether [10] and methylene dichloride [11]. Solvents are removed from the resulting biodiesel together with excess alcohol.
Biodiesel is used in the transport sector in mixtures with mineral diesel. Researchers have investigated the application of an in situ process for the simultaneous production of mineral diesel and biodiesel blends using an alcohol and mineral diesel mixture. In this process, mineral diesel worked as an oil extractant; it was not removed from the resulting product—a mixture of fatty acid ethyl esters and mineral diesel [12]. Kojima et al. investigated the in situ transesterification process of waste-activated bleaching earth with methanol using mineral diesel and catalyst Candida cylindracea and obtained 100% fatty acid methyl ester (FAME) yield [13]. Using rapeseed for transesterification using butanol with catalyst Lipozyme RM IM and mineral diesel as an extractant, a 97.6% rapeseed oil methyl ester (RME) yield was obtained [14]. The use of low-quality rapeseed with higher acidity in the in situ transesterification process using various alcohols (methanol, ethanol and butanol) and mineral diesel was also investigated. It was found that the process is most efficiently catalyzed by immobilized lipase Lipozyme TL IM. The obtained mixtures of mineral diesel and 10% esters met the requirements of the standards and suggested that they could be used as fuel for diesel engines [15].
Both conventional and in situ biodiesel production using oil and alcohols produces biodiesel—fatty acid esters and about 10% by-product—technical glycerol [16]. Recently, there has been interest in the innovative process of the interesterification of oil with short-chain carboxylate esters. A mixture of fatty acid esters and triacylglycerol is produced as the final product. This process does not produce glycerol, resulting in a higher product yield. Most studies are conducted using methyl acetate [17] and ethyl acetate [18] for interesterification and propyl acetate, isopropyl acetate or tert-butyl acetate [19]. Methyl formate is used less often.
The in situ interesterification process using a mixture of methyl formate and mineral diesel has not been studied, so the optimal process conditions are unknown.
The aim of these studies was to evaluate the process of direct enzymatic biodiesel synthesis using rapeseed instead of rapeseed oil as raw material, using a mixture of methyl formate and mineral diesel for interesterification, in order to obtain a reaction product containing rapeseed methyl esters in a mixture with mineral diesel. This research aimed to determine the optimal conditions of such a process and to evaluate the properties of the obtained product.
2. Materials and Methods
2.1. Materials
Rapeseeds used as a source of oil were grown at Agricultural Academy of Vytautas Magnus University. They were ground in a laboratory mill and mechanically sieved to obtain a fraction of 0.315 mm. Mineral diesel was obtained from a local market. It met the quality requirements of EN 590 [20]. For the industrial enzyme preparation, Lipozyme TL IM (Novozymes A/S (Copenhagen, Denmark)) was used as enzymatic catalyst. All reagents used for analysis and synthesis met the requirements of the standards and were analytically pure.
2.2. Methods
2.2.1. Determination of Oil Content in Rapeseed
The amount of oil in rapeseed was determined according to the requirements of standard EN ISO 659 [21]. Ground rapeseeds were placed in a Soxhlet apparatus, and the oil was extracted with hexane. After evaporation of the hexane, the mass of the oil was determined by weight method and the percentage of oil in rapeseed was calculated.
2.2.2. In Situ Interesterification Studies
Ground and sieved rapeseeds were mixed with mineral diesel so that the volume ratio of oil and mineral diesel was 1:9. This ratio was taken so that the product obtained during interesterification would be close to the mixture of mineral diesel and conventional biodiesel currently used in transport.
In situ interesterification studies were performed in a laboratory reactor, into which ground required amounts of rapeseed and mineral diesel were placed. The enzyme Lipozyme TL IM was added, and methyl formate was poured into the same reactor. The resulting suspension was stirred for the specified time. After the set time, rapeseed residues were separated from the liquid fraction by filtration and unreacted methyl formate was evaporated from the resulting solution via vacuum evaporator. The content of mono-, di- and triformyl glycerides and the content of esters in the reaction product were investigated using Perkin Elmer Clarus 500 gas chromatograph equipped with an FID detector and a Restek MXT-Biodiesel TG (15 m–0.32 mm–0.10 μm) column. From the amount of partial formylglycerides and triglycerides, the amount of rapeseed oil methyl esters in the mixture was calculated. The yield of rapeseed methyl esters was calculated based on the theoretically possible amount of esters and the actual amount of esters as a percentage.
2.2.3. Optimization of the In Situ Interesterification Process
Surface response methodology was used for process optimization. Using the Design Expert 7.01 version (Stat-Ease, Meneapolis, MN, USA) program, an experimental plan was drawn up, which included 31 experiments (Table 1). The mass ratio of methyl formate and rapeseed, the amount of catalyst (% from the oil content in rapeseed) and the duration of the process were selected as independent variables. Considering the low boiling point of methyl formate, the process was carried out at 20 °C. The values of the variables were chosen according to the results of previous studies. After the experiments, a second-order polynomial model describing the interesterification process was created, in which response was rapeseed methyl ester yield.
where:
- Y is the response (dependent variable);
- Xi and Xj are independent variables;
- β0, βi, βii and βj, βij are constant coefficients.

Table 1.
Central composite design matrix for in situ interesterification process and predicted and actual values of RME yield.
Table 1.
Central composite design matrix for in situ interesterification process and predicted and actual values of RME yield.
| Coded Factor | Actual Factor | RME Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | A (Mass Ratio of Methyl Formate to Seed, g) | B (Catalyst Amount, %) | C (Duration, h) | Actual Value | Predicted Value |
| 1.0 | 1.0 | 1.0 | 50 | 24 | 72 | 8.20 | 7.88 |
| −1.0 | 1.0 | −1.0 | 10 | 24 | 48 | 55.63 | 53.56 |
| 1.0 | 1.0 | −1.0 | 50 | 24 | 48 | 10.27 | 12.05 |
| −1.0 | 1.0 | −1.0 | 10 | 24 | 48 | 53.08 | 53.56 |
| −1.0 | 1.0 | 1.0 | 10 | 24 | 72 | 69.47 | 70.37 |
| 0 | −1.2 | 0 | 30 | 13 | 60 | 34.19 | 32.88 |
| 0 | 0 | 0 | 30 | 19 | 60 | 39.67 | 40.48 |
| −1.0 | −1.0 | −1.0 | 10 | 14 | 48 | 35.26 | 36.02 |
| 1.0 | −1.0 | −1.0 | 50 | 14 | 48 | 6.09 | 5.39 |
| 1.0 | 1.0 | 1.0 | 50 | 24 | 72 | 9.18 | 7.88 |
| 0 | 0 | 1.2 | 30 | 19 | 74.4 | 35.10 | 36.46 |
| 0 | −1.2 | 0 | 30 | 13 | 60 | 32.34 | 32.88 |
| 1.2 | 0 | 0 | 54 | 19 | 60 | 8.67 | 9.59 |
| 0 | 0 | −1.2 | 30 | 19 | 45.6 | 27.41 | 26.32 |
| −1.0 | 1.0 | 1.0 | 10 | 24 | 72 | 69.15 | 70.37 |
| −1.0 | −1.0 | 1.0 | 10 | 14 | 72 | 56.63 | 57.09 |
| 0 | 0 | 0 | 30 | 19 | 60 | 38.86 | 40.48 |
| 1.0 | −1.0 | −1.0 | 50 | 14 | 48 | 6.39 | 5.39 |
| −1.0 | −1.0 | −1.0 | 10 | 14 | 48 | 34.75 | 36.02 |
| 0 | 0 | 0 | 30 | 19 | 60 | 41.14 | 40.48 |
| −1.0 | −1.0 | 1.0 | 10 | 14 | 72 | 58.82 | 57.09 |
| 1.2 | 0 | 0 | 54 | 19 | 60 | 10.67 | 9.59 |
| 0 | 1.2 | 0 | 30 | 25 | 60 | 44.96 | 44.84 |
| 0 | 1.2 | 0 | 30 | 25 | 60 | 45.09 | 44.84 |
| 0 | 0 | −1.2 | 30 | 19 | 45.6 | 25.45 | 26.32 |
| −1.2 | 0 | 0 | 6 | 19 | 60 | 64.78 | 65.46 |
| 0 | 0 | 1.2 | 30 | 19 | 74.4 | 38.72 | 36.46 |
| 1 | −1.0 | 1.0 | 50 | 14 | 72 | 3.78 | 5.48 |
| 1 | −1.0 | 1.0 | 50 | 14 | 72 | 5.19 | 5.48 |
| −1.2 | 0 | 0 | 6 | 19 | 60 | 67.11 | 65.46 |
| 1 | 1.0 | −1.0 | 50 | 24 | 48 | 12.16 | 12.05 |
Statistical analysis of the model was performed using the Design Expert 7.01 version (Stat-Ease, Meneapolis, MN, USA) program to evaluate the variance (ANOVA) and test the fit of the empirical model. Using the fitted model, contour plots were constructed for each pair of study factors, holding the third factor constant at the estimated stationary point. To validate the model, optimization of the reaction conditions was performed using combinations of independent variables that were not included in the original experimental design.
2.2.4. Interesterification Product Quality Studies
The studies of the quality indicators of the interesterification in situ product obtained under optimal conditions were performed using standardized methods in accordance with the requirements of standards EN 14214 [22] and EN 590 [20].
3. Results and Discussion
3.1. Modelling of Interesterification Process
After oil extraction, it was determined that rapeseeds contain 43.5 ± 0.5% oil. The volume of oil calculated by density and the amount of rapeseed taken for interesterification experiments were such that the volume ratio of oil contained in the rapeseed sample and mineral diesel was 1:9.
Interesterification optimization studies were performed according to the experimental plan. The values of the independent variables varied within the following limits: the amount of catalyst varied from 14 to 25% depending on the amount of oil in the rapeseed sample, the mass ratio of methyl formate to rapeseed was from 6 to 54 and the duration varied from 45 to 74 h. The results of our previous research showed that a high yield of the product is obtained only by using higher amounts of catalyst and interesterification reagents. The test conditions are given in Table 1. It also shows the experimental values of the rapeseed methyl ester yield (RME).
The process was modeled using surface response methodology. By applying multiple regression analyses and analyzing the obtained experimental results, a second-order regression model was created, which describes the dependence of rapeseed methyl ester yield on three independent variables, which are expressed as coded factors (Equation (2)).
where:
Y = 40.4844 − 23.2806 A + 4.98348 B + 4.22478 C − 2.71893 AB − 5.24383 AC − 1.06619 BC − 2.05677 A2 − 1.1278 B2 − 6.31875 C2
- Y—rapeseed methyl ester yield (%);
- A—mass ratio of methyl formate to rapeseed (m/m);
- B—amount of lipase Lipozyme TL IM (% of oil content);
- C—process duration (h).
The relative influence of individual independent variables on the process output can be estimated using the given equation and the coefficients specified therein. From the data presented, it can be seen that the amount of methyl formate has the greatest influence on the yield of rapeseed methyl esters; the amount and duration of the catalyst have a smaller influence. In order to evaluate the accuracy of the developed model, actual and predicted RME yield values were compared (Figure 1). It can be seen that there is a linear dependence between them, which means that the actual and predicted values correlate sufficiently, and the model describing the dependence of the RME yield on the three independent variables is accurate.
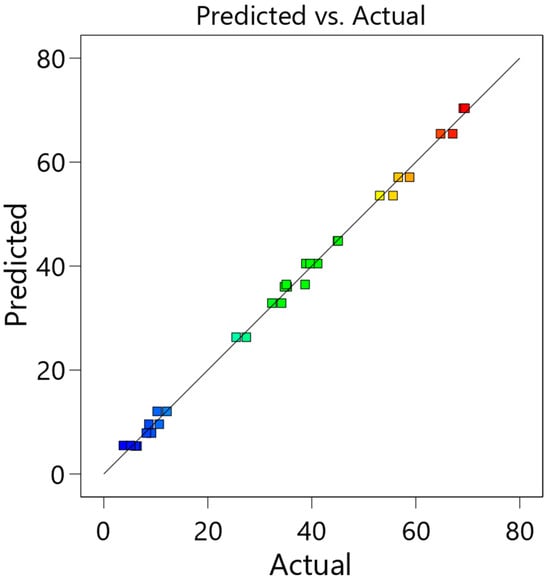
Figure 1.
Quadratic regression plot for 23 factorial design of in situ interesterification process (color points by value of rapeseed methyl ester yield from blue—56.06% to red—94%).
The suitability of the model was also confirmed by analysis of variance (ANOVA), which was used to estimate the variance (Table 2).

Table 2.
ANOVA of in situ interesterification of rapeseed oil with methyl formate in diesel fuel medium.
The F-value of the model, 772.48, shows that the model is significant, and there is only a 0.01% chance that such a value could be due to noise (Table 2). The importance of individual independent variables is shown by F-values, which also allow us to assess the significance of their interaction. The presented data show that the p values of the variables and their interaction are lower than 0.0500, so it can be concluded that models A, B, C, AB, AC, BC, A2, B2 and C2 are significant model terms. None of the p values exceed 0.1000; such a value indicates that the terms are insignificant. The Lack-of-Fit F-value of 1.78 implies that the Lack of Fit is not significant relative to the pure error. There is a 17.39% chance that a Lack-of-Fit F-value this large could occur due to noise. The predicted R2 of 0.9932 is in reasonable agreement with the adjusted R2 of 0.9957, i.e., the difference is less than 0.2. Adeq Precision measures the signal to noise ratio. A ratio greater than 4 is desirable. Your ratio of 81.377 indicates an adequate signal. This model can be used to navigate the design space.
Equation (3) shows the influence of independent variables on the RME yield in terms of actual factors.
Y = −218.622 + 0.972038 A + 4.59282 B + 6.6108 C − 0.0271893 AB − 0.0218493 AC − 0.0177699 BC − 0.00514193 A2 − 0.0451119 B2 − 0.0438802 C2
This equation is intended to determine the influence of relevant factors on the yield of esters. Using this equation, calculations are performed based on the specific values of the relevant factors. However, it is impossible to assess the relative influence of individual independent variables on RME output, because the coefficients are scaled to accommodate the units of each factor and the intercept is not at the center of the design space.
3.2. The Interaction of Independent Variables and Their Effect on the Interesterification Process
The mutual interaction between the two independent variables and its influence on the RME yield at a constant value of the third variable is presented in response surface plots. Figure 2 shows the interaction between the amount of catalyst and the mass ratio of methyl formate and rapeseed at a process time of 72 h.
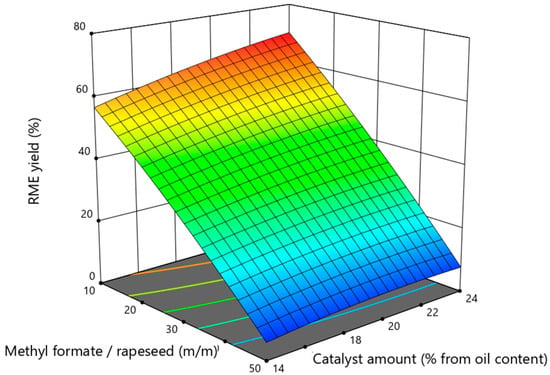
Figure 2.
The influence of the interaction of methyl formate to rapeseed mass ratio and catalyst amount on RME yield at constant duration of 72 h duration.
The presented data show that the amount of the enzyme catalyst Lipozyme TL IM has a direct positive influence on the yield of RME. Meanwhile, increasing the amount of methyl formate has the opposite effect. As the mass ratio of methyl formate and rapeseed increases, the yield of RME decreases. The highest yield of rapeseed methyl esters of 70.4% was obtained with a 10:1 ratio of methyl formate to rapeseed. In conventional biodiesel production, the ratio of methanol to oil of 6:1 is considered optimal, because an excess of methanol is required to shift the equilibrium of the transesterification reaction, which is taken twice as high as the stoichiometric alcohol-to-oil molar ratio equal to 3:1. In the interesterification reaction, one molecule of the oil also reacts with three molecules of the acyl receptor. In the in situ interesterification experiments in mineral diesel fuel media, it was observed that the excess of methyl formate has a negative effect on the yield of RME. Previous studies have shown that the use of methyl formate for the interesterification of rapeseed oil requires a relatively large amount of methyl formate, which does not have such a large negative impact on the process. The addition of mineral diesel to the reaction medium showed a negative trend. This could be explained by the fact that when a large amount of mineral diesel is added to the reaction medium in such a way that the ratio of it to rapeseed oil is 9:1, the reaction medium is greatly diluted and the extracted oil molecules disperse in space and hardly find contact with the enzyme catalyst on the which surface interacts with methyl formate, which is also diluted with mineral diesel.
The fact that larger amounts of acyl receptors complicate the course of the interesterification reaction was also discussed by other authors who studied the process of biodiesel synthesis, especially by applying enzymatic catalysis [23]. Another reason why the course of the intersterification reaction is complicated in the presence of a large amount of the acyl receptor can be the sensitivity of enzyme preparations to a large amount of carboxylate esters with methyl groups and their inactivation by losing their enzymatic activity.
The influence of the amount of catalyst on the yield of RME at a high mass ratio of methyl formate to rapeseed is not so clearly expressed compared to the amount of methyl formate. More significantly, only at the lowest tested amount of methyl formate, increasing the amount of enzyme, the yield of RME increased slightly.
The interesterification process using enzymatic catalysts and methyl formate as an acyl receptor is little studied, but most authors state that when using other acyl receptors and enzymatic heterogeneous catalysts, the process is more complicated than the transesterification process and the interesterification process using chemical homogeneous catalysts. Higher amounts of enzymatic catalysts are required to obtain a high yield of esters. The interesterification of oil with methyl acetate achieved a 92% yield; with only 30% catalyst Novozyme 435 [24], a 90% yield of esters was obtained for interesterification using 10 to 20% methyl and ethyl acetate [25,26]. For interesterification using methyl formate, the optimal determined amount of the catalyst Lipozyme TL IM was 14.6% of the oil content [27]. The process of simultaneous oil extraction from seeds and transesterification was found to be more complicated than transesterification. The results of initial studies using methyl formate showed that the highest RME yields were only obtained with very high catalyst contents.
Figure 3 shows the effect of the duration of the process and the mass ratio of methyl formate to rapeseed on the yield of rapseed oil methyl esters. The results of the studies show that the yield of RME decreases consistently by increasing the level of methyl formate. Meanwhile, the influence of duration is different. At any mass ratio of methyl formate to rapeseed and with an increase in duration up to 62 h, the yield of RME increases, and with an even greater increase in reaction time, the yields that are obtained are both lower. The influence of duration on the yield of the product obtained during interesterification has been analyzed by many authors. It is often mentioned that they are slower than the processes catalyzed by chemical catalysts. The same trend was observed in enzymatic transesterification in the production of conventional biodiesel. The process of enzymatic interesterification using methyl or ethyl acetate has been more extensively studied, and it has been established that high ester yields are achieved only in 12 h to 84 h [25,28]. Only a few studies have been conducted on the use of methyl formate for interesterification of oil. When using Lipozyme TL IM as catalysts, the authors indicate the optimal duration of the 60 h process, and when using the catalyst Lipozyme RM IM, the optimal process duration was 40 h. The process of in situ interesterification in a mineral diesel medium has not been studied, and there are no data on its optimal duration.
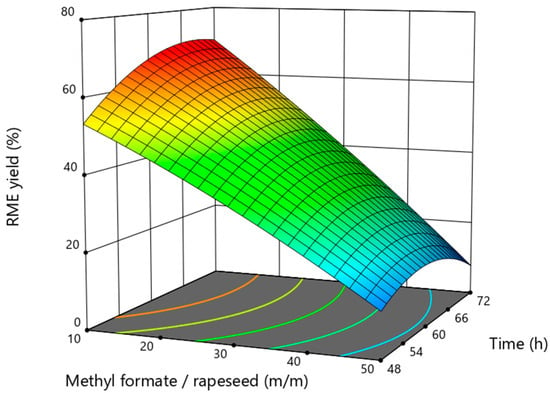
Figure 3.
The influence of the interaction of methyl formate to rapeseed mass ratio and process duration on the rapeseed oil methyl esters yield at a constant catalyst amount of 24%.
The influence of the interaction between the amount of catalyst and the duration of the process on the yield of rapeseed methyl esters is shown in Figure 4. It can be observed that with an increasing amount of catalyst Lipozyme TL IM, the RME yield increases, and the highest was obtained at the highest amount of catalyst tested. This is characteristic of most enzymatic processes: increasing the amount of catalyst increases the surface area of the catalyst on which catalytic reactions take place [29].
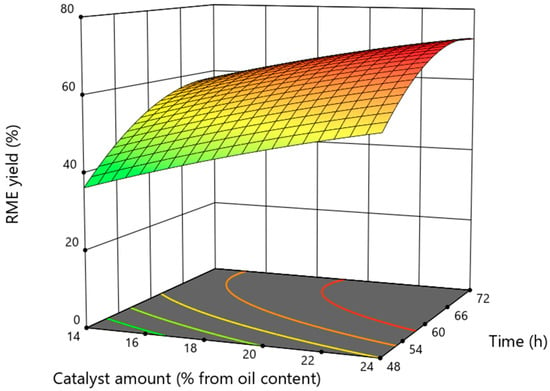
Figure 4.
The influence of the interaction of catalyst ammount and process duration on the rapeseed oil methyl esters yield at a constant mass ratio of methyl formate to oil of 10.
The effect of the process duration is ambiguous and lower compared to the effect of the amount of catalyst on the RME yield. As in the case of the interaction of the process duration with the mass ratio of metformate to rapeseed, increasing the duration up to a certain time increases the RME yield, but further increasing the duration (above 66 h) results in a lower RME yield. This phenomenon can be explained by the fact that the interesterefication reaction is reversible. As the process continues for a longer time, the amount of methyl formate in the reaction medium decreases, the reaction rate remains constant and the reaction rate of the reverse reaction increases compared to the rate of the direct reaction. Another process that could lead to a lower yield of RME is the hydrolysis process of esters, which decomposes unsaturated fatty acids and their side reactions, decreasing the rate of the main reaction [30]. A low reaction rate is characteristic of enzymatic processes, because these reactions take place on the surface of the catalyst, whose size, surface area and porosity are one of the most important rate-limiting factors. Using acyl acceptors other than methyl formate and homogeneous alkaline catalysts required from 20 min up to 20 h to achieve a high yield of esters [31], and when using a heterogeneous catalyst (magnetic solid acid catalyst), the optimal duration was 10.8 h [32].
It is also important that in the studied case, the in situ process involves two processes: the extraction of the oil in the rapeseed at the initial stage and the interesterification of extracted oil in the second stage. The extraction of oil requires a certain time before beginning interesterification. It has been observed that the direct interesterification of rapeseed oil requires a shorter time to obtain a higher yield of RME [27]. The final optimal rate of the simultaneous oil extraction and interesterification process is determined by the rate of the more complex and slower process.
3.3. Optimisation of In Situ Interesterification Process
According to the prediction, it was determined that an RME yield of 70.34% is obtained at a 10:1 mass ratio of methyl formate to rapeseed, taking 24% of the biocatalyst (from oil mass) and performing the process for 72 h. The process was optimized to find optimal reaction conditions that allow for obtaining the highest RME yield with the least amount of methyl formate and lipase in the shortest time. The regression solutions are presented in Table 3. They were validated experimentally by running the process under simulated conditions. Three repeated experiments were performed, and the average of the obtained RME yield was taken as a result. As can be seen from the presented data, the experimental results agree with the predicted result with the smallest error. This indicates that the regression equation accurately predicts the process.

Table 3.
Solutions of regression equation.
3.4. Physicochemical Properties of the In Situ Interesterification Product
The requirements for biodiesel used in the transport sector are given in standards EN 14214 and ASTM 6751 [33]. The quality of mineral diesel is defined by standard EN 590. The product of in situ interesterification should be close to the quality of mineral diesel, since rapeseed was used as raw material, in which the oil content was such that the oil and the volume ratio of mineral diesel would be 1:9. The reaction product contains about 84% of RME and 16% formyl glycerides; therefore, the physical and chemical properties of this product are slightly different from those of mineral diesel and biodiesel (Table 4).

Table 4.
Physical and chemical characteristics of interesterification products.
The presented data show that the interesterification product does not meet the requirements for mineral diesel. It has a slightly higher viscosity and density, although it meets the requirements for biodiesel in terms of viscosity and density. Also, the synthesis product contains compounds such as formylglycerides, which are not formed in conventional biodiesel production, where the formed glycerol and/or its derivatives are separated from the reaction product. In the studied case, the formylglycerides obtained by interesterification are soluble in biodiesel and mineral diesel, so they remain in the obtained product. On the one hand, they increase the viscosity and density, but on the other hand, formylglycerides have been found to improve the low-temperature properties of biodiesel. In addition, not separating the glycerol derivatives increases the fuel amount.
In summary, it can be said that the interesterification process, unlike the transesterification process, gives a product with different characteristics than biodiesel. Its properties and suitability for use in diesel engines have not been studied much, and it is unlikely that the physical and chemical properties of innovative fuel will meet the current requirements for fuel, but from an economic point of view, such a product may have future prospects. It is necessary to further study the interesterification in situ process in order to achieve the highest product yield, to perform operational tests of the innovative product and assess its impact on the environment.
4. Conclusions
The in situ interesterification process avoids the oil extraction operation by saving material and energy costs; the oil is extracted and interesterified at the same time. As an additional solvent, mineral diesel can be used by choosing its amount so that the ratio of rapeseed oil and mineral diesel is 1:10. In this way, a mixture of mineral diesel and biodiesel is produced, which is suggested to be used in the transport sector. This is a promising and environmentally friendly way to catalyze the interesterification reaction with the enzyme preparation Lipozyme LT IM and to use methyl formate as an acyl agent, replacing the synthetic toxic methanol used in conventional biodiesel synthesis.
The efficiency of the in situ interesterification in a diesel fuel medium is influenced by such independent variables as the mass ratio of methyl formate and rapeseed, the amount of catalyst and the duration of the process. It is not appropriate to increase the temperature of the process, because the boiling point of methyl formate is relatively low. The interaction of three independent variables was evaluated using the surface response methodology. The constructed second-order regression model showed that the amount of methyl formate has a greater negative influence on the yield of rapeseed methyl esters, while the duration of the process and the amount of the catalyst Lipozyme RM IM have a positive influence. The optimal conditions of interesterification were determined: the mass ratio of methyl formate and rapeseed was 6:1, the duration of the process was 70 h and the amount of the enzyme preparation Lipozyme TL IM was 23% of the amount of oil. Under these conditions, a 73% yield of rapeseed methyl esters was obtained. The obtained results were verified by a laboratory method. It was established that in order to obtain a high yield of RME when applying the enzymatic process, the duration of the process must be long, and a relatively large amount of the enzyme preparation is required. Meanwhile, increasing the amount of methyl formate decreases the RME yield due to dilution of the reaction medium with mineral diesel and methyl formate. The physical and chemical indicators of the obtained interesterification product do not fully meet the requirements for mineral diesel fuel. Due to the formylglycerol derivatives contained in the product, the density and viscosity exceed the requirements set by the biodiesel standard; therefore, in order to use the innovative product as fuel, further research is necessary, including the analysis of operational and environmental indicators.
Author Contributions
Conceptualization, V.M. and E.S.; methodology, M.G., V.M. and K.K.; software, K.K.; validation, V.M. and E.S.; formal analysis, investigation, K.K. and M.G.; resources, K.K.; data curation, V.M.; writing—original draft preparation, V.M. and E.S.; writing—review and editing, V.M. and E.S.; visualization, M.G. and E.S.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the Research Council of Lithuania (LMTLT), agreement No MIP-22/59, Synthesis of innovative biodiesel.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because it also forms part of an ongoing study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dawood, S.; Ahmad, M.; Ullah, K.; Zafar, M.; Khan, K. Synthesis and characterization of methyl esters from non-edible plant species yellow oleander oil, using magnesium oxide (MgO) nano-catalyst. Mater. Res. Bull. 2018, 101, 371–379. [Google Scholar] [CrossRef]
- Moraes, P.S.; Engelmann, J.I.; Igansi, A.V.; Sant Anna Cadaval, T.R., Jr.; Antonio De Almeida Pinto, L. Nile tilapia industrialization waste: Evaluation of the yield, quality and cost of the biodiesel production process. J. Clean. Prod. 2021, 287, 125041. [Google Scholar] [CrossRef]
- Vishal, D.; Dubey, S.; Goyal, R.; Dwivedi, G.; Baredar, P.; Chhabra, M. Optimization of alkali-catalyzed transesterification of rubber oil for biodiesel production & its impact on engine performance. Renew. Energy 2020, 158, 167–180. [Google Scholar]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Snail shells as a heterogeneous catalyst for biodiesel fuel production. Processes 2023, 11, 260. [Google Scholar] [CrossRef]
- Ferrero, G.O.; Faba, E.M.S.; Eimer, G.A. Biodiesel production from alternative raw materials using a heterogeneous low ordered biosilicified enzyme as biocatalyst. Biotechnol. Biofuels 2021, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, X.; Zhao, B.; Sun, J.; Wang, Y. Rapid in situ transesterification of sunflower oil. Ind. Eng. Chem. Res. 2009, 48, 850–856. [Google Scholar] [CrossRef]
- Benni, S.D.; Munnolli, D.R.S.; Katagi, K.S.; Kadam, N.S.; Akki, M.C. Liquid fuel synthesis from Leonotis nepetifolia seeds through in-situ transesterification method. Energy Sources A Recovery Util. Environ. Eff. 2020, 1–18. [Google Scholar] [CrossRef]
- Kavitha, M.S.; Murugavelh, S. In situ acid catalysed transesterification of biodiesel production from Sterculia foetida oil and seed. Int. J. Green Energy 2019, 16, 1465–1474. [Google Scholar]
- Tran, D.T.; Yeh, K.L.; Chen, C.L.; Chang, J.S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Lei, H.; Ding, X.; Zhang, H.; Chen, X.; Li, Y.; Zhang, H.; Wang, Z. In situ production of fatty acid methyl ester from low quality rice bran: An economical route for biodiesel production. Fuel 2010, 89, 1475–1479. [Google Scholar] [CrossRef]
- Li, P.; Miao, X.; Li, R.; Zhong, J. In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J. Biomed. Biotechnol. 2011, 2011, 141207. [Google Scholar] [CrossRef] [PubMed]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel production by lipase-catalyzed in situ transesterification of rapeseed oil containing a high free fatty acid content with ethanol in diesel fuel media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- Kojima, S.; Du, D.; Sato, M.; Park, E.Y. Efficient production of fatty acid methyl esterfrom waste activated bleaching earth using diesel oil as organic solvent. J. Biosci. Bioeng. 2004, 98, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sendzikiene, E.; Makareviciene, V.; Gumbyte, M. Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel. Process Technol. 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Santaraite, M. Simultaneous extraction of rapeseed oil and enzymatic transesterification with butanol in the mineral diesel medium. Energies 2022, 15, 6837. [Google Scholar] [CrossRef]
- Govindaraju, R.; Chen, S.S.; Wang, L.P.; Chang, H.M.; Pasawan, M. Significance of membrane applications for high-quality biodiesel and byproduct (glycerol) in biofuel Industries—Review. Curr. Pollut. Rep. 2021, 7, 128–145. [Google Scholar] [CrossRef]
- Esan, A.O.; Smith, S.M.; Ganesan, S. A non-conventional sustainable process route via methyl acetate esterification for glycerol-free biodiesel production from palm oil industry wastes. Process Saf. Environ. Prot. 2022, 166, 402–413. [Google Scholar] [CrossRef]
- Supang, W.; Ngamprasertsith, S.; Sakdasri, W.; Sawangkeaw, R. Ethyl acetate as extracting solvent and reactant for producing biodiesel from spent coffee grounds: A catalyst- and glycerol-free process. J. Supercrit. Fluids 2022, 186, 105586. [Google Scholar] [CrossRef]
- Sustere, Z.; Kampars, V. The influence of acyl and alkohol moieties of carboxylate esters on rapeseed oil chemical interesterification. Mater. Methods Technol. 2017, 11, 1–7. [Google Scholar]
- EN 590; Automotive Fuels. Diesel. Requirements and Test Methods. International Organization for Standardization: Geneva, Switzerland, 2022.
- EN ISO 659; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- EN 14214; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods (Includes Amendment: 2019). International Organization for Standardization: Geneva, Switzerland, 2019.
- Surendhiran, D.; Vijay, M.; Sirajunnisa, A.R. Biodiesel production from marine microalga Chlorella salina using whole cell yeast immobilized on sugarcane bagasse. J. Environ. Chem. Eng. 2014, 2, 1294–1300. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lian, S.H.; Chen, S.S.; Suc, C.H.; Lin, J.H.; Chienc, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Tavares, G.R.; Goncalves, J.E.; dos Santos, W.D.; da Silva, C. Enzymatic interesterification of crambe oil assisted by ultrasound. Ind. Crops Prod. 2017, 97, 218–223. [Google Scholar] [CrossRef]
- Makarevičienė, V.; Kazancev, K.; Sendžikienė, E.; Gumbytė, M. Glycerol free biodiesel synthesis by application of methyl formate in enzymatic interesterification of rapeseed oil. Green Chem. Lett. Rev. 2023, 16, 2215259. [Google Scholar] [CrossRef]
- Modi, M.K.; Reddy, J.R.C.; Rao, B.V.S.K.; Prasad, R.B.N. Lipase-mediated conversion of vegetable oils into biodiesel using ethyl acetate as acyl acceptor. Bioresour. Technol. 2007, 98, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jan, Y. Lipase-Catalyzed Biodiesel Production with Methyl Acetate as Acyl Acceptor. Naturforsch. C J. Biosci. 2008, 63, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Imahara, H.; Minami, E.; Hari, S.; Saka, S. Thermal stability of biodiesel in supercritical methanol. Fuel 2008, 87, 1–6. [Google Scholar] [CrossRef]
- Galia, A.; Centineo, A.; Saracco, G.; Schiavo, B.; Scialdone, O. Interesterification of rapeseed oil catalyzed by tin octoate. Biomass Bioenergy 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zhang, J.; Li, G. In situ reactive extraction of cottonseeds with methyl acetate for biodiesel production using magnetic solid acid catalysts. Bioresour. Technol. 2014, 174, 182–189. [Google Scholar] [CrossRef]
- ASTM 6751; Standard Specification for Biodiesel Fuel Blendstock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).