Gaze Characteristics Using a Three-Dimensional Heads-Up Display During Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
3. Results
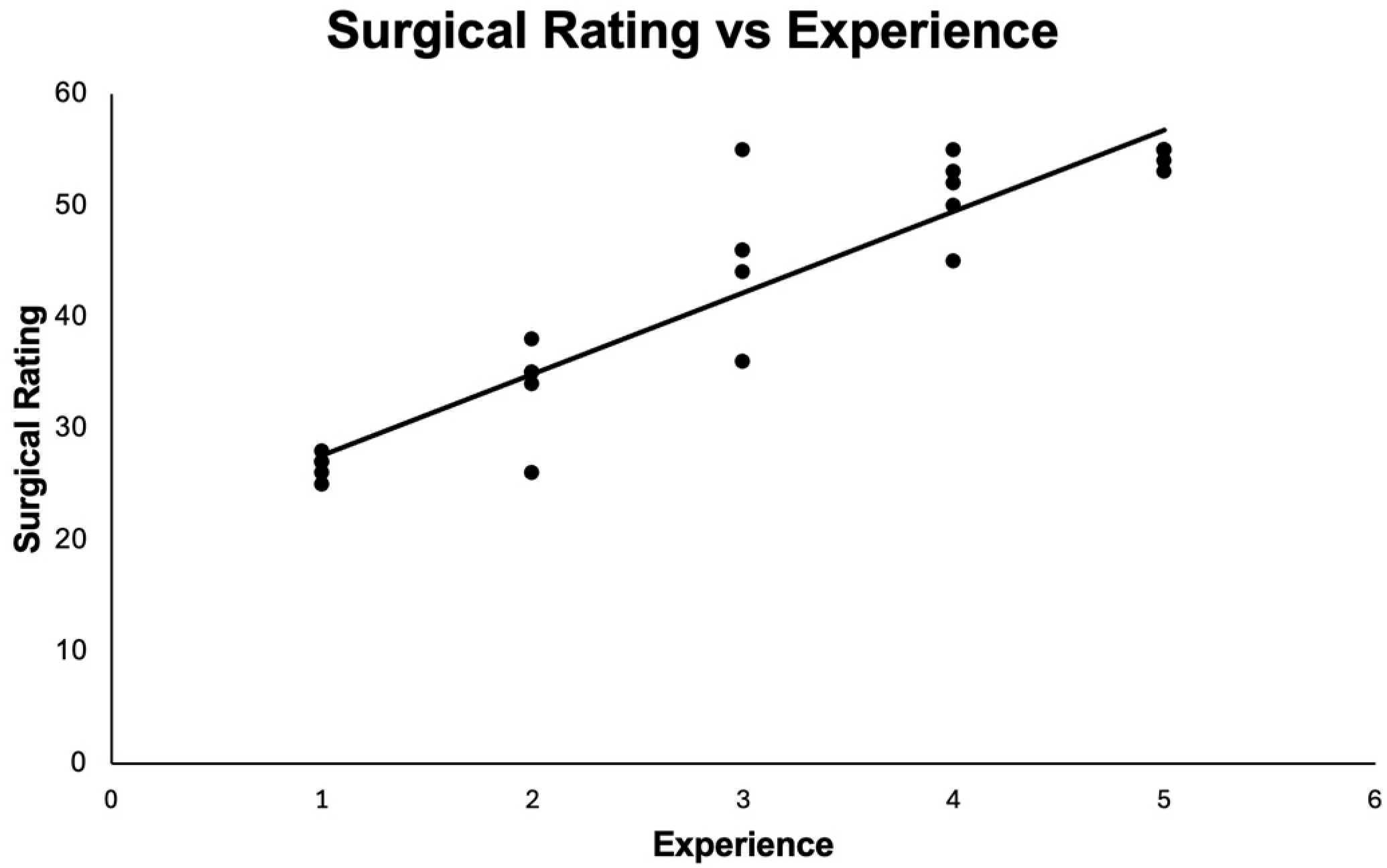
3.1. Surgical Outcomes
3.2. Fixations
3.3. Saccade Length
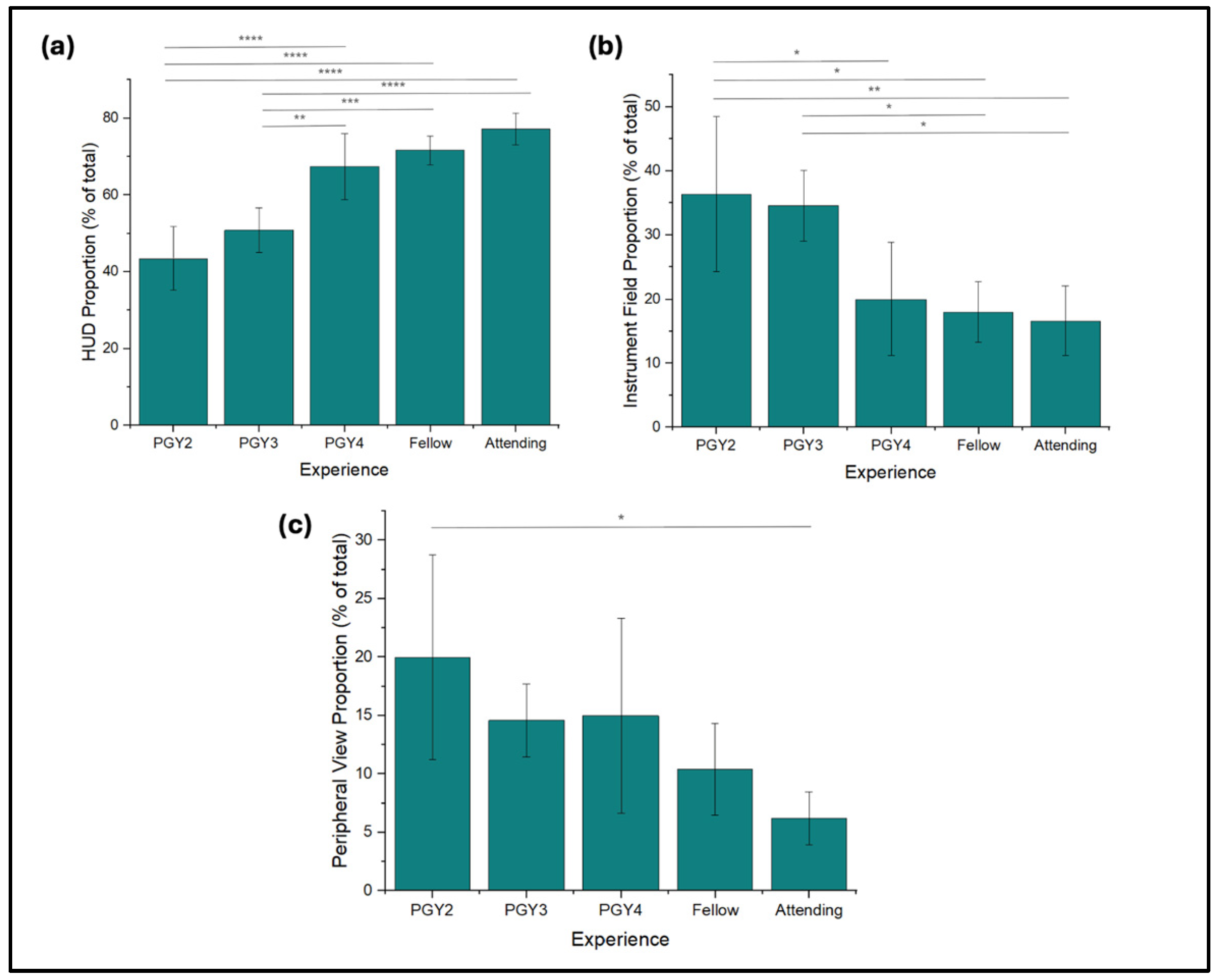
3.4. Gaze Distribution
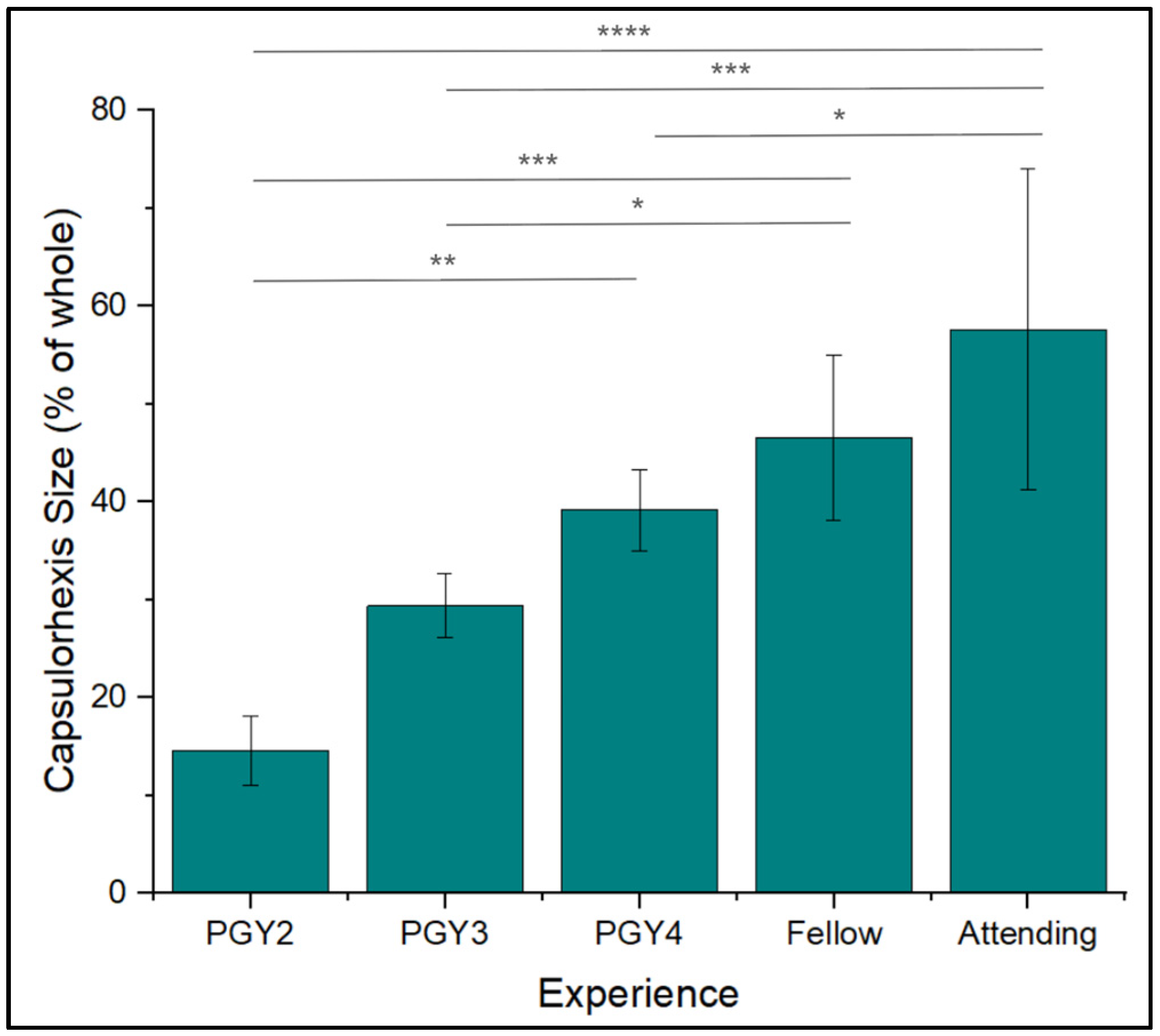
3.5. Capsulorhexis Diameter
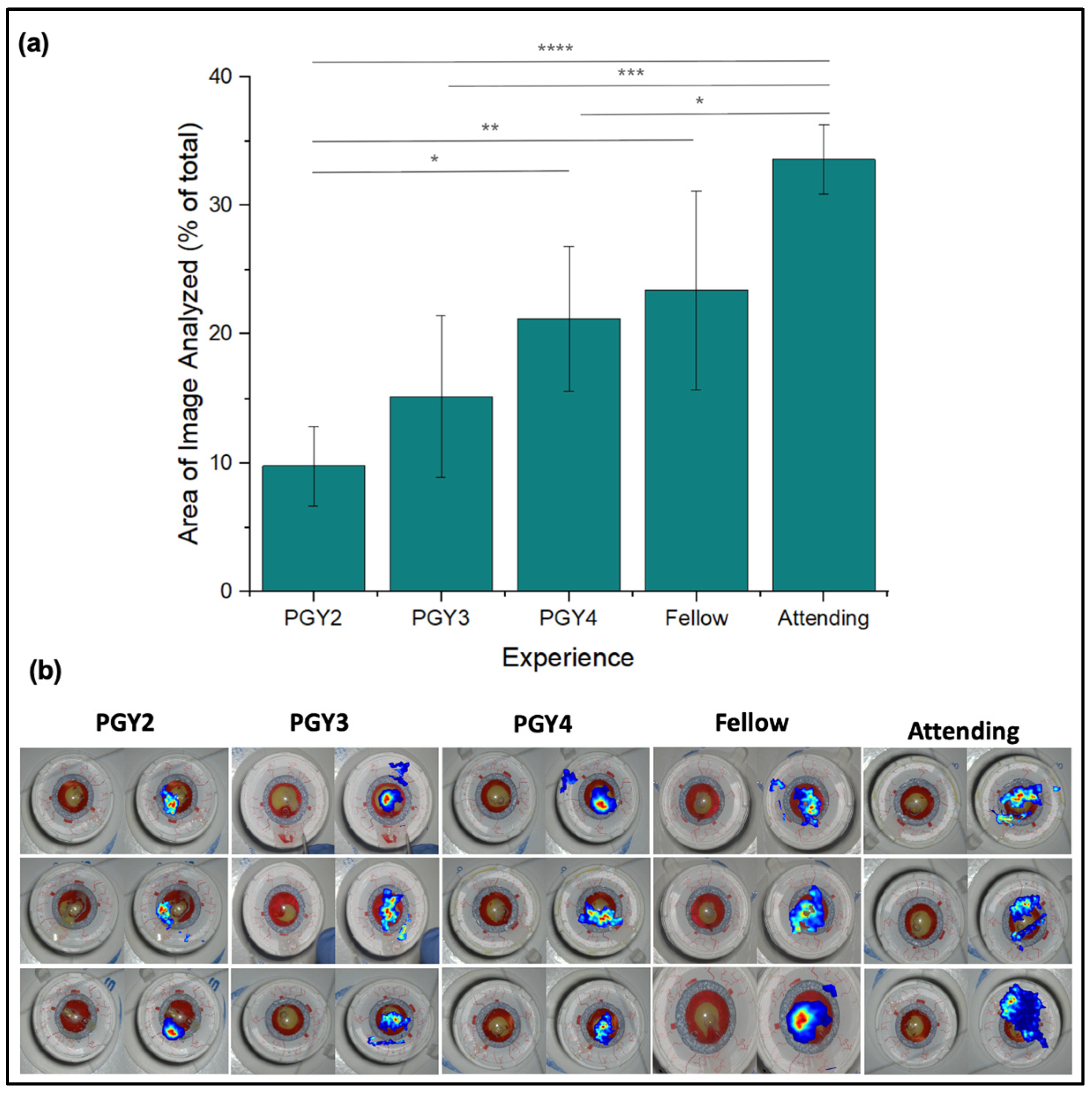
3.6. Area of Fixation
3.7. Angle of Tear Relative to Forceps
3.8. Tear Morphology
3.9. Qualitative Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HUD | Heads-up display |
| CCC | Continuous curvilinear capsulorhexis |
References
- Chen, X.; Xu, J.; Chen, X.; Yao, K. Cataract: Advances in surgery and whether surgery remains the only treatment in future. Adv. Ophthalmol. Pr. Res. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Nieddu, D.; Chighine, A.; Carai, A.; d’Aloja, E. From Routine to Risk: Medical Liability and the Legal Implications of Cataract Surgery in the Age of Trivialization. J. Clin. Med. 2025, 14, 6838. [Google Scholar] [CrossRef]
- Vajsbaher, T.; Schultheis, H.; Francis, N.K. Spatial cognition in minimally invasive surgery: A systematic review. BMC Surg. 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Grantcharov, T.; Moorthy, K.; Hance, J.; Darzi, A. A competency-based virtual reality training curriculum for the acquisition of laparoscopic psychomotor skill. Am. J. Surg. 2006, 191, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Donaldson, R.; Subramaniam, A.; Palmer, H.; Champion, C.D.; Cox, M.L.; Appelbaum, L.G. Developing Expert Gaze Pattern in Laparoscopic Surgery Requires More than Behavioral Training. J. Eye Mov. Res. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Chetwood, A.S.A.; Kwok, K.W.; Sun, L.-W.; Mylonas, G.P.; Clark, J.; Darzi, A.; Yang, G.-Z. Collaborative eye tracking: A potential training tool in laparoscopic surgery. Surg. Endosc. 2012, 26, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Menekse Dalveren, G.G.; Cagiltay, N.E. Using Eye-Movement Events to Determine the Mental Workload of Surgical Residents. J. Eye Mov. Res. 2018, 11, 28. [Google Scholar] [CrossRef]
- Harvey, A.; Vickers, J.N.; Snelgrove, R.; Scott, M.F.; Morrison, S. Expert surgeon’s quiet eye and slowing down: Expertise differences in performance and quiet eye duration during identification and dissection of the recurrent laryngeal nerve. Am. J. Surg. 2014, 207, 187–193. [Google Scholar] [CrossRef]
- Nespolo, R.G.; Cole, E.; Wang, D.; Yi, D.; Leiderman, Y.I. A platform for tracking surgeon and observer gaze as a surrogate for attention in ophthalmic surgery. Artif. Intell. Big Data 2023, 3, 100246. [Google Scholar] [CrossRef]
- Tien, T.; Pucher, P.H.; Sodergren, M.H.; Sriskandarajah, K.; Yang, G.Z.; Darzi, A. Eye tracking for skills assessment and training: A systematic review. J. Surg. Res. 2014, 191, 169–178. [Google Scholar] [CrossRef]
- Chen, H.-E.; Sonntag, C.C.; Pepley, D.F.; Prabhu, R.S.; Han, D.C.; Moore, J.Z.; Miller, S.R. Looks can be deceiving: Gaze pattern differences between novices and experts during placement of central lines. Am. J. Surg. 2019, 217, 362–367. [Google Scholar] [CrossRef]
- Weinstock, R.J.; Diakonis, V.F.; Schwartz, A.J.; Weinstock, A.J. Heads-up Cataract Surgery: Complication Rates, Surgical Duration, and Comparison With Traditional Microscopes. J. Refract. Surg. 2019, 35, 318–322. [Google Scholar] [CrossRef]
- Topalli, D.; Cagiltay, N.E. Eye-Hand Coordination Patterns of Intermediate and Novice Surgeons in a Simulation-Based Endoscopic Surgery Training Environment. J. Eye Mov. Res. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.P.; Young, A.L.; Behndig, A.; Chang, D.F.; Dhubhghaill, S.N.; Eom, Y.; Fan, A.H.; Findl, O.; Gundersen, K.G.; Khanna, R.C.; et al. Controversies, consensuses and guidelines on modern cataract surgery by the Academy of Asia-Pacific Professors of Ophthalmology (AAPPO). Asia Pac. J. Ophthalmol. 2025, 14, 100224. [Google Scholar] [CrossRef] [PubMed]
- Bapna, T.; Valles, J.; Leng, S.; Pacilli, M.; Nataraja, R.M. Eye-tracking in surgery: A systematic review. ANZ J. Surg. 2023, 93, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Zaman, N.; Kamran, S.A.; Sarker, P.; Tavakkoli, A.; Lee, A.G. Head-mounted display cataract surgery: A new frontier with eye tracking and foveated rendering technology. Eye 2024, 38, 1022–1023. [Google Scholar] [CrossRef]
- Gupta, P.; Sheth, N.; AlAhmadi, R.; Yao, X.; Heiferman, M.J. The Effect of Experience on Visual Search Patterns in Retinal Imaging Analysis. Ophthalmic Surg. Lasers Imaging Retin. 2025, 56, 336–344. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Gao, X.; Chen, Y. The eye activity measurement of mental workload based on basic flight task. In Proceedings of the IEEE 10th International Conference on Industrial Informatics, Beijing, China, 25–27 July 2012; pp. 502–507. [Google Scholar] [CrossRef]
- Marquart, G.; Cabrall, C.; de Winter, J. Review of Eye-related Measures of Drivers’ Mental Workload. Procedia Manuf. 2015, 3, 2854–2861. [Google Scholar] [CrossRef]
- Iqbal, S.T.; Zheng, X.S.; Bailey, B.P. Task-evoked pupillary response to mental workload in human-computer interaction. In Proceedings of the CHI ’04 Extended Abstracts on Human Factors in Computing Systems, Vienna, Austria, 24–29 April 2004; pp. 1477–1480. [Google Scholar] [CrossRef]
- Zheng, B.; Jiang, X.; Bednarik, R.; Atkins, M.S. Action-related eye measures to assess surgical expertise. BJS Open 2021, 5, zrab068. [Google Scholar] [CrossRef]
- Tahri Sqalli, M.; Aslonov, B.; Gafurov, M.; Mukhammadiev, N.; Sqalli Houssaini, Y. Eye tracking technology in medical practice: A perspective on its diverse applications. Front. Med. Technol. 2023, 5, 1253001. [Google Scholar] [CrossRef]
- Gallagher, A.G.; Richie, K.; McClure, N.; McGuigan, J. Objective psychomotor skills assessment of experienced, junior, and novice laparoscopists with virtual reality. World J. Surg. 2001, 25, 1478–1483. [Google Scholar] [CrossRef]
- Aghazadeh, F.; Zheng, B.; Tavakoli, M.; Rouhani, H. Motion Smoothness-Based Assessment of Surgical Expertise: The Importance of Selecting Proper Metrics. Sensors 2023, 23, 3146. [Google Scholar] [CrossRef]
- Ghasemloonia, A.; Maddahi, Y.; Zareinia, K.; Lama, S.; Dort, J.C.; Sutherland, G.R. Surgical Skill Assessment Using Motion Quality and Smoothness. J. Surg. Educ. 2017, 74, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Westling, G.; Bäckström, A.; Flanagan, J.R. Eye–Hand Coordination in Object Manipulation. J. Neurosci. 2001, 21, 6917–6932. [Google Scholar] [CrossRef] [PubMed]
- Sailer, U.; Flanagan, J.R.; Johansson, R.S. Eye–Hand Coordination during Learning of a Novel Visuomotor Task. J. Neurosci. 2005, 25, 8833–8842. [Google Scholar] [CrossRef]
- Magyar, M.; Sándor, G.L.; Ujváry, L.; Nagy, Z.Z.; Tóth, G. Intraoperative complication rates in cataract surgery performed by resident trainees and staff surgeons in a tertiary eyecare center in Hungary. Int. J. Ophthalmol. 2022, 15, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wairagade, N.A.; Devasia, J.; Deshmukh, M.; Surana, S.D. Complication rates in phacoemulsification surgeries performed by trainee residents. Global J. Cataract. Surg. Res. Ophthalmol. 2024, 3, 28–33. [Google Scholar] [CrossRef]
- Ellis, E.M.; Lee, J.E.; Saunders, L.; Haw, W.W.; Granet, D.B.; Heichel, C.W. Complication rates of resident-performed cataract surgery: Impact of early introduction of cataract surgery training. J. Cataract. Refract. Surg. 2018, 44, 1109–1115. [Google Scholar] [CrossRef]
- Kasa, K.; Burns, D.; Goldenberg, M.G.; Selim, O.; Whyne, C.; Hardisty, M. Multi-Modal Deep Learning for Assessing Surgeon Technical Skill. Sensors 2022, 22, 7328. [Google Scholar] [CrossRef]
- Shafiei, S.B.; Shadpour, S.; Mohler, J.L.; Attwood, K.; Liu, Q.; Gutierrez, C.; Toussi, M.S. Developing surgical skill level classification model using visual metrics and a gradient boosting algorithm. Ann. Surg. Open 2023, 4, e292. [Google Scholar] [CrossRef]
- Lavanchy, J.L.; Zindel, J.; Kirtac, K.; Twick, I.; Hosgor, E.; Candinas, D.; Beldi, G. Automation of surgical skill assessment using a three-stage machine learning algorithm. Sci. Rep. 2021, 11, 5197. [Google Scholar] [CrossRef]
- Nair, A.G.; Ahiwalay, C.; Bacchav, A.E.; Sheth, T.; Lansingh, V.C.; Vedula, S.S.; Bhatt, V.; Reddy, J.C.; Vadavalli, P.K.; Praveen, S.; et al. Effectiveness of simulation-based training for manual small incision cataract surgery among novice surgeons: A randomized controlled trial. Sci. Rep. 2021, 11, 10945. [Google Scholar] [CrossRef]
- Ritter, E.M.; Scott, D.J. Design of a proficiency-based skills training curriculum for the fundamentals of laparoscopic surgery. Surg. Innov. 2007, 14, 107–112. [Google Scholar] [CrossRef]
- Wilson, M.R.; McGrath, J.S.; Vine, S.J.; Brewer, J.; Defriend, D.; Masters, R.S.W. Perceptual impairment and psychomotor control in virtual laparoscopic surgery. Surg. Endosc. 2011, 25, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Vine, S.J.; Bright, E.; Masters, R.S.W.; Defriend, D.; McGrath, J.S. Gaze training enhances laparoscopic technical skill acquisition and multi-tasking performance: A randomized, controlled study. Surg. Endosc. 2011, 25, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Fichtel, E.; Lau, N.; Park, J.; Henrickson Parker, S.; Ponnala, S.; Fitzgibbons, S. Eye-tracking in surgical education: Gaze-based dynamic area of interest can discriminate adverse events and expertise. Surg. Endosc. 2019, 33, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Berges, A.J.; Vedula, S.S.; Chara, A.; Hager, G.D.; Ishii, M.; Malpani, A. Eye tracking and motion data predict endoscopic sinus surgery skill. Laryngoscope 2023, 133, 500–505. [Google Scholar] [CrossRef]
- Chainey, J.; Elomaa, A.P.; O’Kelly, C.J.; Kim, M.J.; Bednarik, R.; Zheng, B. Eye-hand coordination of neurosurgeons: Evidence of action-related fixation in microsuturing. World Neurosurg. 2021, 155, e196–e202. [Google Scholar] [CrossRef]
- Koskinen, J.; Torkamani-Azar, M.; Hussein, A.; Huotarinen, A.; Bednarik, R. Automated tool detection with deep learning for monitoring kinematics and eye-hand coordination in microsurgery. Comput. Biol. Med. 2022, 141, 105121. [Google Scholar] [CrossRef]
- Richstone, L.; Schwartz, M.J.; Seideman, C.; Cadeddu, J.; Marshall, S.; Kavoussi, L.R. Eye metrics as an objective assessment of surgical skill. Ann. Surg. 2010, 252, 177–182. [Google Scholar] [CrossRef]


| Current Position | PGY2 | PGY3 | PGY4 | Fellows | Attendings |
|---|---|---|---|---|---|
| Sample (n) | 5 | 5 | 5 | 5 | 5 |
| Age (yrs; mean ± sd) | 29.3 ± 1.4 | 30.2 ± 1.21 | 31.5 ± 2.12 | 33.1 ± 2.54 | 44.5 ± 8.32 |
| Years of Experience | 0.5 ± 0.13 | 1.4 ± 0.23 | 2.2 ± 0.42 | 4.4 ± 0.72 | 14.5 ± 3.61 |
| Males/Females | 3/2 | 2/3 | 3/2 | 4/1 | 2/3 |
| Current Position | PGY2 | PGY3 | PGY4 | Fellows | Attendings |
|---|---|---|---|---|---|
| Sample (n) | 5 | 5 | 5 | 5 | 5 |
| No. of Capsulorhexis Procedures | 0 ± 0 | 15 ± 1.31 | 115 ± 14.12 | 292.9 ± 85.41 | 478.31 ± 121.48 |
| No. of Corneal | 0.6 ± 0.91 | 12 ± 1.11 | 123 ± 21.71 | 345 ± 132.13 | 632 ± 292.22 |
| No. of Wound Closure | 0 ± 0 | 7.37 ± 4.69 | 56 ± 32.53 | 176 ± 224.97 | 363 ± 287.09 |
| Modified ICO-OSCAR Score | 26 ± 1.14 | 33.6 ± 4.51 | 45.4 ± 6.76 | 51 ± 3.81 | 54.4 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, P.; Kao, E.; Sheth, N.; Alahmadi, R.; Heiferman, M.J. Gaze Characteristics Using a Three-Dimensional Heads-Up Display During Cataract Surgery. J. Eye Mov. Res. 2025, 18, 68. https://doi.org/10.3390/jemr18060068
Gupta P, Kao E, Sheth N, Alahmadi R, Heiferman MJ. Gaze Characteristics Using a Three-Dimensional Heads-Up Display During Cataract Surgery. Journal of Eye Movement Research. 2025; 18(6):68. https://doi.org/10.3390/jemr18060068
Chicago/Turabian StyleGupta, Puranjay, Emily Kao, Neil Sheth, Reem Alahmadi, and Michael J. Heiferman. 2025. "Gaze Characteristics Using a Three-Dimensional Heads-Up Display During Cataract Surgery" Journal of Eye Movement Research 18, no. 6: 68. https://doi.org/10.3390/jemr18060068
APA StyleGupta, P., Kao, E., Sheth, N., Alahmadi, R., & Heiferman, M. J. (2025). Gaze Characteristics Using a Three-Dimensional Heads-Up Display During Cataract Surgery. Journal of Eye Movement Research, 18(6), 68. https://doi.org/10.3390/jemr18060068







