Eye Movement Impairment in Women Undergoing Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Statements
2.3. Participants and Eligibility Criteria
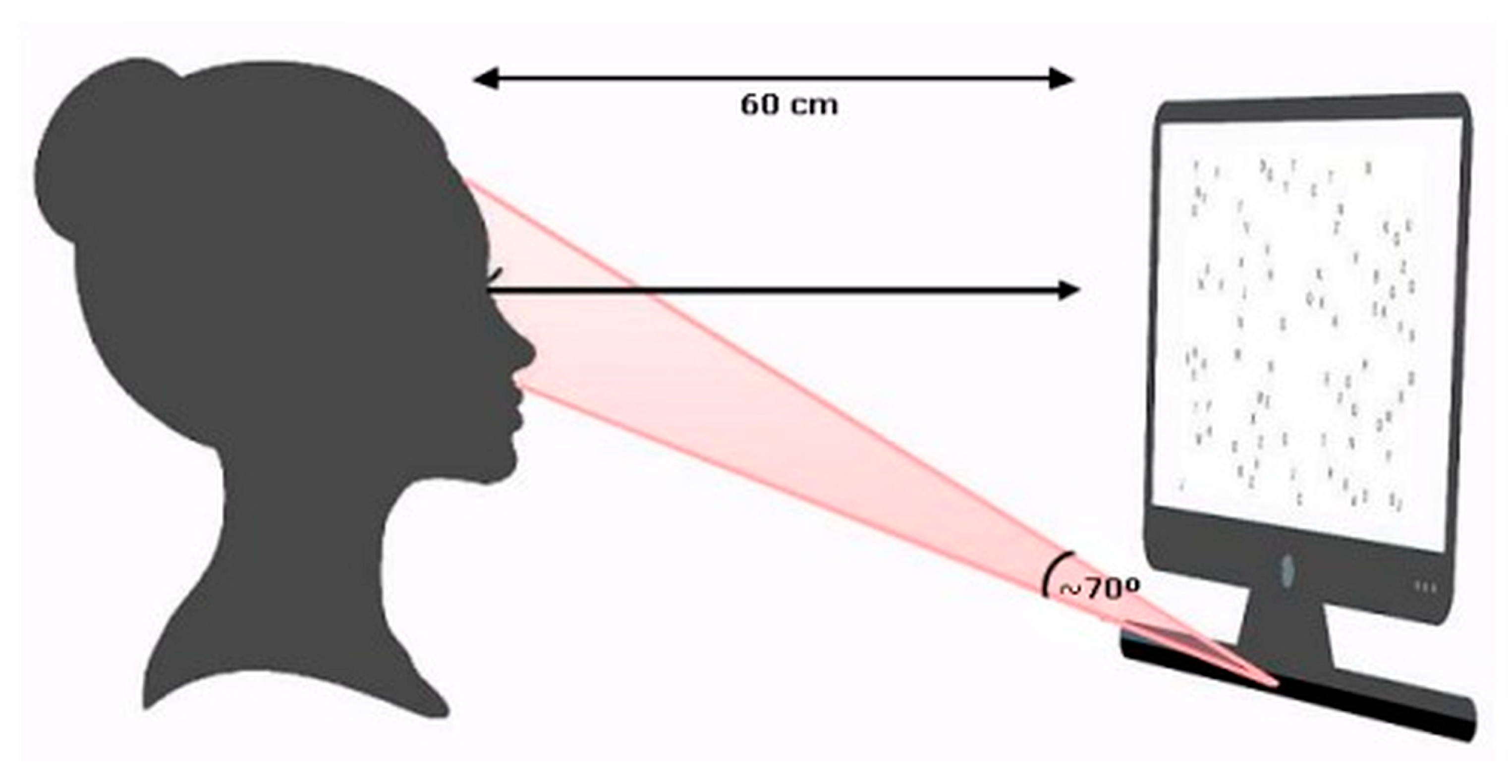
2.4. Procedure and Stimulus
2.5. Visual Search Task
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| CRCI | Chemotherapy-related Cognitive Impairment |
| CT | Chemotherapy Treatment |
| HC | Healthy Controls |
| QL | Quality of Life |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Amjad, M.; Chidharla, A.; Kasi, A. Cancer Chemotherapy; StatPearls Publishing: St. Petersburg, FL, USA, 2023; Volume 1. Available online: https://pubmed.ncbi.nlm.nih.gov/33232037/ (accessed on 23 February 2025).
- Santomasso. Anticancer Drugs and the Nervous System. Contin. Lifelong Learn. Neurol. 2020, 26, 732–764. [Google Scholar] [CrossRef]
- Durán-Gómez, N.; López-Jurado, C.F.; Nadal-Delgado, M.; Pérez-Civantos, D.; Guerrero-Martín, J.; Cáceres, M.C. Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis. J. Clin. Med. 2022, 11, 2363. [Google Scholar] [CrossRef]
- Magnuson, A.; Lei, L.; Gilmore, N.; Kleckner, A.S.; Lin, F.V.; Ferguson, R.; Hurria, A.; Wittink, M.N.; Esparaz, B.T.; Giguere, J.K.; et al. Longitudinal Relationship Between Frailty and Cognition in Patients 50 Years and Older with Breast Cancer. J. Am. Geriatr. Soc. 2019, 67, 928–936. [Google Scholar] [CrossRef]
- Grotheer, M.; Kubota, E.; Grill-Spector, K. Establishing the functional relevancy of white matter connections in the visual system and beyond. Brain Struct. Funct. 2022, 227, 1347–1356. [Google Scholar] [CrossRef]
- Frattini, D.; Wibble, T. Alertness and Visual Attention Impact Different Aspects of the Optokinetic Reflex. Investig. Opthalmology Vis. Sci. 2021, 62, 16. [Google Scholar] [CrossRef]
- Song, W.; Zhang, G. Risky-Driving-Image Recognition Based on Visual Attention Mechanism and Deep Learning. Sensors 2022, 22, 5868. [Google Scholar] [CrossRef] [PubMed]
- Alwi, S.M.S.; Narayanan, V.; Taib, N.A.M.; Din, N.C. Chemotherapy-related cognitive impairment (CRCI) among early-stage breast cancer survivors in Malaysia. J. Clin. Exp. Neuropsychol. 2021, 43, 534–545. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Tao, L.; Cheng, H.; Li, J.; Zhang, J.; Qiu, B.; Yu, Y.; Wang, K. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: Evidence from an arterial spin labeling perfusion study. Sci. Rep. 2017, 7, 42684. [Google Scholar] [CrossRef]
- De Oliveira, M.E.C.; Silva, G.M.; Lima, E.S.H.; Almeida, N.L.; Fernandes, T.; Negreiros, N.d.S.; Trombetta, B.d.N.T.; Santos, N.A. Consequences of Antineoplastic Treatment on Visual Processing of Women with Breast Cancer: A Systematic Review. Trends Psychol. 2023, 33, 536–560. [Google Scholar] [CrossRef]
- Souto, D.; Kerzel, D. Visual selective attention and the control of tracking eye movements: A critical review. J. Neurophysiol. 2021, 125, 1552–1576. [Google Scholar] [CrossRef]
- Wong, O.W.H.; Fung, G.P.C.; Chan, S. Characterizing the Relationship Between Eye Movement Parameters and Cognitive Functions in Non-demented Parkinson’s Disease Patients with Eye Tracking. J. Vis. Exp. 2019, e60052. [Google Scholar] [CrossRef]
- Lanonis, F.R.; Jiménez, M.A.; Gasaneo, G.; Rosso, O.A.; Delrieux, C.A. Ordinal pattern transition networks in eye tracking reading signals. Chaos 2023, 33, 053101. [Google Scholar] [CrossRef]
- Brajkovich, H.L. Dr. Snellen’s 20/20: The development and use of the eye chart. J. Sch. Health 1980, 50, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, J.; Ren, X.; Yu, Z.; Zhang, R.; Li, X. Evaluation of eye movements and visual performance in patients with cataract. Sci. Rep. 2020, 10, 9875. [Google Scholar] [CrossRef]
- Rösler, A.; Mapstone, M.E.; Hays, A.K.; Mesulam, M.M.; Rademaker, A.; Gitelman, D.R.; Weintraub, S. Alterations of visual search strategy in Alzheimer’s disease and aging. Neuropsychology 2000, 14, 398–408. [Google Scholar] [CrossRef]
- Sun, S.; Hongbo, Y.; Wang, S.; Yu, R. Cognitive and neural bases of visual-context-guided decision-making. Neuroimage 2023, 15, 120170. [Google Scholar] [CrossRef]
- Liu, J.C.; Li, K.A.; Yeh, S.L.; Chien, S.Y. Assessing Perceptual Load and Cognitive Load by Fixation-Related Information of Eye Movements. Sensors 2022, 22, 1187. [Google Scholar] [CrossRef]
- Anderson, D.E.; Holstein, S.A.; Kedar, S. Visual Pathway Degeneration in Chemotherapy-Related Neurotoxicity: A Review and Directions for Future Research. Neuro-Ophthalmology 2020, 44, 139–147. [Google Scholar] [CrossRef]
- Chelala, E.; Arej, N.; Antoun, J.; Kourie, H.R.; Zaarour, K.; Haddad, F.G.; Farhat, F.; El Karak, F.; Kattan, J. Central Macular Thickness Monitoring after a Taxane-Based Therapy in Visually Asymptomatic Patients. Chemotherapy 2017, 62, 199–204. [Google Scholar] [CrossRef]
- Raffa, R.B.; Tallarida, R.J. Effects on the visual system might contribute to some of the cognitive deficits of cancer chemotherapy-induced ‘chemo-fog’: Ocular toxicity and ‘chemo-fog’. J. Clin. Pharm. Ther. 2010, 35, 249–255. [Google Scholar] [CrossRef]
- Chung, S.T.L. Training to improve temporal processing of letters benefits reading speed for people with central vision loss. J. Vis. 2021, 21, 14. [Google Scholar] [CrossRef]
- Goldberg, J.H.; Kotval, X.P. Computer interface evaluation using eye movements: Methods and constructs. Int. J. Ind. Ergon. 1999, 24, 631–645. [Google Scholar] [CrossRef]
- Andryszak, P.; Wiłkość, M.; Żurawski, B.; Izdebski, P. Verbal fluency in breast cancer patients treated with chemotherapy. Breast Cancer 2017, 24, 376–383. [Google Scholar] [CrossRef]
- Apple, A.C.; Schroeder, M.P.; Ryals, A.J.; Wagner, L.I.; Cella, D.; Shih, P.-A.; Reilly, J.; Penedo, F.J.; Voss, J.L.; Wang, L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clin. 2018, 20, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Poppelreuter, M.; Bartsch, H.H. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ‘subjective’ complaints and ‘objective’ neuropsychological test results. Psycho-Oncology 2009, 18, 775–782. [Google Scholar] [CrossRef]
- Seigers, R.; Loos, M.; Van Tellingen, O.; Boogerd, W.; Smit, A.B.; Schagen, S.B. Neurobiological changes by cytotoxic agents in mice. Behav. Brain Res. 2016, 299, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wagawade, J.D.; Roy, S.; Hullatti, K.; Khode, N.R.; Pednekar, H.; Hegde, H.V.; Kholkute, S.D. Doxorubicin-induced cognition impairment in a rat model. Asian J. Pharm. Clin. Res. 2015, 8, 301–304. Available online: https://journals.innovareacademics.in/index.php/ajpcr/article/view/5711 (accessed on 13 April 2025).
- McDonald, B.C.; Saykin, A.J. Alterations in brain structure related to breast cancer and its treatment: Chemotherapy and other considerations. Brain Imaging Behav. 2013, 7, 374–387. [Google Scholar] [CrossRef]
- Simó, M.; Rifà-Ros, X.; Rodriguez-Fornells, A.; Bruna, J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 1311–1321. [Google Scholar] [CrossRef]
- Brown, T.; Sykes, D.; Allen, A.R. Implications of Breast Cancer Chemotherapy-Induced Inflammation on the Gut, Liver, and Central Nervous System. Biomedicines 2021, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Stüve, O. Review: Cyclophosphamide in multiple sclerosis: Scientific rationale, history and novel treatment paradigms. Ther. Adv. Neurol. Disord. 2009, 2, 357–368. [Google Scholar] [CrossRef]
- Formica, V.; Leary, A.; Cunningham, D.; Chua, Y.J. 5-Fluorouracil can cross brain–blood barrier and cause encephalopathy: Should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma. Cancer Chemother. Pharmacol. 2006, 58, 276–278. [Google Scholar] [CrossRef]
- Montague, K.; Malcangio, M. The Therapeutic Potential of Monocyte/Macrophage Manipulation in the Treatment of Chemotherapy-Induced Painful Neuropathy. Front. Mol. Neurosci. 2017, 10, 397. [Google Scholar] [CrossRef]
- Vyas, D.; Laput, G.; Vyas, A. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets Ther. 2014, 7, 1015–1023. [Google Scholar] [CrossRef]
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef]
- Farrell, C.; Brearley, S.G.; Pilling, M.; Molassiotis, A. The impact of chemotherapy-related nausea on patients’ nutritional status, psychological distress and quality of life. Support. Care Cancer 2012, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, L.; Zheng, X.; Shi, Y. Quality of life in patients with breast cancer with neoadjuvant chemotherapy: A systematic review. BMJ Open 2022, 12, e061967. [Google Scholar] [CrossRef]
- Bachman, P.; Reichenberg, A.; Rice, P.; Woolsey, M.; Chaves, O.; Martinez, D.; Maples, N.; Velligan, D.I.; Glahn, D.C. Deconstructing processing speed deficits in schizophrenia: Application of a parametric digit symbol coding test. Schizophr. Res. 2010, 118, 6–11. [Google Scholar] [CrossRef] [PubMed]


| Chemotherapy (N = 12) | Control (N = 12) | ||
|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | p Value * |
| Age, years | 47.17 ± 6.79 | 42.92 ± 6.00 | 0.11 |
| Chemotherapy cycles | 20.58 ± 14.14 | - | - |
| QL- Functional capacity | 43.75 ± 24.22 | 82.50 ± 12.70 | 0.00 * |
| QL-Physical limitation | 6.25 ± 11.30 | 60.42 ± 36.08 | 0.00 * |
| QL-Pain | 46.75 ± 18.60 | 59.83 ± 24.28 | 0.15 |
| QL-General health status | 49.75 ± 19.58 | 59.75 ± 20.35 | 0.23 |
| QL-Vitality | 48.75 ± 17.98 | 54.58 ± 10.54 | 0.34 |
| QL-Social aspects | 50.00 ± 21.32 | 74.00 ± 25.28 | 0.02 * |
| QL-Emotional limitation | 25.96 ± 39.90 | 57.08 ± 41.88 | 0.07 |
| QL-Mental health | 58.67 ± 25.60 | 70.67 ± 13.02 | 0.16 |
| Duration of application | 26.06 ± 20.25 | 11.97 ± 5.43 | 0.03 * |
| Duration of fixation (seconds) | 0.23 ± 0.20 | 0.19 ± 0.21 | 0.00 * |
| Total duration of fixation (seconds) | 14.18 ± 6.38 | 9.12 ± 3.43 | 0.02 * |
| Total visit duration (seconds) | 17.52 ± 7.63 | 12.10 ± 5.07 | 0.05 * |
| Test Duration | Duration of Fixation | Total Duration of Fixation | Total Visit Duration | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | r | p Value | r | p Value | r | p Value | r | p Value |
| Chemotherapy cycles | 0.51 | 0.01 * | 0.68 | 0.00 * | 0.49 | 0.01 * | 0.50 | 0.01 * |
| QL–Functional capacity | −0.48 | 0.01 * | −0.48 | 0.01 * | −0.56 | 0.00 * | −0.56 | 0.00 * |
| QL–Physical limitation | −0.47 | 0.01 * | −0.60 | 0.00 * | −0.31 | 0.13 | −0.19 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.E.C.; Sousa, J.M.N.d.; Da Silva Vieira Torres, G.; de Brito, R.P.S.; Negreiros, N.d.S.; da Nóbrega Tomaz Trombetta, B.; Alexandrino, K.A.L.G.; Nóbrega, W.F.S.; Polimeni, L.L.S.S.; Braga, C.C.; et al. Eye Movement Impairment in Women Undergoing Chemotherapy. J. Eye Mov. Res. 2025, 18, 41. https://doi.org/10.3390/jemr18050041
de Oliveira MEC, Sousa JMNd, Da Silva Vieira Torres G, de Brito RPS, Negreiros NdS, da Nóbrega Tomaz Trombetta B, Alexandrino KALG, Nóbrega WFS, Polimeni LLSS, Braga CC, et al. Eye Movement Impairment in Women Undergoing Chemotherapy. Journal of Eye Movement Research. 2025; 18(5):41. https://doi.org/10.3390/jemr18050041
Chicago/Turabian Stylede Oliveira, Milena Edite Casé, José Marcos Nascimento de Sousa, Gerlane Da Silva Vieira Torres, Ruanna Priscila Silva de Brito, Nathalia dos Santos Negreiros, Bianca da Nóbrega Tomaz Trombetta, Kedma Anne Lima Gomes Alexandrino, Waleska Fernanda Souto Nóbrega, Letícia Lorena Soares Silva Polimeni, Catarina Cavalcanti Braga, and et al. 2025. "Eye Movement Impairment in Women Undergoing Chemotherapy" Journal of Eye Movement Research 18, no. 5: 41. https://doi.org/10.3390/jemr18050041
APA Stylede Oliveira, M. E. C., Sousa, J. M. N. d., Da Silva Vieira Torres, G., de Brito, R. P. S., Negreiros, N. d. S., da Nóbrega Tomaz Trombetta, B., Alexandrino, K. A. L. G., Nóbrega, W. F. S., Polimeni, L. L. S. S., Braga, C. C., Lima, C. M. S. d. S., Fernandes, T. P., & Santos, N. A. d. (2025). Eye Movement Impairment in Women Undergoing Chemotherapy. Journal of Eye Movement Research, 18(5), 41. https://doi.org/10.3390/jemr18050041






