A Review of Digital Eye Strain: Binocular Vision Anomalies, Ocular Surface Changes, and the Need for Objective Assessment
Abstract
1. Introduction
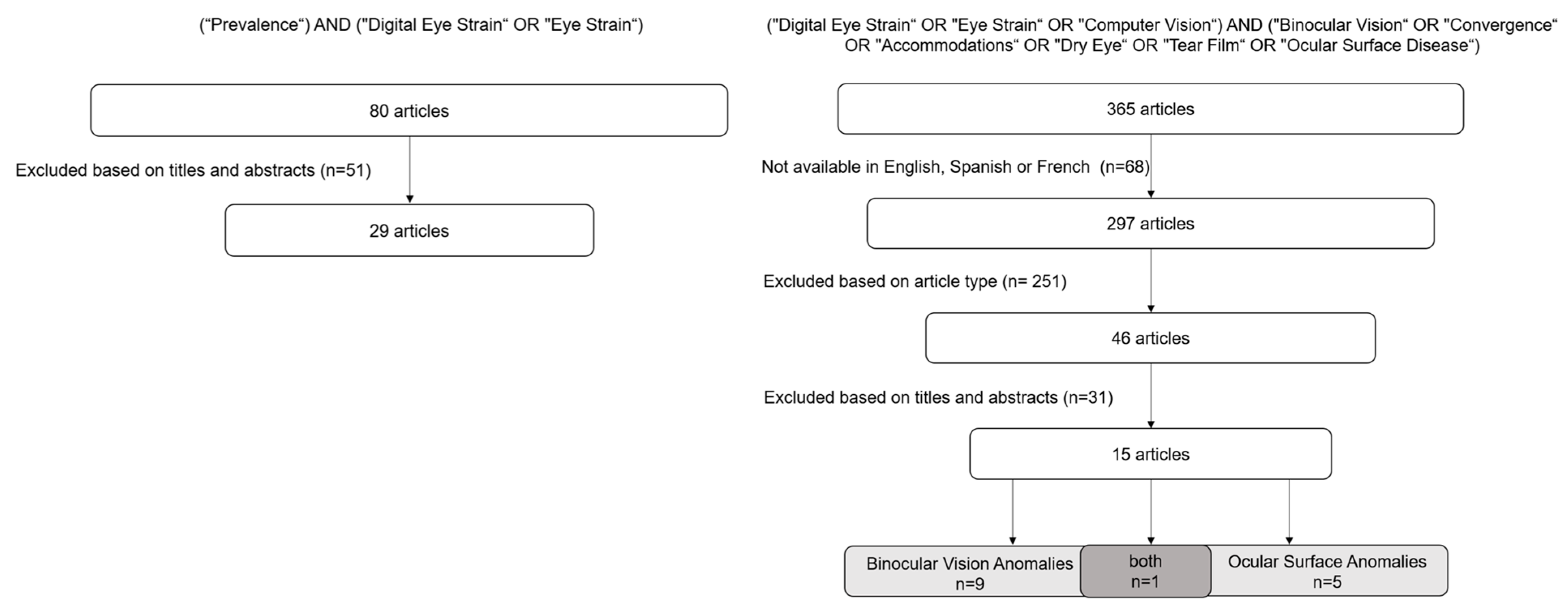
2. Materials and Methods
3. Results and Discussion
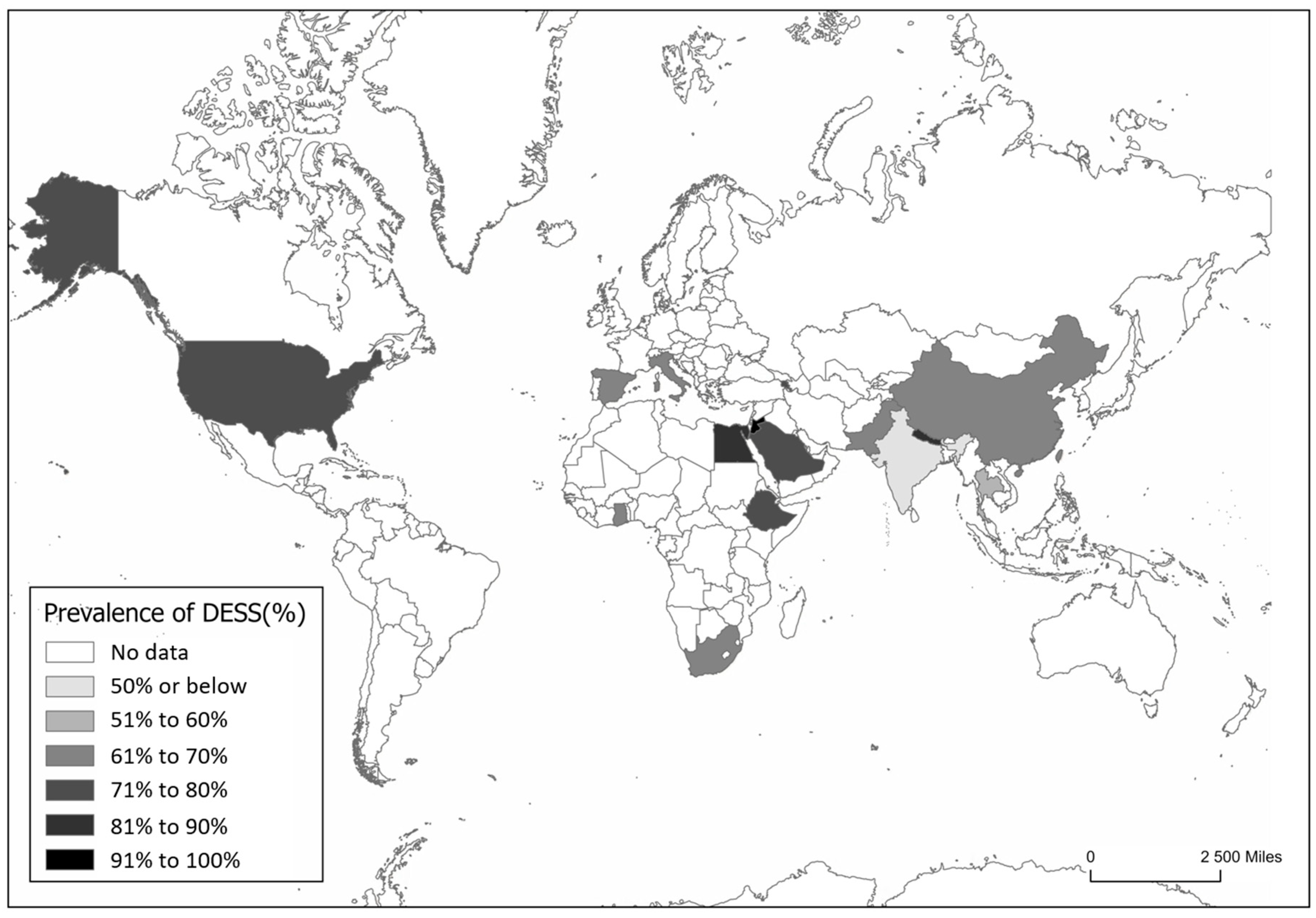
3.1. Prevalence of DESS
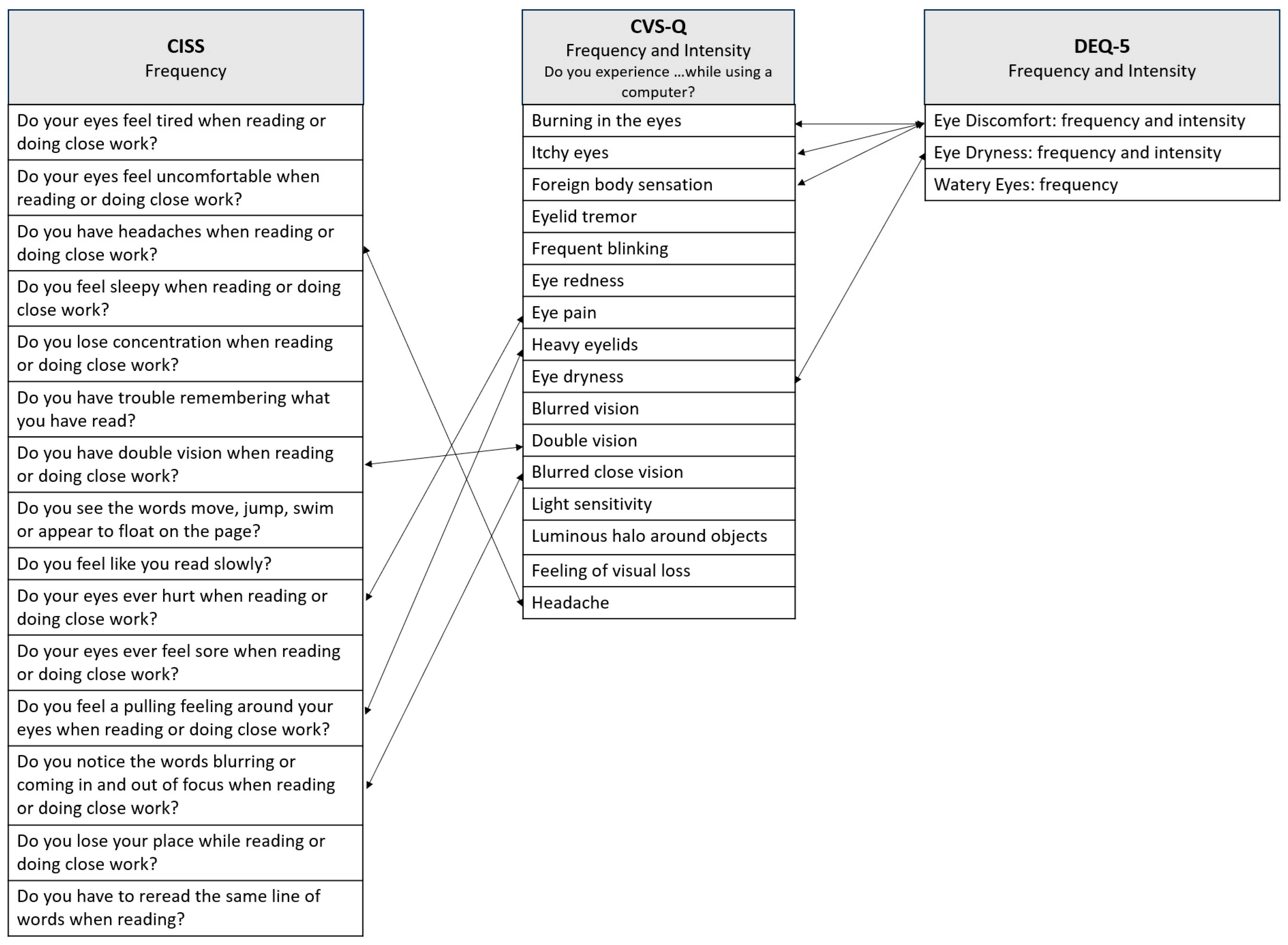
3.2. Symptoms of DESS
3.3. Binocular Vision Anomalies
- Accommodative Dysfunction and Vergence Dysfunction
3.4. Ocular Surface Anomalies
- Display-Related Factors Influencing Ocular Surface
- Blinking
- Dry Eye Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Accommodation Amplitude |
| BAF | Binocular Accommodative Facility |
| BCVA | Best-Corrected Visual Acuity |
| CI | Confidence Interval |
| CISS | Convergence Insufficiency Symptom Survey |
| CFF | Critical Flicker Fusion Frequency |
| CFS | Corneal Fluorescein Staining |
| cm | Centimeters |
| cpm | Cycles per Minute |
| CT | Cover Test |
| CVS | Computer Visual Syndrome |
| CVS-Q | Computer Vision Syndrome Questionnaire |
| CVSS17 | Computer Vision Symptom Scale 17 |
| D | Diopters |
| DED | Dry Eye Disease |
| DEQ-5 | Dry Eye Questionnaire version 5 |
| DESS | Digital Eye Strain Syndrome |
| eINK | Electronic INK |
| FTBUT | Fluorescein Tear Break-up Time |
| FV | Fusional Vergence |
| HDDD | High-Demand Digital Devices |
| iBUT | Invasive Tear Break-up Time |
| LDDD | Low Demand Digital Devices |
| MAF | Monocular Accommodative Facility |
| MEM | Monocular Estimate Method |
| mm | Millimeters |
| NIBUT | Non-Invasive Tear Break-up Time |
| NFV | Negative Fusional Vergence |
| NPA | Near Point Accommodation |
| NPC | Near Point of Convergence |
| NRA | Negative Relative Accommodation |
| OLED | Organic Light-Emitting Diode; |
| OR | Odds Ratio |
| OSDI | Ocular Surface Disease Index |
| PFV | Positive Fusional Vergence |
| PRA | Positive Relative Accommodation |
| RCT | Randomized Controlled Trials |
| RS | Reading Speed |
| SANDE | Symptom Assessment in Dry Eye |
| SQVD | Symptom Questionnaire for Visual Dysfunctions |
| SD | Standard Deviation |
| TBUT | Tear Break-Up Time |
| TFD | Total Fixation Duration |
| TFF | Time First Fixation |
| TMH | Tear Meniscus Height |
| TVD | Total Visit Duration |
| VA | Visual Acuity |
| VAS | Visual Analogue Scale |
| VDT | Video Digital Terminal |
| WRRT | Wilkins Rate of Reading Test |
| ΔFTBUT | Range of Fluorescein Tear Break-up Time |
References
- Sheppard, A.L.; Wolffsohn, J.S. Digital eye strain: Prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018, 3, e000146. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, J. Manual De Ergoftalmologia; Sociedade Portuguesa de Oftalmologia: Lisbon, Portugal, 2016; p. 40. Available online: https://spoftalmologia.pt/wp-content/uploads/2016/12/MANUAL-DE-ERGOFTALMOLOGIA.pdf (accessed on 14 September 2024).
- Abusamak, M.; Jaber, H.M.; Alrawashdeh, H.M. The Effect of Lockdown Due to the COVID-19 Pandemic on Digital Eye Strain Symptoms Among the General Population: A Cross-Sectional Survey. Front. Public Health 2022, 10, 895517. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Tripathi, A.; Khan, I.A.; Agarwal, M. Effect of increased screen time on eyes during COVID-19 pandemic. J. Family Med. Prim. Care 2022, 11, 3642–3647. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Pinitpuwadol, W.; Angsriprasert, D.; Chonpimai, P.; Saiman, M. Tear Film Change and Ocular Symptoms After Reading Printed Book and Electronic Book: A Crossover Study. Jpn. J. Ophthalmol. 2019, 63, 137–144. [Google Scholar] [CrossRef]
- Zambarbieri, D.; Carniglia, E. Eye movement analysis of reading from computer displays, eReaders and printed books. Ophthalmic Physiol. Opt. 2012, 32, 390–396. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Alabdulkader, A.M.; Alghamdi, A.N.; Altayeb, J.; Jabaan, R.; Assaf, L.; Alanazi, R.A. Prevalence of Digital Eye Strain Among University Students and Its Association with Virtual Learning During the COVID-19 Pandemic. Clin. Ophthalmol. 2023, 17, 1755–1768. [Google Scholar] [CrossRef]
- Almudhaiyan, T.M.; Aldebasi, T.; Alakel, R.; Marghlani, L.; Aljebreen, A.; Moazin, O.M. The Prevalence and Knowledge of Digital Eye Strain Among the Undergraduates in Riyadh, Saudi Arabia. Cureus J. Med. Sci. 2023, 15, e37081. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, Y. Digital Eye Strain and Its Risk Factors Among a University Student Population in Jordan: A Cross-Sectional Study. Cureus 2021, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, R.; El Meski, N.; Saba, J.; Lahoud, C.; Saab, L.; Haouili, M.; Shatila, M.; Aidibe, Z.; Musharrafieh, U. Asthenopia among university students: The eye of the digital generation. J. Family Med. Prim. Care 2020, 9, 3921–3932. [Google Scholar]
- Kaur, K.; Gurnani, B.; Nayak, S.; Deori, N.; Kaur, S.; Jethani, J.; Singh, D.; Agarkar, S.; Hussaindeen, J.R.; Sukhija, J.; et al. Digital Eye Strain—A Comprehensive Review. Ophthalmol. Ther. 2022, 11, 1655–1680. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, M. Computer vision syndrome: A review of ocular causes and potential treatments. Ophthalmic Physiol. Opt. 2011, 31, 502–515. [Google Scholar] [CrossRef]
- Rossi, G.C.M.; Scudeller, L.; Bettio, F.; Pasinetti, G.M.; Bianchi, P.E. Prevalence of Dry Eye in Video Display Terminal Users: A Cross-Sectional Caucasian Study in Italy. Int. Ophthalmol. 2019, 39, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Chidi-Egboka, N.C.; Jalbert, I.; Golebiowski, B. Smartphone gaming induces dry eye symptoms and reduces blinking in school-aged children. Eye 2023, 37, 1342–1349. [Google Scholar] [CrossRef]
- Golebiowski, B.; Long, J.; Harrison, K.; Lee, A.; Chidi-Egboka, N.; Asper, L. Smartphone Use and Effects on Tear Film, Blinking and Binocular Vision. Curr. Eye Res. 2020, 45, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valerio, M.D.R.; Mohamed-Noriega, K.; Zamora-Ginez, I.; Duarte, B.G.B.; Vallejo-Ruiz, V. Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin. Ophthalmol. 2020, 14, 4311–4317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhu, H.; Mou, Y.; Wu, Y.; He, J.; Huang, X.; Jin, X. Effects on the Ocular Surface from Reading on Different Smartphone Screens: A Prospective Randomized Controlled Study. Clin. Transl. Sci. 2021, 14, 829–836. [Google Scholar] [CrossRef]
- Mou, Y.; Shen, X.; Yuan, K.; Wang, X.; Fan, F.; Wu, Y.; Wang, C.; Jin, X. Comparison of the influence of light between circularly polarized and linearly polarized smartphones on dry eye symptoms and asthenopia. Clin. Transl. Sci. 2022, 15, 994–1002. [Google Scholar] [CrossRef]
- Yammouni, R.; Evans, B.J.W. Is reading rate in digital eyestrain influenced by binocular and accommodative anomalies? J. Optom. 2021, 14, 229–239. [Google Scholar] [CrossRef]
- Mohan, A.; Sen, P.; Shah, C.; Datt, K.; Jain, E. Binocular Accommodation and Vergence Dysfunction in Children Attending Online Classes During the COVID-19 Pandemic: Digital Eye Strain in Kids (DESK) Study-2. J. Pediatr. Ophthalmol. Strabismus 2021, 58, 224–231. [Google Scholar] [CrossRef]
- Maharjan, U.; Rijal, S.; Jnawali, A.; Sitaula, S.; Bhattarai, S.; Shrestha, G.B. Binocular vision findings in normally-sighted school aged children who used digital devices. PLoS ONE 2022, 17, e0266068. [Google Scholar] [CrossRef]
- Talens-Estarelles, C.; García-Marqués, J.V.; Cervino, A.; García-Lázaro, S. Use of digital displays and ocular surface alterations: A review. Ocul. Surf. 2021, 19, 252–265. [Google Scholar] [CrossRef]
- Singh, S.; McGuinness, M.B.; Anderson, A.J.; Downie, L.E. Interventions for the Management of Computer Vision Syndrome: A Systematic Review and Meta-analysis. Ophthalmology 2022, 129, 1192–1215. [Google Scholar] [CrossRef]
- Mylona, I. Spotlight on Digital Eye Strain. Clin. Ophthalmol. 2023, 15, 29–36. [Google Scholar] [CrossRef]
- Pucker, A.D.; Kerr, A.M.; Sanderson, J.; Lievens, C. Digital Eye Strain: Updated Perspectives. Clin. Optom. 2024, 16, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Asper, L.; Long, J.; Lee, A.; Harrison, K.; Golebiowski, B. Ocular and visual discomfort associated with smartphones, tablets and computers: What we do and do not know. Clin. Exp. Optom. 2019, 102, 463–477. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Lingham, G.; Downie, L.E.; Huntjens, B.; Inomata, T.; Jivraj, S.; Kobia-Acquah, E.; Muntz, A.; Mohamed-Noriega, K.; Plainis, S.; et al. TFOS Lifestyle: Impact of the Digital Environment on the Ocular Surface. Ocul. Surf. 2023, 28, 213–252. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2021; Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 19 April 2024).
- Sterne, J.; Savović, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Seguí, M.; Cabrero-garcía, J.; Crespo, A.; Verdú, J.; Ronda, E. A Reliable and Valid Questionnaire Was Developed to Measure Computer Vision Syndrome at the Workplace. J. Clin. Epidemiol. 2015, 68, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Jamal, N. Convergence insufficiency—A major review. Optometry 2012, 83, 137–158. [Google Scholar]
- Cacho-Martínez, P.; Cantó-Cerdán, M.; Lara-Lacárcel, F.; García-Muñoz, Á. Assessing the role of visual dysfunctions in the association between visual symptomatology and the use of digital devices. J. Optom. 2024, 17, 100510. [Google Scholar] [CrossRef]
- Sánchez-Brau, M.; Domenech-Amigot, B.; Brocal-Fernández, F.; Quesada-Rico, J.A.; Seguí-Crespo, M. Prevalence of Computer Vision Syndrome and Its Relationship with Ergonomic and Individual Factors in Presbyopic VDT Workers Using Progressive Addition Lenses. Int. J. Environ. Res. Public Health 2020, 17, 1003. [Google Scholar] [CrossRef]
- De-Hita-Cantalejo, C.; García-pérez, Á.; Capote-puente, R.; Sánchez-gonzález, M.C. Accommodative and binocular disorders in preteens with computer vision syndrome: A cross-sectional study. Ann. N. Y. Acad. Sci. 2021, 1492, 73–81. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, K.Y.; Gao, S.A.; Yang, J.R.; Qiu, W.Q. Correlation between Eye Movements and Asthenopia: A Prospective Observational Study. J. Clin. Med. 2022, 11, 7043. [Google Scholar] [CrossRef]
- Auffret, E.; Mielcarek, M.; Bourcier, T.; Delhommais, A.; Sauer, A. Stress oculaire induit par les écrans. Analyses des symptômes fonctionnels et de l’équilibre binoculaire chez des utilisateurs intensifs. J. Fr. Ophtalmol. 2022, 45, 438–445. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, P.; Deng, X.W.; Liang, J.Q.; Liao, Y.R.; Fan, S.X.; Xiao, J.H. Eye Habits Affect the Prevalence of Asthenopia in Patients with Myopia. J. Ophthalmol. 2022, 2022, 8669217. [Google Scholar] [CrossRef]
- De-Hita-Cantalejo, C.; Sanchez-Gonzalez, J.M.; Silva-Viguera, C.; Sanchez-Gonzalez, M.C. Tweenager Computer Visual Syndrome Due to Tablets and Laptops during the Postlockdown COVID-19 Pandemic and the Influence on the Binocular and Accommodative System. J. Clin. Med. 2022, 11, 5317. [Google Scholar] [CrossRef] [PubMed]
- Porcar, E.; Montalt, J.C.; Pons, Á.M.; España-Gregori, E. Symptomatic accommodative and binocular dysfunctions from the use of flat-panel displays. Int. J. Ophthalmol. 2018, 11, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Auffret, E.; Gomart, G.; Bourcier, T.; Gaucher, D.; Speeg-Schatz, C.; Sauer, A. Digital eye strain. Symptoms, prevalence, pathophysiology, and management. J. Fr. Ophtalmol. 2021, 44, 1605–1610. [Google Scholar] [CrossRef]
- Alvarez, T.L.; Scheiman, M.; Santos, E.M.; Yaramothu, C.; D’Antonio-Bertagnolli, J.V. Convergence Insufficiency Neuro-mechanism in Adult Population Study Randomized Clinical Trial: Clinical Outcome Results. Optom. Vis. Sci. 2020, 97, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Jain, R.; Kamath, M.A.; Bappal, A. A study on correlation of computer vision syndrome and dry eye disease and knowledge regarding its associated factors amongst health professionals. Indian J. Ophthalmol. 2023, 71, 1441–1445. [Google Scholar] [CrossRef]
- Chidi-Egboka, N.C.; Jalbert, I.; Wagner, P.; Golebiowski, B. Blinking and normal ocular surface in school-aged children and the effects of age and screen time. BMJ Ophthalmol. 2023, 107, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Brau, M.; García González, G. Prevalencia del síndrome visual informático (SVI) en trabajadores présbitas. Arch. Prev. Riesgos Labor. 2021, 24, 200–203. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Wang, M.T.M.; Vidal-Rohr, M.; Menduni, F.; Dhallu, S.; Ipek, T.; Acar, D.; Recchioni, A.; France, A.; Kingsnorth, A.; et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul. Surf. 2021, 21, 58–63. [Google Scholar] [CrossRef]
- Sharbini, S.; Hamid, Z.; Abdul Rahman, H.; Idris, F.; Naing, L. The Development and Validation of a Questionnaire Measuring Digital Eye Strain and Risk Level (DESRIL-27). Res. Sq. 2023, 1–19. [Google Scholar] [CrossRef]

| Authors | Year | Country | Sample (n, Age, Years) | Type of Study | Methods | Questionnaire | Main Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Golebiowski, B., et al. [15] | 2020 | Australia | 12 (18–23) | Cross-sectional (pilot study) | Evaluation before and after reading for 60 min on smartphone): ocular surface, horizontal fixation disparity and ease of binocular accommodation. | Questionnaires on eye strain and ocular surface symptoms. | ↑ Symptoms after smartphone use (comfort, tiredness and drowsiness; p ≤ 0.02). Binocular accommodative facility (cycles/min) difference (CI 95%) = −3.0 (−5.7, −0.5), p = 0.01. Accommodative facility the median pre-task was 11.3 cycles/min (IQR 6.6), and the post-task was 7.8 cycles/min (IQR 2.5), p = 0.01. | Small sample size Short-term intervention |
| Sánchez-Brau, M., et al. [33] | 2020 | Spain | 109 VDT workers mean = 54 ± 4.8 | Cross-sectional | VDT exposure daily duration (hours), cumulative years of use, and screen technology. Viewing distance, eye-screen angle and neck posture. Frontocometer, refraction and binocular VA. | CVS-Q | ↑ risk of CVS symptoms: Female: OR = 3.40; 95% CI, 1.12–10.33; p = 0.031. Non-neutral neck posture: OR = 3.27; 95% CI, 1.03–10.41; p = 0.045. Altered lighting: OR = 3.64; 95% CI, 1.22–10.81; p = 0.020. | Single Visit Self-reported screen time Limited assessment of lens characteristics |
| Yammouni, R., & Evans, B. [19] | 2021 | United Kingdom | 107 mean = 31 | Cross-sectional | Ophthalmoscopy, slit lamp biomicroscopy, retinoscopy, subjective refraction and accommodation and binocular tests. | CVS Q SANDE | Kendall’s tau correlation revealed no significant associations between amplitude of accommodation and % WRRT and CVS-Q. Other measures, such as accommodation lag, ±1.50 facility, NPC and vergence facility were also non-significant. | Lacks a control group without DES |
| De-Hita-Cantalejo, C., et al. [34] | 2021 | Spain | 309 mean = 10.75 ± 0.67 | Cross-sectional | Two groups: mild and severe CVS. Visual acuity testing, cover test, accommodation and vergence. | CVSS17 | Breakdown (p = 0.03) and recovery (p = 0.02) of the NPC. Distance of break and recovery of NFV (p = 0.02 and p < 0.01, respectively). | Uneven distribution of symptom severity Lack of follow-up Limited generalizability due to sample setting |
| Liu, Z., et al. [35] | 2022 | China | 93 mean = 24.09 ± 2.57 | Cohort | Before and after a 20 min reading period: demographic characteristics and daily computer use. Eye movements and accommodation parameters. | CVS Q | CVS-Q score and PRA: r = 0.206 (p = 0.047); difficulty focusing for near vision was positively correlated with: TFF r = 0.279 (p = 0.007), TFD r = 0.235 (p = 0.023), TVD r = 0.253 (p = 0.014), and RS r = 0.237 (p = 0.022). Feeling of sight worsening was positively correlated with regressive saccades r = 0.27 (p = 0.011). | Small sample size Lack of ocular surface assessment Short-term intervention |
| Auffret, E., et al. [36] | 2022 | France | 24 control mean = 28.6 ± 9.2 28 exposed mean = 35.2 ± 11.4 | Cohort (pilot study) | Changes in binocularity in short-term exposure to the screen in 2 groups at 2 points in time: a group exposed <5/d and a group exposed >5 h/d. Assesses the consequences of chronic exposure—exposure to the screen >5 h/d, 5 days a week, for 1 year. Ocular discomfort questionnaire and binocular function. | Ocular discomfort questionnaire | Ocular discomfort score > in the exposed group: 0.3 vs. 0.6 (p = 0.04); blurred intermediate group control 0.3 and group exposed 1 (p = 0.02) vision; light sensitivity control group 0.8 and exposed group 1.4 (p = 0.04). FV in synoptophore—group control 18.09° and group exposed 13.42° (p = 0.045). BAF—group control 10.17 cycles/min and group exposed 6.00 cycles/min (p = 0.038). No significant differences were found between the control and exposed groups in NPC or NPA. | Small sample size Inclusion of participants with suboptimal optical correction |
| Wang, J. et al. [37] | 2022 | China | 65 myopic mean = 20 ± 14.5 | Cross-sectional | Subjective refraction, BCVA, surface ocular and binocular vision tests. | Questionnaire of asthenopia symptoms | 57% of myopic patients had asthenopia; ↑ prevalence in older patients (p = 0.004). Asthenopia prevalence rate in myopic patients with <2 h of outdoor activities: 69%. Asthenopia prevalence in myopic patients with dry eye: 87%. Daily screen time (p = 0.003), continuous near work time (p < 0.001), eye care education (p = 0.002) and dry eye (p = 0.008) were positively correlated with asthenopia. Eye care education OR = 0.115 (p = 0.006) is a protective factor and continuous time working nearby OR = 4.227 (p = 0.046), indicating an increased risk of asthenopia. | Small sample size Non-standardized questionnaire |
| De-Hita-Cantalejo, C., et al. [38] | 2022 | Spain | 118 (10–12) | Case–control | Two groups: low demand digital devices and high-demand digital devices. Visual acuity; accommodation amplitude, posture and facility. | CVS-Q | Only visual acuity showed a statistically significant difference between groups: visual acuity both eyes LDDD 1.22 ± 0.01 HDDD 0.62 ± 0.05 (p < 0.01). | Lack of follow-up Self-reported screen time Age group differences |
| Maharjan, U., et al. [21] | 2022 | Nepal | 180 (7–17) | Cross-sectional | Ophthalmology and binocular vision examinations. Two groups: user group—digital devices in the last 6 months; non-user group—not used digital devices in the last 6 months. The user group was subdivided: low users (<3 h/d and 1 day a week) and high users (>3 h/d and every day of the week). | Parents were asked about the amount of time children use digital devices | Accommodative amplitudes, accommodative ease, and positive fusional vergence for both near and distance were significantly reduced in the high digital device user group (p < 0.01). ↑ Prevalence of accommodative and vergence anomalies (except vergence insufficiency) in the subgroup of high users of digital devices (p < 0.01). | Non-standardized questionnaire No assessment of subjective symptoms Focus solely on objective measurements |
| Cacho-Martínez, P., et al. [32] | 2024 | Spain | 346 mean = 32.95 ± 14.56 | Prospective study | Refractive examination: retinoscopy and subjective examination. Accommodative tests: Monocular AA, MAF and BAF (±2.00D), MEM dynamic retinoscopy, and PRA e NRA. Binocular vision tests: CT; PFV e NFV, Worth test and stereopsis. | SQVD | 57.2% reported visual symptoms. 65.3%, some form of visual dysfunction (objective measure). <35 years, an association was found between having visual symptoms and digital device use (OR = 2.10, p = 0.01), adjusting for visual dysfunctions; this association disappeared (OR = 1.44, p = 0.27). >35 years, no association was found between symptoms and the use of digital devices (OR = 1.27, p = 0.49). | Self-reported screen time |
| Authors | Year | Country | Sample (n and Age, Years) | Type of Study | Methods | Questionnaire | Main Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Golebiowski, B., et al. ([15]) | 2020 | Australia | 12 (18–23) | Cross-sectional pilot study | Evaluation before and after exposure (reading for 60 min on smartphone) NIBUT, lipid layer appearance, TMH. | Questionnaires for eye fatigue and ocular surface symptoms. | ↑ incomplete blinks per minute (median of 6 at baseline to 15 after 60 min of screen use; p = 0.0049). No significant differences were observed in TMH, NIBUT, lipid layer grade, or total blink rate over time. | Small sample size Non-standardized questionnaire Short-term intervention |
| Sanchez-Valerio, M., et al. [16] | 2020 | México | 108 VDT workers mean = 32.1 ± 7.8 | Cross-sectional online | Three groups: Mild Exposure Group (less than 3 h/d); Moderate Exposure Group (between 3 and 7 h/d); Severe Exposure Group (more than 8 h/d). TBUT, ocular surface staining and Schirmer test. | CVSS17 OSDI Questionnaire: time of exposure to the computer and the type of VDT used. | 50.9% used a laptop, 38% desktop and 11.1% used both Average computer exposure time: 5.96 ± 2.5 h/d. 79.7% had symptoms of DED according to the OSDI. 97.2% had changes in tear breakup time, 44.4% had damage to the ocular surface and 26.9% had reduced watery tear production. Computer exposure time was positively correlated with DED and negatively correlated with TBUT (p < 0.001). Accumulated screen exposure time negatively correlated with TBUT (p < 0.001) and positively correlated with ocular surface damage (p < 0.001). No significant correlation was found between exposure time and Schirmer. | Self-reported screen time |
| Yuan, K., et al. [17] | 2021 | China | 119 university students mean = 24.76 | Prospective randomized controlled study | Continued reading for 2 h on different smartphone screens. Evaluation groups: Light + OLED; Light + eINK; Dark + OLED; Dark + eINK. Eye examinations: VA; NIBUT; lacrimal meniscus alterations; hyperemia; FBUT; CFS; Meibomius gland assessment, Shirmer I test; blink frequency. Parameters assessed BEFORE and AFTER 2 h reading tasks at 40 cm. | OSDI CVS-Q | iBUT after 2 h reading on the OLED screen compared to the baseline in light and dark environments. ↓ in tear meniscus after OLED reading (p < 0.001). ↑ blink rate with OLED screen (p < 0.001). ↑ocular hyperemia in the nasal and temporal area of the bulbar conjunctiva (p < 0.001). The eINK screen had a minor effect on tear film stability, tear volume and eye redness. Reading on an eINK screen did not exacerbate eye symptoms. | Short-term intervention |
| Mou, Y., et al. [18] | 2022 | China | 120 university students mean = 25.86 ± 2.31 | Prospective randomized controlled study | Evaluation groups with 30 participants: 4 groups: Circular + Dark; Circular + Light; Linear + Dark; Linear + Light. Eye examinations: CFF; TMH, NTBUT, redness of the conjunctiva, iBUT, CFS, Schimer I test. | OSDI CVS-17 CISS VAS | ↑ OSDI scores of the linearly polarized light and dark (p < 0.001 and p < 0.001). ↓ NIBUT after reading in linearly polarized (p < 0.001). ↓ meniscus and Schirmer on linear. ↓ FTBUT in all groups, with the difference in ΔFTBUT present only between the linear and dark circular polarization (p < 0.05). | No adjustment for statistical multiplicity |
| Agarwal, R., et al. [4] | 2022 | India | 435 mean = 35 | Cross-sectional online | Online, questionnaire-based | Sociodemographic details, reason for increased screen time, number of hours, time spent on screens, common complaints and measures taken to overcome fatigue. | ↑ screen time with lockdown (89% of participants); 81.4% had at least one symptom related to screens. Common symptoms were as follows: 52.8% eye pain/fatigue, 31.3% headache and 19.7% dry eyes/frequent blinking. 42.9% of participants reported that symptoms occurred frequently (daily or 2–3 times a week). 45.7% of participants adopted measures such as anti-reflective glasses, screen filters, increasing room lighting and decreasing screen brightness. Positive and significant correlation between the number and frequency of symptoms and the use of multiple screens, time spent on digital screens and continuous screen time. | Non-standardized questionnaire |
| Patel, H., et al. [42] | 2023 | India | 501 mean = 23.14 ± 2.47 | Cross-sectional study | Visual acuity using and anterior segment examination with a slit lamp. Patients with a score of 6 or more underwent dry eye examination tests: TMH, TBUT, and the Schirmer I test. | CVS-Q; questionnaire on awareness of CVS and use of digital gadgets. Questionnaire assessing the frequency and intensity of symptoms over time. Questionnaire on knowledge, attitude and prevalence of dry eye. | Most common symptom was headache, 66.7% occasionally and 6.8% always. 47.7% reported that closing their eyes provided relief from dry eye. 56.1% used mobile phones and laptops. 46.3% reported using digital devices for 3–5 h and 35.7% using them for 1–3 h. | Self-reported screen time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata, M.J.; Aguiar, P.; Grzybowski, A.; Moreira-Rosário, A.; Lança, C. A Review of Digital Eye Strain: Binocular Vision Anomalies, Ocular Surface Changes, and the Need for Objective Assessment. J. Eye Mov. Res. 2025, 18, 39. https://doi.org/10.3390/jemr18050039
Barata MJ, Aguiar P, Grzybowski A, Moreira-Rosário A, Lança C. A Review of Digital Eye Strain: Binocular Vision Anomalies, Ocular Surface Changes, and the Need for Objective Assessment. Journal of Eye Movement Research. 2025; 18(5):39. https://doi.org/10.3390/jemr18050039
Chicago/Turabian StyleBarata, Maria João, Pedro Aguiar, Andrzej Grzybowski, André Moreira-Rosário, and Carla Lança. 2025. "A Review of Digital Eye Strain: Binocular Vision Anomalies, Ocular Surface Changes, and the Need for Objective Assessment" Journal of Eye Movement Research 18, no. 5: 39. https://doi.org/10.3390/jemr18050039
APA StyleBarata, M. J., Aguiar, P., Grzybowski, A., Moreira-Rosário, A., & Lança, C. (2025). A Review of Digital Eye Strain: Binocular Vision Anomalies, Ocular Surface Changes, and the Need for Objective Assessment. Journal of Eye Movement Research, 18(5), 39. https://doi.org/10.3390/jemr18050039










