Abstract
By employing a specific particle interaction theory and a high-precision equation of states for the liquid and vapor phases of H2, respectively, a new H2 solubility model in pure water and aqueous NaCl solutions is proposed in this study. The model established by fitting the experimental data of H2 solubility can be used to estimate H2 solubility in pure water at temperatures and pressures of 273.15–423.15 K and 0–1100 bar, respectively, and in salt solutions (NaCl concentration = 0–5 mol/kg) at temperatures and pressures of 273.15–373.15 K and 0–230 bar, respectively. By adopting the theory of liquid electrolyte solutions, the model can also be used to predict H2 solubility in seawater without fitting the experimental data of a seawater system. Within or close to experimental data uncertainty, the mean absolute percentage error between the model-predicted and experimentally obtained H2 solubilities was less than 1.14%.
1. Introduction
Hydrogen (H2) is an important natural gas because it is light, storable, and reactive [1]. H2 is considered the best energy carrier for the efficient storage of renewable primary energy sources such as solar and wind energy [2]. On the one hand, the combustion of H2 does not emit pollutants and greenhouse gases; the only combustion product is H2O; on the other hand, H2 has a high calorific value of ~140 MJ/kg [3]. H2 is potentially suitable for large-scale geological storage in porous formations, saline aquifers, caverns, or depleted oil and gas reservoirs, all of which can provide significant storage capacity [4,5,6]. To assess the stability and safety of the long-term operation of hydrogen storage reservoirs and the efficiency of energy storage, one should study the solubility and volumetric properties of H2 in gas−liquid systems for the migration of fluids and the alteration of minerals induced during storage [7]. Moreover, H2 is abundantly present in nature. Hydrogen production can be divided into inorganic and organic geneses. Inorganic hydrogen is usually produced via earth degassing, water–rock reactions, and water radiolysis [8,9,10], whereas organic hydrogen is primarily produced via biogenesis and the thermal decomposition of organic matter [11,12]. Natural hydrogen is abundant in the formation areas of terrestrial volcanic rocks, large fault basins, marine serpentinized areas, and hydrothermal vents [9,13,14]. Hydrogen can be utilized as an electron donor in the reactions of photoautotrophic, photoheterotrophic, chemoautotrophic, and chemoheterotrophic organisms [15]. Most typically, autotrophic hydrogen bacteria consume hydrogen to produce life-sustaining methane, which explains the abundance of hydrogen-consuming organisms in submarine hydrothermal vents [16,17,18]. In studies on hydrogen-related biological activities or physicochemical processes, the hydrogen supply rate and hydrogen concentration in fluids must be determined. Moreover, H2 solubility in a fluid largely determines the hydrogen transport rate and hydrogen concentration in the dissolved state.
Experiments using various solutions have yielded a large amount of H2 solubility data at various temperatures and pressures. Additionally, H2 solubility data have been accumulating since 1855. Using the pure physical absorption method, Bunsen [19] measured the absorption coefficients of H2 in pure water at various temperatures (277.15–296.75 K) and atmospheric pressure. However, because H2 shows low solubility in water at atmospheric pressure and the experimental conditions were limited, the measurement results were very similar. Using the same method, Wiebe and Gaddy [20] measured the absorption coefficients of hydrogen in pure water over a wide temperature range (273.15–373.15 K) and different pressures (25–1000 atm). They treated nitrogen impurities in the hydrogen so that gas composition was close to pure hydrogen and the influence of the water-vapor partial pressure on solubility was corrected. Chabab et al. [21] employed the static analysis method to measure H2 solubility in pure water and aqueous NaCl solutions (1, 3, and 5 mol/kg) at different temperatures (323.18–372.76 K) and pressures (28.623–229.720 bar).
A model based on experimental data can be used to predict H2 solubility in an unmeasured system. Jauregui-Haza et al. [22] studied H2 solubility in water and organic solvents such as octene, toluene, and nonanal. They applied regular solution theory using the polar solvent factor correction method reported by Lemcoff [23]. Moreover, they derived the Henry constant of H2 at temperatures of 353, 363, and 373 K. The H2 solubility error in the aforementioned solvents was ~2.6%; however, the model was only applicable to pure aqueous solutions and the Henry constant of H2 was not determined in aqueous NaCl solutions. Li et al. [7] considered the system pressure, temperature, and formation fluid salinity in an H2 solubility model. Their model reproduced all available experimental data and accurately predicted H2 solubility in formation fluids under a range of typical geological hydrogen storage conditions (273–373 K, 1–500 bar, and 0–5 mol/kg NaCl). Within or close to the experimental data uncertainty, H2 solubility was predicted with a maximum relative error of 5% in pure water; however, the error increased to 15% in brine. Chabab et al. [21] estimated the H2 solubility using a fast method based on a Setschenow-type relation [24], which predicted H2 solubility in pure water and aqueous NaCl solutions with average deviations of 0.5% and 2%, respectively. This model was adapted to the temperature and pressure ranges of 273.15–373.15 K and 1–203 bar, respectively, in pure water, and 323.15–373.15 K and 10–230 bar, respectively, in aqueous NaCl solutions. However, in aqueous NaCl, the lower bound of this model was 323.15 K, which is unsuitable for studying H2 solubility in nature. Torín-Ollarves and Trusler [25] proposed a simple model based on an analysis method for predicting H2 solubility in aqueous solutions at temperatures and pressures of 273.15–423.15 K and 1–1010 bar, respectively. For reasons that defy a logical explanation, the prediction results of this model quite differed from those reported by Chabab et al. [21].
Duan et al. [26] established a solubility model of methane gas in aqueous solutions. Their model applies a specific theory of particle interactions for the liquid phase and a high-precision equation of state for the vapor phase. The methane solubility in both pure water and aqueous NaCl solutions was predicted for the temperature range of 273.15–523.15 K and the pressure range of 0–1600 bar. The error between the calculated and experimental data was ~7%. The parameters in this model were fitted to the experimental data and represented the interactions between substances. The values of different parameters were closely related, suggesting that the model is applicable to complex brines (e.g., CaCl2, KCl, and seawater) using the approximation principle. The calculated results were consistent with the experimental data. Later, the solubilities of N2, CO2, C2H6, and O2 in pure water to aqueous NaCl solutions were calculated using this model [27,28,29,30,31] and were also consistent with the experimental data. In conclusion, this model was widely applicable and can accurately calculate gas solubility in pure water and was easily employed in multiple ionic systems. Herein, we establish H2 solubility models for the H2 + H2O system and the H2 + H2O + NaCl system, as well as for other ionized water systems that are applicable to a wide range of temperatures, pressures, and salinities. The gas-phase chemical potential of hydrogen was computed using the equation of state proposed by Peng and Robinson [32], whereas the liquid-phase chemical potential of hydrogen was defined using the theory of liquid electrolyte solutions proposed by Pitzer [33]. The relevant parameters of this model were fitted to as many experimental data as possible. During comparison with experimental data, the model achieved high accuracy, thus providing a foundation for related marine geochemistry research.
2. H2 Solubility Model
H2 solubility in aqueous solutions was determined based on the balance between the chemical potentials of H2 in the liquid and vapor phases. The potential can be expressed in terms of fugacity in the vapor phase (Equation (1)) and activity in the liquid phase (Equation (2)):
where and represent the standard chemical potentials of H2 in liquid and vapor phases, respectively. Here, denotes the chemical potential in a hypothetical ideal solution of unit molality [34], and denotes the chemical potential when the pressure of a hypothetical ideal gas is set to 1 bar.
At phase equilibrium , subsequently, we obtain Equation (3).
In parameterization, reference value can be set to 0 for convenience as only the difference between and is important. Since the vapor phase has low water content, the fugacity coefficient of H2 in gaseous mixtures is approximate to that of pure H2 in the studied region. Therefore, can be approximated from the equation of state of pure H2 (refer to Appendix A) [32]. The mole fraction . of H2 in the gas is calculated as follows.
If the partial pressure of water in the vapor phase is approximated as the saturated pressure of pure water [26,28,29,30,31], and will contain errors of up to 5%. However, these errors can be largely canceled by parameterization. Herein, the mole fraction of water in the vapor phase is estimated using the following semiempirical equation:
where represents the mole fraction of H2O in the liquid phase, which is approximated as 1 and 1–2 in the H2 + H2O and H2 + H2O + NaCl systems, respectively, when dissolved hydrogen is neglected. The saturation pressure (in bar) of water was calculated using an empirical equation (refer to Appendix B). The molar volume of water in the liquid phase was approximated to the saturated liquid-phase volume of water and was calculated using the equation proposed by Sun et al. [35]. The fugacity coefficient of water was calculated using the following equation, which is obtained by fitting the methane–water experimental data [30].
The values of are listed in Table 1. The water content in the vapor phase can be calculated accurately using Equations (5) and (6). The results for different temperatures are plotted in Figure 1.

Table 1.
Parameters of Equation (6) [30].
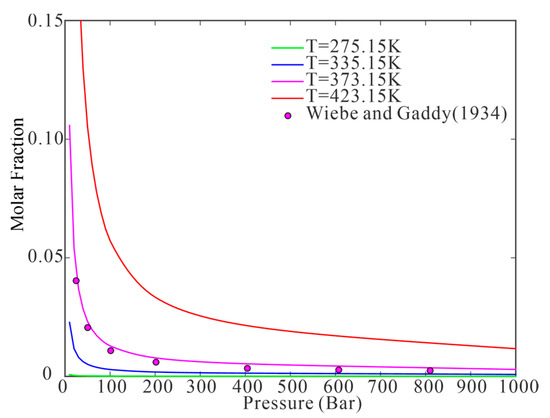
Figure 1.
Pressure-dependent water content in the vapor phase as predicted using the model (The point reported by Wiebe and Gaddy [20]; The curves calculated using the proposed model).
is expressed as a virial expansion of excess Gibbs energy [33]:
where and represent the second-order and third-order interaction parameters, respectively. The subscripts c and a denote cations and anions, respectively. Substituting Equation (7) into Equation (3) yields the following.
Following Pitzer et al. [36], we selected the following equation for the T–P dependences of , , and .
The basis of our model parameterization consists of Equations (8) and (9).
3. Results and Discussion
3.1. Review of H2 Solubility Data
H2 solubility in pure water and aqueous NaCl solutions was measured in a wide range of temperatures, pressures, and ionic strengths (Table 2). The remaining experimental data in pure water show good continuity and correlation, with the exception of some obvious deviations and relative dispersions. After including most of the experimental pure water data in the parameterization, the optimal ranges of temperatures and pressures for the H2 + H2O system in this model were determined to be 273.15–423.15 K and 0–1100 bar, respectively.
Alternatively, the experimental data of H2 solubility in aqueous NaCl solutions showed poorer continuity and a narrower range than those in pure water. The measured values of H2 solubility reported by Torín-Ollarves and Trusler [25] are obviously inconsistent with those reported by Chabab et al. [21]. These abnormal data were excluded, and the experimental data reported by Braun [37], Croizer and Yamamoto [38], and Chabab et al. [20] were selected for the parameterization. Finally, the solubility models for the H2 + H2O + NaCl system yielded temperature, pressure, and salinity ranges of 273.15–373.15 K, 0–230 bar, and 0–5 mol/kg, respectively.
The H2 solubility has been measured in solutions other than aqueous NaCl solutions. For example, Braun [37] measured the H2 solubility in 0.16–0.34 mol/kg BaCl2 solution. Thomas et al. [32] and Gordon et al. [39] measured H2 solubility in seawater with different salinities. Although the temperature ranges in these experiments were wide, the pressure was kept constant (1 atm). Because the aforementioned parameterization requires the combined effect of temperature, pressure, and salinity, these data were excluded from parameterization.

Table 2.
Aqueous H2 solubility measurements in the literature.
Table 2.
Aqueous H2 solubility measurements in the literature.
| References | System | T (K) | P (bar) | Na |
|---|---|---|---|---|
| Bunsen [19] | Water | 277.15–296.75 | 1+ | 7 |
| Timofejew [40] | Water | 274.55–298.85 | 1+ | 5 |
| Bohr and Bock [41] | Water | 273.15–373.15 | 1+ | 48 |
| Winkler [42] | Water | 273.65–323.58 | 1+ | 6 |
| Braun [37] | Water | 278.15–298.15 | 1+ | 5 |
| 0.21–4.03 m NaCl | 278.15–298.15 | 1+ | 5 | |
| 0.16–0.34 m BaCl2 | 278.15–298.15 | 1+ | 5 | |
| Ipatiew jun et al. [43] | Water | 273.65–318.15 | 20.265–141.855 | 17 |
| Wiebe and Gaddy [20] | Water | 273.65–373.15 | 25.331–1013.250 | 40 |
| Morrison and Billett. [44] | Water | 285.65–345.65 | 1+ | 12 |
| Pray et al. [45] | Water | 324.82–588.71 | 6.9–24.150 | 9 |
| Ruetschi and Amlie [46] | Water | 303.15 | 1+ | 1 |
| 0.0011–15.2% H2SO4 | 303.15 | 1+ | 10 | |
| 0.0091–10.23% KOH | 303.15 | 1+ | 9 | |
| Shoor et al. [47] | Water | 298.15-333.15 | 1+ | 3 |
| Longo et al. [48] | Water | 310.15 | 1+ | 1 |
| Power and Stegall [49] | Water | 310.15 | 1+ | 1 |
| Croizer and Yamamoto [38] | Water | 274.60–302.47 | 1+ | 42 |
| 27.665–39.927‰ Seawater | 274.65–303.49 | 1+ | 180 | |
| 10.950–27.376‰ NaCl | 274.03–301.51 | 1+ | 10 | |
| Gordon et al. [39] | Water | 273.29–302.40 | 1+ | 7 |
| 4.919–39.096‰ Seawater | 272.80–302.41 | 1+ | 32 | |
| Chou D Hary et al. [50] | Water | 323.15–373.15 | 25.331–101.325 | 4 |
| Alvarez et al. [51] | Water | 318.90–636.10 | 6.78–284.5 | 26 |
| Kling and Maurer [52] | Water | 323.15–423.15 | 31.8–153.7 | 10 |
| Jauregui-Haza et al. [22] | Water | 353.15–373.15 | 1+ | 3 |
| Chabab et al. [21] | Water | 323.18–372.73 | 29.272–121.706 | 6 |
| 1–5 m NaCl | 323.19–372.78 | 28.623–229.720 | 31 | |
| Torín-Ollarves and Trusler [25] | 2.5 m NaCl | 323.15–423.15 | 116.4–458.1 | 10 |
Note. 1+ denotes that the partial pressure of hydrogen is 1 atm. Na, number of measurements.
3.2. Construction and Validation of the Model
3.2.1. Determination of Model and Calculation of H2 Solubility
To estimate H2 solubility as a function of temperature, pressure, and salinity, we must determine parameters and of Na+ and Cl− in the liquid phase and the standard chemical potential in Equation (8). Because measurements can only be performed in electronically neutral solutions, one of the parameters must be assigned arbitrarily [53]. We set to zero and fitted the remaining parameters. First, was evaluated using the H2 solubility data for pure water (92 related experimental data values), with a root mean square error of 1.62. Next, and were evaluated simultaneously by the least-squares fitting of the solubility data for the aqueous NaCl solutions (41 related experimental data values), with a root mean square error of 1.42. The temperature- and pressure-dependent coefficients are listed in Table 3.

Table 3.
Values of the interaction parameters in Equation (9).
Substituting the parameters (Table 3) into Equation (8), we obtain the H2 solubilities in pure water and aqueous NaCl solutions. The calculated solubilities in pure water and in 1, 3, and 5 mol/kg NaCl solutions are displayed in Table 4, Table 5, Table 6 and Table 7.

Table 4.
Calculated H2 solubility (mol/kg) in pure water.

Table 5.
Calculated H2 solubility (mol/kg) in a 1 mol/kg NaCl solution.

Table 6.
Calculated H2 solubility (mol/kg) in a 3 mol/kg NaCl solution.

Table 7.
Calculated H2 solubility (mol/kg) in a 5 mol/kg NaCl solution.
3.2.2. Model Validation
Figure 2 and Figure 3 show a comparison of the experimental data with the results predicted using our model. The model adequately represented most of the experimental data, remaining within or close to the experimental uncertainty (~1.14%).
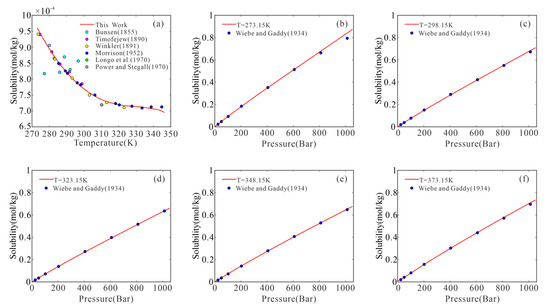
Figure 2.
H2 solubility versus pressure in pure water: model predictions (red lines) and experimental data (colored circles). ((a) The point reported by Bunsen [19], Timofejew [40], Winkler [42] Morrison [44], Longo et al. [48] and Power and Stegall [49]; (b–f) The point reported by Wiebe and Gaddy [20]; All the curves calculated using the proposed model).
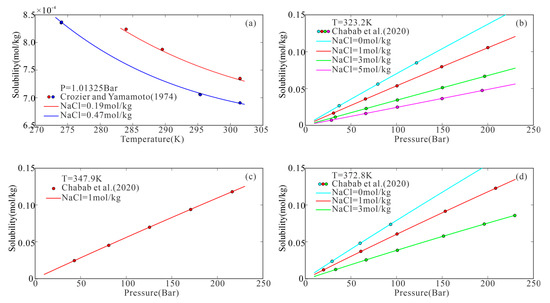
Figure 3.
H2 solubility versus pressure in aqueous NaCl solutions with different concentrations: model predictions (colored lines) and experimental data (colored circles). ((a) The point reported by Crozier and Yamamoto [38]; (b–d) The point reported by Chabab et al. [21]; All the curves calculated using the proposed model).
In the T–P–m range covered by our model, H2 solubility increased with increasing pressure and decreased with increasing ionic strength. The temperature dependence of H2 solubility was more drastic (Figure 4). H2 solubility was slightly dependent on the temperature at low pressures (<200 bar) but decreased and then increased with increasing temperature at high pressures (>200 bar). The isobaric minimum solubility point was observed at ~320 K and 200 bar (Figure 4).
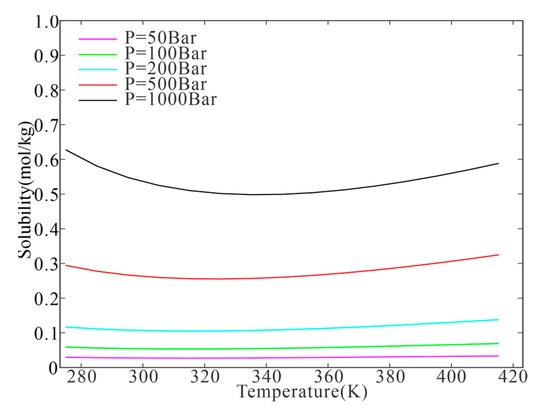
Figure 4.
Isobaric minimum solubilities of H2 in pure water.
The aforementioned solubility model may also be used to calculate the partial molar volume , Henry’s constant , and the heat of solution of H2 in aqueous NaCl solutions. At a given temperature, we can set P to 20 times of for the calculation of Henry’s constant. The functions are expressed using Equations (10)–(14).
Table 8 and Table 9 list the molar heat of the solution and Henry’s constants of H2 in water, respectively, obtained experimentally and calculated using our model. When the temperature was high, Henry’s constants were closer only at higher pressures. There will still be small errors, but this is an acceptable range. Both sets of results demonstrated good agreement, affirming the reliability of the model from another perspective.

Table 8.
Molar heat of solution of H2 in water.

Table 9.
Henry’s constant () of H2 in pure water ( reported by Fernandez-Prini and Roberto [54]; calculated using the proposed model).
3.3. H2 Solubility in Seawater: Extrapolation of the Model
A model developed using the specific interaction approach can be evaluated using binary and ternary data and then applied to more complicated systems [55]. Seawater often contains NaCl, KCl, MgCl2, CaCl2, and sulfate, as well as carbonate salts, although NaCl commonly dominates. As an example, Table 10 lists the main components in seawater with a salinity of 34.7‰. As data were limited, the model could only be directly fitted to the experimental results for the H2–NaCl–H2O system. We incorporated Ca2+, K+, Mg2+, and SO42− into this model to tackle more complicated systems, utilizing the approximation suggested by Duan et al. [26].

Table 10.
Main components of seawater with a salinity of 34.7‰.
The interaction parameters ( and ) of ions with the same charge achieved approximately the same values. Within experimental accuracy, the interaction parameters of CH4–bivalent cations were approximately double those of CH4–monovalent cations at different temperatures and pressures. The interaction parameters of CH4–anions were relatively small and therefore contributed little to the calculations. Hence, Duan et al. approximated all interaction parameters of CH4–monovalent cations and CH4–bivalent cations using and 2, respectively [26]. By adopting the same approach, we approximated the H2 solubility in seawater-type brines by setting the interaction parameters of H2–monovalent cations and H2–bivalent cations as and 2, respectively. All ternary parameters were treated similarly. With this simplification, we achieved the following:
where = −3.572. To check the accuracy of the approximation, we compared the calculated results of Equation (15) with the experimental data on the solubility of H2 in seawater (Figure 5). The modeled results (p = 1 atm and T < 290 K) demonstrated excellent agreement with the experimental measurements.
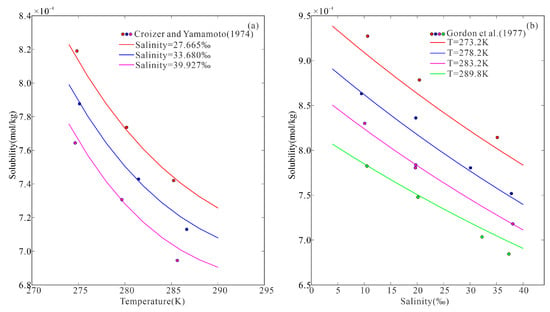
Figure 5.
H2 solubility in seawater versus temperature (a) and salinity (b). (Solid lines represent the modeled data, and the discrete symbols represent the experimental data [38,39]).
4. Conclusions
By applying a high precision equation of state to the vapor phase and the theory of liquid electrolyte solutions proposed by Pitzer to the liquid phase, we developed an accurate model of H2 solubility in pure water and aqueous NaCl solutions. The results were within or close to the experimental uncertainty in pure water (273.15–423.15 K and 0–1100 bar) and aqueous NaCl solutions (273.15–373.15 K, 0–230 bar, and 0–5 mol/kg). After a simple extrapolation, the model predicted H2 solubility in complex aqueous solutions such as seawater containing Na+, K+, Mg2+, Ca2+, Cl−, and SO42− with remarkable accuracy. The mean absolute percentage error between this model and experimentally obtained H2 solubilities was less than 1.14%. The model can calculate hydrogen solubility not only in the subsea environments but also under several typical hydrogen geological storage conditions.
Author Contributions
Conceptualization, Z.Z. (Zhiwei Zhu); investigation, Z.Z. (Zhiwei Zhu); methodology, Z.Z. (Zhiwei Zhu). and Y.C.; software, Z.Z. (Zhiwei Zhu) and Y.C.; supervision, Y.C. and D.C.; validation, Z.Z. (Zhiwei Zhu); writing—original draft, Z.Z. (Zhiwei Zhu); writing—review and editing, Z.Z. (Zhiwei Zhu) and Z.Z. (Zihan Zheng) All authors have read and agreed to the published version of the manuscript.
Funding
This paper is financially supported by the National Natural Science Foundation of China (No. 91858208, No. 41776050, and No. 41776080).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| List of symbols | |
| T | Absolute temperature (K) |
| P | Total pressure (bar): |
| R | Universal gas constant (83.14 bar·cm3/mol/K) |
| Mole fraction of component i in the vapor phase | |
| Mole fraction of component i in the liquid phase | |
| Molality of component i in the liquid phase (mol/kg) | |
| The partial molar volume(cm3/mol) | |
| Greek letters | |
| Activity | |
| Fugacity coefficient | |
| Activity coefficient | |
| Chemical potential | |
| Interaction parameter | |
| Interaction parameter | |
| Subscripts | |
| a | Anion |
| c | Cation |
| Superscripts | |
| v | Vapor |
| l | Liquid |
| (0) | Standard state |
Appendix A
Peng et al. [27] calculated the fugacity coefficient of hydrogen using the equation of state.
By setting the following:
Equation (A1) can be rewritten as follows.
Here, R denotes the general gas constant (8.314 J/mol/K), and a can be regarded as a measure of the intermolecular attraction force; b is a constant related to the size of the gas molecules. Both a and b can be obtained using the critical properties of hydrogen at the critical point.
The critical temperature Tc and critical pressure Pc of hydrogen were 33.2 K and 1.3 × 106 Pa, respectively, and the acentric factor was −0.216. First, a and b values in the critical state were determined from the state parameters of hydrogen using Equation (A4). Then, a and b values at other temperatures and pressures were calculated using Equations (A5)–(A7). Finally, the fugacity coefficient of hydrogen was estimated using Equation (A8), which is derived from Equation (A1).
Appendix B
The pure water pressure was calculated using the following empirical model [28]:
where
and Tc and Pc represent the critical temperature and critical pressure of water, respectively (Tc = 647.29 K and Pc = 220.85 bar). The values of parameters c1–c5 in Equation (A9) are listed in Table A1.

Table A1.
Parameters of Equation (A9).
Table A1.
Parameters of Equation (A9).
| Parameters | Values |
|---|---|
| c1 | −38.640844 |
| c2 | 5.8948420 |
| c3 | 59.876516 |
| c4 | 26.654627 |
| c5 | 10.637097 |
References
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Osaka, Japan, 2019; Volume 3. [Google Scholar]
- Sørensen, B. Energy and Resources: A plan is outlined according to which solar and wind energy would supply Denmark’s needs by the year 2050. Science 1975, 189, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria. New York. Mineral. Mag. 1966, 35, 1024–1025. [Google Scholar]
- Foh, S.; Novil, M.; Rockar, E.; Randolph, P. Underground Hydrogen Storage. Final Report. [Salt Caverns, Excavated Caverns, Aquifers and Depleted Fields]; Brookhaven National Lab.: New York, NY, USA, 1979.
- Carden, P.; Paterson, L. Physical, chemical and energy aspects of underground hydrogen storage. Int. J. Hydrogen Energy 1979, 4, 559–569. [Google Scholar] [CrossRef]
- Evans, D. An Appraisal of Underground Gas Storage Technologies and Incidents, for the Development of Risk Assessment Methodology; British Geological Survey: Nottingham, UK, 2007; Volume 1. [Google Scholar]
- Li, D.; Beyer, C.; Bauer, S. A unified phase equilibrium model for hydrogen solubility and solution density. Int. J. Hydrogen Energy 2018, 43, 512–529. [Google Scholar] [CrossRef]
- Goebel, E.D.; Coveney, R.M.; Angino, E.E.; Zeller, E.J.; Dreschhoff, G.M. Geology, Composition, Isotopes of Naturally Occurring Rich Gas from Wells near Junction City, Kans. Oil Gas J. 1984, 82, 215–222. [Google Scholar]
- Coveney, R.M., Jr.; Goebel, E.D.; Zeller, E.J.; Dreschhoff, G.A.; Angino, E.E. Serpentinization and the Origin of Hydrogen Gas in Kansas1. AAPG Bull. 1987, 71, 39–48. [Google Scholar]
- Klein, F.; Tarnas, J.D.; Bach, W. Abiotic Sources of Molecular Hydrogen on Earth. Elements 2020, 16, 19–24. [Google Scholar] [CrossRef]
- Seewald, J.S. Organic–inorganic interactions in petroleum-producing sedimentary basins. Nature 2003, 426, 327–333. [Google Scholar] [CrossRef]
- Piché-Choquette, S.; Constant, P. Molecular Hydrogen, a Neglected Key Driver of Soil Biogeochemical Processes. Appl. Environ. Microbiol. 2019, 85, e02418-18. [Google Scholar] [CrossRef]
- Marty, B.; Gunnlaugsson, E.; Jambon, A.; Oskarsson, N.; Ozima, M.; Pineau, F.; Torssander, P. Gas Geochemistry of Geothermal Fluids, the Hengill Area, Southwest Rift Zone of Iceland. Chem. Geol. 1991, 91, 207–225. [Google Scholar] [CrossRef]
- Petersen, J.; Zielinski, F.U.; Pape, T.; Seifert, R.; Moraru, C.; Amann, R.; Hourdez, S.; Girguis, P.; Wankel, S.D.; Barbe, V.; et al. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 2011, 476, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Reveillaud, J.; Reddington, E.; Delmont, T.O.; Eren, A.M.; McDermott, J.M.; Seewald, J.S.; Huber, J.A. Genomic variation in microbial populations inhabiting the marine subseafloor at deep-sea hydrothermal vents. Nat. Commun. 2017, 8, 1114. [Google Scholar] [CrossRef] [PubMed]
- Amend, J.P.; Shock, E.L. Energetics of Overall Metabolic Reactions of Thermophilic and Hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001, 25, 175–243. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, H.G. Production, Modification, and Consumption of Atmospheric Trace Gases by Microorganisms. Tellus 2016, 26, 11–20. [Google Scholar]
- Scheuermann, P.P.; Xing, Y.; Ding, K.; Seyfried, W.E. Experimental measurement of H2(aq) solubility in hydrothermal fluids: Application to the Piccard hydrothermal field, Mid-Cayman Rise. Geochim. Cosmochim. Acta 2020, 283, 22–39. [Google Scholar] [CrossRef]
- Bunsen, R. Ueber Das Gesetz Der Gasabsorption. Eur. J. Org. Chem. 1855, 93, 1–50. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The Solubility of Hydrogen in Water at 0, 50, 75 and 100° from 25 to 1000 Atmospheres. J. Am. Chem. Soc. 1934, 56, 76–79. [Google Scholar] [CrossRef]
- Chabab, S.; Theveneau, P.; Coquelet, C.; Corvisier, J.; Paricaud, P. Measurements and Predictive Models of High-Pressure H2 Solubility in Brine (H2o+Nacl) for Underground Hydrogen Storage Application. Int. J. Hydrog. Energy 2020, 45, 32206–32220. [Google Scholar] [CrossRef]
- Jáuregui-Haza, U.J.; Pardillo-Fontdevila, E.J.; Wilhelm, A.M.; Delmas, H. Solubility of Hydrogen and Carbon Monoxide in Water and Some Organic Solvents. Lat. Am. Appl. Res. 2004, 34, 71–74. [Google Scholar]
- Lemcoff, N. Liquid phase catalytic hydrogenation of acetone. J. Catal. 1977, 46, 356–364. [Google Scholar] [CrossRef]
- Setschenow, J. Über Die Konstitution Der Salzlösungen Auf Grund Ihres Verhaltens Zu Kohlensäure. Z. Für Phys. Chem. 1889, 4, 117–225. [Google Scholar] [CrossRef]
- Torín-Ollarves, G.A.; Trusler, J.M. Solubility of hydrogen in sodium chloride brine at high pressures. Fluid Phase Equilibria 2021, 539, 113025. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Greenberg, J.; Weare, J.H. The Prediction of Methane Solubility in Natural Waters to High Ionic Strength from 0 to 250 °C and from 0 to 1600 Bar. Geochim. Cosmochim. Acta 1992, 56, 1451–1460. [Google Scholar] [CrossRef]
- Sun, R.; Hu, W.; Duan, Z. Prediction of Nitrogen Solubility in Pure Water and Aqueous Nacl Solutions up to High Temperature. Solut. Chem. 2001, 30, 561–573. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, Z.; Hu, J.; Sun, R.; Duan, Z. An accurate model for calculating C2H6 solubility in pure water and aqueous NaCl solutions. Fluid Phase Equilibria 2005, 238, 77–86. [Google Scholar] [CrossRef]
- Duan, Z.; Mao, S. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523K and from 1 to 2000bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Geng, M.; Duan, Z. Prediction of oxygen solubility in pure water and brines up to high temperatures and pressures. Geochim. Cosmochim. Acta 2010, 74, 5631–5640. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1970, 15, 3069–3078. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of Electrolytes. Ⅰ. Theoretical Basis and General Equations. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Denbigh, K.G. The Principles of Chemical Equilibrium: With Applications in Chemistry and Chemical Engineering; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Sun, R.; Huang, Z.; Duan, Z. A new equation of state and Fortran 77 program to calculate vapor–liquid phase equilibria of CH4–H2O system at low temperatures. Comput. Geosci. 2003, 29, 1291–1299. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Peiper, J.C.; Busey, R.H. Thermodynamic Properties of Aqueous Sodium Chloride Solutions. J. Phys. Chem. Ref. Data 1984, 13, 1–102. [Google Scholar] [CrossRef]
- Braun, L. Über Die Absorption Von Stickstoff Und Von Wasserstoff in Wässerigen Lösungen Verschieden Dissociierter Stoffe. Z. Für Phys. Chem. 1900, 33, 721–739. [Google Scholar] [CrossRef]
- Crozier, T.E.; Yamamoto, S. Solubility of Hydrogen in Water, Sea Water, and Sodium Chloride Solutions. J. Chem. Eng. Data 1974, 19, 242–244. [Google Scholar] [CrossRef]
- Gordon, L.I.; Cohen, Y.; Standley, D.R. The solubility of molecular hydrogen in seawater. Deep Sea Res. 1977, 24, 937–941. [Google Scholar] [CrossRef]
- Timofejew, W. Über Die Absorption Von Wasserstoff Und Sauerstoff in Wasser Und Alkohol. Z. Für Phys. Chem. 1890, 6, 141–152. [Google Scholar] [CrossRef][Green Version]
- Bohr, C.; Bock, J. Bestimmung der Absorption einiger Gase in Wasser bei den Temperaturen zwischen 0 und 100°. Ann. Phys. 1891, 280, 318–343. [Google Scholar] [CrossRef]
- Winkler, L.W. Die Löslichkeit Der Gase in Wasser. Ber. Dtsch. Chem. Ges. 1891, 24, 89–101. [Google Scholar] [CrossRef]
- Ipatiew, W.W.; Drushina-Artemowitsch, S.I.; Tichomirow, W.I. Löslichkeit Des Wasserstoffs in Wasser Unter Druck. Ber. Dtsch. Chem. Ges. 1932, 65, 568–571. [Google Scholar] [CrossRef]
- Morrison, T.J.; Billett, F. The Salting-out of Non-Electrolytes. Part Ii. The Effect of Variation in Non-Electrolyte. J. Chem. Soc. 1952, 1952, 3819–3822. [Google Scholar] [CrossRef]
- Pray, H.A.; Schweickert, C.E.; Minnich, B.H. Solubility of Hydrogen, Oxygen, Nitrogen, and Helium in Water at Elevated Temperatures. Ind. Eng. Chem. 1952, 44, 1146–1151. [Google Scholar] [CrossRef]
- Ruetschi, P.; Amlie, R.F. Solubility of Hydrogen in Potassium Hydroxide and Sulfuric Acid. Salting-out and Hydration. J. Phys. Chem. 1966, 70, 718–723. [Google Scholar] [CrossRef]
- Shoor, S.K.; Walker, R.D.; Gubbins, K.E. Salting out of nonpolar gases in aqueous potassium hydroxide solutions. J. Phys. Chem. 1969, 73, 312–317. [Google Scholar] [CrossRef]
- Longo, L.D.; Delivoria-Papadopoulos, M.; Power, G.G.; Hill, E.P.; Re, F. Diffusion equilibration of inert gases between maternal and fetal placental capillaires. Am. J. Physiol. Content 1970, 219, 561–569. [Google Scholar] [CrossRef]
- Power, G.G.; Stegall, H. Solubility of gases in human red blood cell ghosts. J. Appl. Physiol. 1970, 29, 145–149. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Parande, M.G.; Brahme, P.H. Simple apparatus for measuring solubility of gases at high pressures. Ind. Eng. Chem. Fundam. 1982, 21, 472–474. [Google Scholar] [CrossRef]
- Alvarez, J.; Crovetto, R.; Fernández-Prini, R. The Dissolution of N2 and of H2 in Water from Room Temperature to 640 K. Ber. Der Bunsenges. Für Phys. Chem. 1988, 92, 935–940. [Google Scholar] [CrossRef]
- Kling, G.; Maurer, G. The solubility of hydrogen in water and in 2-aminoethanol at temperatures between 323 K and 423 K and pressures up to 16 MPa. J. Chem. Thermodyn. 1991, 23, 531–541. [Google Scholar] [CrossRef]
- Harvie, C.E.; Møller, N.; Weare, J.H. The Prediction of Mineral Solubilities in Natural Waters: The Na-K-Mg-Ca-H-Cl-So4-Oh-Hco3-Co3-Co2-H2o System to High Ionic Strengths at 25 °C. Geochim. Cosmochim. Acta 1984, 48, 723–751. [Google Scholar] [CrossRef]
- Fernández-Prini, R.; Alvarez, J.L.; Harvey, A.H. Henry’s Constants and Vapor-Liquid Distribution Constants for Gaseous Solutes in H2o and D2o at High Temperatures. J. Phys. Chem. Ref. Data 2003, 32, 903–916. [Google Scholar] [CrossRef]
- Weare, J.H. Models of Mineral Solubility in Concentrated Brines with Application to Field Observations. Rev. Mineral. Geochem. 1987, 17, 143–176. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).